Abstract
In the last few decades, there is an increasing emergence and re-emergence of viruses, such as West Nile virus, Enterovirus 71 and henipaviruses that cause epidemic viral encephalitis and other central nervous system (CNS) manifestations. The mortality and morbidity associated with these outbreaks are significant and frequently severe. While aspects of epidemiology, basic virology, etc., may be known, the pathology and pathogenesis are often less so, partly due to a lack of interest among pathologists or because many of these infections are considered “third world” diseases. In the study of epidemic viral encephalitis, the pathologist’s role in unravelling the pathology and pathogenesis is critical. The novel henipavirus infection is a good example. The newly created genus Henipavirus within the family Paramyxoviridae consists of two viruses, viz., Hendra virus and Nipah virus. These two viruses emerged in Australia and Asia, respectively, to cause severe encephalitides in humans and animals. Studies show that the pathological features of the acute encephalitis caused by henipaviruses are similar and a unique dual pathogenetic mechanism of vasculitis-induced microinfarction and parenchymal cell infection in the CNS (mainly neurons) and other organs causes severe tissue damage. Both viruses can cause relapsing encephalitis months and years after the acute infection due to a true recurrent infection as evidenced by the presence of virus in infected cells. Future emerging viral encephalitides will no doubt continue to pose considerable challenges to the neuropathologist, and as the West Nile virus outbreak demonstrates, even economically advanced nations are not spared.
Keywords: Vasculitis, Encephalitis, West Nile Virus, Japanese Encephalitis Virus, Viral Encephalitis
Introduction
The last few decades saw continuing outbreaks of human infections involving many pathogenic viruses from practically all major families of viruses. Some of these viruses are known and endemic while others are novel. Some of the known viruses are emerging again to cause more outbreaks and in places not previously known for outbreaks to occur. Many of these viruses cause severe human disease and may affect many different organ systems. The most devastating epidemic in the recent past is of course due to the human immunodeficiency virus, which causes infection of many organ systems including the central nervous system (CNS). The influenza viruses (H5N1, H1N1, etc.) pose a serious health threat to millions of people by causing severe respiratory syndromes [1, 3].
Viral encephalitis due to known arboviruses, such as Japanese encephalitis (JE) virus and tick-borne encephalitis (TBE) virus continue to cause widespread infections and deaths in endemic countries. In the Indian subcontinent, thousands of JE virus infections, still occur despite the availability of vaccines [26]. In the Far East, Russia and eastern Europe, TBE recurs regularly to cause deaths [51]. Because TBE is occurring in increasing numbers and in previously unaffected areas, it is now considered to be emerging. West Nile virus (WN), another known arbovirus usually found in Africa, Europe and Asia, recently emerged for the first time to cause severe disease in humans and animals in North America in 1999 [9, 44, 48]. In a short span of a decade [48], it has spread to the whole of North America to be the most important emerging epidemic encephalitis. The virus has caused neuroinvasive disease in more than 11,000 people with a mortality of 9%.
The enteroviruses are still responsible for outbreaks of CNS infections despite the worldwide retreat of poliovirus. The most important of these is probably Enterovirus 71 because of the very high mortality in patients who develop encephalomyelitis, although fortunately this complication is rare [40, 81]. Nonetheless, it is emerging in many previously unaffected areas, particularly in Asia, where typically, children develop CNS disease in a background of epidemic hand, foot and mouth disease [90].
Another important group of viruses that has recently emerged to cause epidemic encephalitis are the henipaviruses. This group of viruses from the genus Henipavirus (family Paramyxoviridae) comprises the Hendra (HeV) virus and Nipah virus (NiV). Like other paramyxoviruses, henipaviruses are enveloped, negative-strand RNA viruses. Because of the novel nature of this new genus, the high mortality and the unique pathogenesis of the disease, this review will focus specially on henipavirus infection as previous reviews has focused mainly on NiV alone [81, 86]. The pathology and pathogenesis shall be highlighted and compared with other viral encephalitides. For other important epidemic viral encephalitides, there are existing good reviews [26, 32, 51].
HeV was first isolated after an outbreak in horses and two humans in the town of Hendra, Australia in 1994 [56, 72]. Following this, there were more outbreaks in horses and humans and to date a total of six cases with three fatalities have been reported [35, 60, 64]. All the human cases have had close contact with infected horses which are now thought to be the intermediate hosts for HeV transmission [52, 80]. HeV cases have not been reported outside Australia.
The first NiV outbreak started in northern Malaysia in 1998 [7], involving about 27 patients with 15 fatalities [8]. The outbreak which started in pig farms then spread to other farms in the south following transfer of infected pigs [54]. This area became the second and most severely affected epicenter [7], and the virus was named after Kampung Sungai Nipah (Nipah river village) located here. A prevalence of 265 cases of acute NiV encephalitis with 105 fatalities in Malaysia has been reported [62], but if asymptomatic or mildly symptomatic cases are also included, the total number is probably more than 350 cases [86]. Infected pigs exported to Singapore spread the infection to some abattoir workers [7, 63]. After 1999, in Malaysia and Singapore, there are no reports of new NiV outbreaks until 2001 onwards when several recurrent outbreaks of NiV in Bangladesh and India [36, 38, 49] have involved more than 120 people.
Henipavirus transmission to humans
The natural host of henipaviruses has been confirmed to be the fruit bat (Pteropus species), and bat-to-human transmission may be direct or indirect via intermediate hosts [13, 34]. In HeV transmission, the horse is the intermediate host [64, 72]. As virus could be detected in oronasal secretions and urine from infected horses, contact with these secretions appears to be the most likely route of transmission [21, 80]. Although person-to-person HeV transmission has not been reported, involvement of the lung and kidney in acute infection and the presence of virus in nasopharyngeal secretions strongly suggest this possibility [64, 85]. The mode of bat-to-horse transmission remains unclear, but may be due to the ingestion of feed or pasture contaminated by bat-derived foetal tissues or urine [80]. Outside Australia, HeV has not been isolated from bats.
In the first known NiV outbreak in Malaysia and Singapore, the intermediate host is the pig as it is clear that direct contact with infected pigs or fresh pig products was responsible for viral transmission. In Malaysia, a higher prevalence of infection was found among pig farmers, abattoir workers, pork sellers and army personnel involved in culling of pigs [2, 10, 62, 65, 69]. Widespread surveillance of pig populations and culling of sick animals stopped the Malaysian epidemic [8], while the banning of pig imports and abattoir closure stopped the outbreak in Singapore [8, 10]. It was suggested that bat-to-pig transmission could result from pigs ingesting half-eaten, contaminated fruits dropped by bats near farms [13].
Person-to-person transmission is very rare in Malaysia. In a large serological survey of health staff, serum neutralisation tests were all negative [55]. However, a nurse who had previously cared for a NiV-infected patient, seroconverted but remained asymptomatic although the brain MRI showed a few discrete lesions typically seen in acute NiV encephalitis [75, 76].
In contrast to the Malaysian outbreak, the Bangladeshi/Indian outbreaks showed a high incidence of person-to-person transmission either to health care workers or other people who had contact with patients [31, 36, 39]. Person-to-person transmission could be explained by the presence of virus in patients’ secretions [14]. Moreover, no animal has been positively identified as intermediate hosts so far, although in some outbreaks, patients were reported to have had contact with sick animals (pig, cow and goat) [49]. Date palm sap, a local delicacy in Bangladesh, has been implicated in some cases [50]. Palm sap drip collected overnight into open pots tied onto palm trees allow foraging fruit bats to feed and contaminate the sap thus transmitting virus to people who drink the raw sap.
Clinical manifestations of henipavirus infection
The incubation period appears to range from a few days to 2 weeks [11, 27, 64, 72]. Milder clinical features include fever, influenza-like illness, headache and drowsiness [11, 27, 35, 72].
Severe HeV infection can manifest either as a neurological or a pulmonary syndrome, but since only very few patients have been involved, it is not well characterised as NiV infection. Neurological signs include confusion, motor deficits and seizures while the pulmonary syndrome comprises an influenza-like illness, hypoxaemia, diffuse alveolar shadowing in chest X-rays [64, 72].
Severe NiV infection is characterised predominantly by an acute febrile encephalitic syndrome. In a cohort of 90 patients with acute NiV encephalitis, the main presenting features were fever, headache, dizziness, vomiting, and reduced level of consciousness [27]. In fact, more than 50% of patients have some degree of reduced consciousness. Clinical signs, such as areflexia, hypotonia, abnormal pupillary response, tachycardia, hypertension, abnormal doll’s eye reflex and segmental myoclonus, suggested involvement of the brainstem and upper cervical cord. Segmental myoclonus was characterised by focal, rhythmic jerking of the diaphragm and muscles in the limbs, neck and face. Meningism and generalised tonic-clonic convulsions were also observed.
A pulmonary syndrome appears to occur in a minority of patients. In the same cohort only 14% was reported to have unproductive cough [27]. In another Malaysian hospital series, 24% of patients had abnormal findings in the chest X-rays, but none had severe lung disease [11]. In the Singapore series of 11 patients, 3 were clinically thought to have atypical pneumonia with abnormal chest X-rays [63].
Brain MRI scans in acute henipavirus encephalitis show multiple, disseminated, small discrete hyperintense lesions mainly in the cortex, subcortical and deep white matter [47, 64, 70]. In three Bangladeshi patients with apparent acute NiV encephalitis and available brain MRI findings, only one patient showed the same discrete hyperintense lesions while other two patients showed multiple confluent lesions [66].
Specific anti-henipavirus IgM and IgG antibodies that can be detected in the serum and CSF in most patients are critical to diagnosis. More is known about seroconversion after NiV infection than HeV infection. Overall, antibodies are more likely to be positive in serum than CSF. In NiV infection, IgM seroconversion by day 4 is about 65%, and by day 12, 100%. IgM can persist for at least 3 months. There is 100% IgG, seroconversion by day 25 [67] and IgG levels may persist for several years [12]. Using either serology or IHC in autopsy tissues, a positive diagnosis can be made in a majority of NiV cases, and when these diagnostic methods are used in combination, the diagnosis can be confirmed in all cases [87]. Specific neutralising antibodies, IgM or IgG have been reported in HeV infected patients [35, 60, 72].
Complications and sequelae in henipavirus infection
Mortality in HeV infection is about 50%, while mortality in severe NiV infection is about 40% (Malaysia) to 70% (Bangladesh/India) [36, 38, 62]. In many of the Malaysian patients who recovered, there were no apparent serious sequelae [27]. Neuropsychiatric sequelae have been reported [59]. Fatal intracerebral haemorrhage is a rare complication [27]. It is not known if post-infectious encephalomyelitis occurs following acute henipavirus infection.
Henipavirus infection may be complicated by relapsing encephalitis following apparent recovery. One case of relapsing HeV encephalitis and more than 20 cases of relapsing NiV encephalitis (probably <10% of survivors) have been reported thus far [12, 60, 74]. Some cases of relapsing NiV encephalitis only had mild symptoms such as fever and headache during the acute phase, and hence have also been called “late-onset” encephalitis. The single case of relapsing HeV encephalitis occurred about 13 months after exposure, while an average of 8 months elapsed before relapsing NiV encephalitis occurred. Clinical and radiological findings suggest that relapsing NiV encephalitis is distinct from acute NiV encephalitis [70, 74]. The brain MRI in relapsing henipavirus encephalitis shows patchy, confluent hyper-intense cortical lesions.
Pathology of henipavirus infection
The macroscopic features of the HeV-infected brain have not been reported while the features in the NiV-infected brain are non-specific; no discrete lesions could be identified. Our current knowledge of the microscopic pathology of henipavirus infection is based on autopsy tissues: 2 autopsies of HeV infection and more than 30 autopsies of NiV infection [85, 87]. In general, the microscopic features in these two infections appear to be very similar, hence they will be discussed together.
Acute henipavirus infection
One of the most important targets in acute henipavirus infection is the endothelium and smooth muscle of blood vessels. True vasculitis characterized by varying degrees of segmental endothelial ulceration, karyorrhexis, intramural necrosis and inflammatory cells is observed in blood vessels of the brain (Fig. 1a), lung, kidney, heart and many other major organs. Milder subendothelial inflammation (endothelitis) may also be seen (Fig. 1b). In some vessels particularly in NiV infection, occasional endothelial multinucleated giant cell (Fig. 1d) can be detected (about 30% of cases). Thrombosis is often associated with vasculitis and some vessels may be completely obliterated by thrombotic plugs (Fig. 1a). Viral antigens (Fig. 1c), RNA and nucleocapsids can be detected in endothelium and multinucleated giant cells, and vascular smooth muscle. In NiV infection, there is a suggestion that CNS vascular susceptibility is the highest compared to vessels in other organs. Typically, small vessels, e.g. capillaries, small arteries and venules show evidence of vasculitis, but not the larger vessels. Focal haemorrhages may be observed near vascular lesions.
Fig. 1.
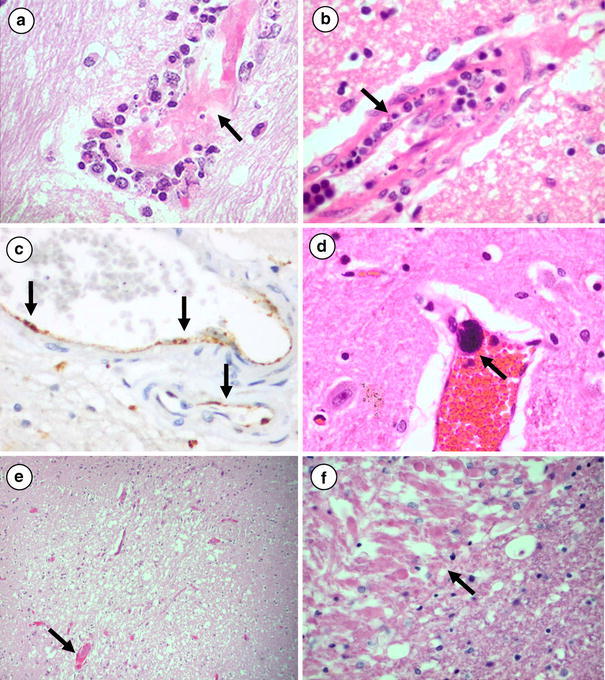
Vascular and CNS pathology in acute henipavirus infection. a Cerebral vasculitis in acute Nipah virus encephalitis characterised by intramural inflammatory cells and necrosis and thrombo-occlusion of the lumen (arrow). b More subtle endotheliitis showing subendothelial infiltration of inflammatory cells (arrow) in acute Hendra virus encephalitis. c Immunolocalisation of Nipah viral antigens in endothelium (arrows). d Multinucleated giant cell (arrow) arising from the endothelium of a non-inflamed blood vessel in acute Nipah encephalitis. e Necrotic plaque in the cerebral cortex of acute Nipah encephalitis with adjacent thrombosed vessel (arrow). f White matter necrotic plaque in acute Hendra encephalitis that consists of eosinophilic, axonal spheroid-like material (arrow). H&E stains: a, b, d, e, h; immunoperoxidase stain using cross-reactive, anti-Hendra polyclonal antibodies with diaminobenzidine substrate and haematoxylin counterstain, c. Original magnifications: ×400 (a–d, f); ×40 (e)
In the CNS, the main pathological findings are vasculitis (with or without thrombosis), parenchymal necrosis and evidence of viral infection in neuroglial cells. Vascular lesions in both grey and white matter are seen throughout the CNS and these are often associated with discrete necrotic or more subtle vacuolar plaque-like lesions. The necrotic plaque-like lesions are characterized by varying degrees of necrosis, neuropil vacuolation/oedema and mild inflammation (Fig. 1e). In neuronal areas, there may be some neuronal loss as well. In the white matter, well-developed plaques consist of eosinophilic, necrotic material similar to axonal spheroids seen in diffuse axonal injury (Fig. 1f). Inflammatory cells when present comprise neutrophils, macrophages, lymphocytes, plasma cells and reactive microglia. In the HeV-infected molecular layer of the cerebellum, subtle vacuolar plaques are paler staining and consist of small fine vacuoles associated with an increase of CD68-positive macrophages/microglia which may be difficult to detect without IHC. In some cases, focal neuronophagia, microglial nodule formation, clusters of foamy macrophages, perivascular cuffing and meningitis can be found. Some neurons show the rare perineuronal microcystic change often in the vicinity of NiV-necrotic plaques.
Viral inclusions, antigens, RNA and nucleocapsids are observed mainly in neurons (soma and processes), although the very rare ependymal cell or astrocyte may be involved [28, 41, 87]. Neuronal viral inclusions can be found in the cytoplasm and nuclei, mostly near vasculitic vessels or necrotic plaques. Cytoplasmic inclusions are usually small, discrete, eosinophilic, and sometimes multiple (Fig. 2a, b). Nuclear inclusions are less commonly found and occupy most of the nucleus. Inclusions were reported in 62% of cases in one series. Viral antigen/RNA positive neurons (Fig. 2c) found at the periphery of necrotic/vacuolar plaques may form concentric or eccentric rings. Some plaque-like, groups of positive neurons are not associated with prominent necrosis, vacuolation or oedema (Fig. 2d). Necrotic plaques in the white matter are generally not associated with viral antigens/RNA. In acute NiV encephalitis, the level of viral antigens peaks at about 6–10 days, and are largely cleared after approximately 14 days. In a case of resolving acute NiV encephalitis who died several months after the infection, the brain showed randomly distributed, discrete slit-like, oval or spherical lesions in the grey and white matter. Most lesions consist of foamy macrophages, lymphocytes and reactive gliosis (unpublished observations). There was no evidence of vasculitis, but some residual perivascular cuffing remained.
Fig. 2.
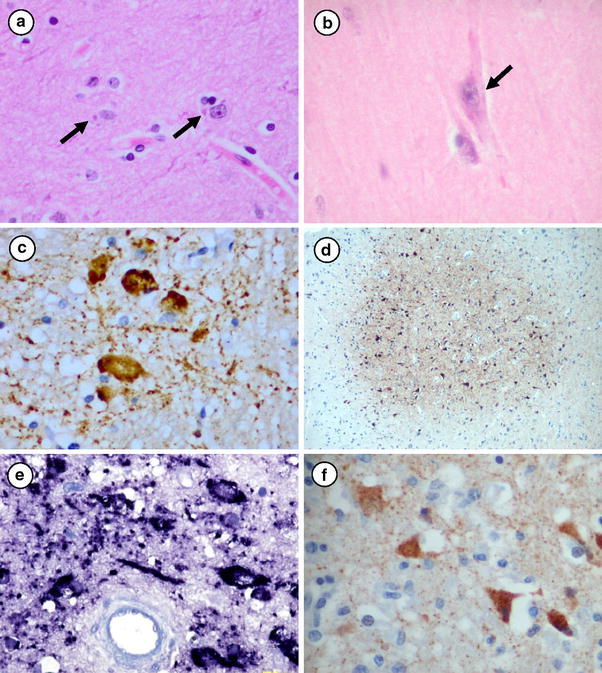
CNS pathology in human henipavirus encephalitis. Cytoplasmic eosinophilic viral inclusions in a acute Nipah virus encephalitis (arrows), and b acute Hendra virus encephalitis (arrow). c Inclusion-like and granular Nipah viral antigens in neurons and neuropil. d Plaque-like, cluster of neurons immunostained for viral antigens in acute Hendra virus encephalitis. e Viral RNA within neurons in relapsing Nipah encephalitis. f Viral antigens within neurons in relapsing Hendra encephalitis. H&E stains, a, b; immunoperoxidase using cross reactive anti-Hendra polyclonal antibodies with diaminobenzidine substrate and haematoxylin counterstain, c, d, f; in situ hybridization using specific Nipah virus riboprobes with NBT/BCIP substrate and haematoxylin counterstain, e. Original magnifications: ×40 (d); ×400 (a–c, e, f)
In the lung, apart from vasculitis, there is parenchymal inflammation, necrosis, intra-alveolar macrophages/inflammatory cells, type II pneumocyte proliferation, alveolar membranes and haemorrhage. Occasionally, intra-alveolar multinucleated giant cells with nuclear inclusions are noted. In the kidney, the rare focal glomerulitis with or without thrombosis or necrosis, and focal inflammation around necrotic tubules may be observed. The heart and lymph node may also show features of infection. Viral antigens are demonstrable in the lung parenchyma including alveolar type II pneumocytes, intra-alveolar macrophages, and renal glomeruli and tubules.
Relapsing henipavirus encephalitis
The pathological features of relapsing henipavirus encephalitis are based on an autopsy case of HeV [85] and two autopsies of relapsing NiV encephalitis [74, 87]. Macroscopically, relapsing NiV encephalitis shows varying degrees of confluent softening and necrosis in the cerebral cortex and subcortical areas such as the thalamus and basal ganglia. The microscopic features of relapsing HeV and NiV encephalitis again appear to be similar and the pathology is confined to the CNS. Other non-CNS organs are essentially normal and vasculitis is absent throughout.
In affected neuronal areas (cerebral cortex, basal ganglia brainstem, etc.), confluent and extensive parenchymal necrosis, oedema and inflammation are seen with some spill over into adjacent white matter. Inflammatory cells consist of macrophages, lymphocytes and some plasma cells, with prominent perivascular cuffing. In many areas, severe neuronal loss is replaced by reactive glial and prominent vascular proliferation. Although viral inclusions can be found, the most prominent inclusions are found in relapsing NiV encephalitis. Focal viral antigens/RNA and nucleocapsids are demonstrated mainly in surviving neurons (Fig. 2e, f), ependyma and possibly in other glial or inflammatory cells as well. Neuronophagia and prominent microglial nodules are rarely observed. Severe meningitis is found in many areas. Vasculitis, endothelial syncytia or thrombosis as seen in acute henipavirus encephalitis are absent. Blood vessels are all negative for antigen/RNA.
Comparative pathology of viral encephalitides
Certain pathological features in henipavirus encephalitis may be distinctive enough to suggest the diagnosis, particularly if this can be confirmed by IHC, ISH, serology, RT–PCR and virus culture. Perhaps the most unique finding in acute henipavirus encephalitis is the multinucleated endothelial cell found in about 30% of NiV cases. This feature has not been described in other viral encephalitides. Extensive vasculitis found in all cases is probably a more useful feature to diagnose acute henipavirus encephalitis. In viral encephalitis, CNS vasculitis is rarely encountered with the exception of varicella-zoster and herpes simplex which may be associated with granulomatous angiitis [23, 71], a feature not seen in acute henipavirus infection. In the case of varicella zoster, usually larger vessels are implicated, but the type of vascular lesions may be variable [43]. Other non-viral pathogens including rickettsiae, and Neisseria [73] may be associated with vasculitis. In rickettsial encephalitis, vasculitis and necrosis are more subtle and less prominent [79]. Other CNS changes in acute henipavirus encephalitis, such as perivascular cuffing, parenchymal inflammation and neuronophagia, are rather non-specific features and can be found in other viral encephalitides [20].
Vasculitis, a key event in the pathogenesis of acute henipavirus infection, follows from vascular endothelial and smooth muscle cell involvement resulting in thrombosis, vascular occlusion, ischaemia, microinfarction, and probably thromboembolism as well. These vascular lesions contribute to the formation of necrotic/vacuolar plaques that seem to correlate with the multiple discrete lesions seen in brain MRI studies [47, 70]. However, neuronal infection also contributes to plaque formation especially in the cerebral grey matter and brain stem. This dual pathogenetic mechanism in the CNS and other organs appear to be unique to henipaviruses. Necrotic/vacuolar plaques in the white matter are caused mainly by ischaemia/microinfarction since glial cells are far less susceptible to infection. Vasculitic vessels probably cause a breach in the blood–brain barrier to facilitate virus escape into the parenchyma to infect neurons. Inter-neuronal spread further into the periphery of the plaque may contribute to dissemination. In subacute sclerosing panencephalitis (SSPE), it is believed that endothelial infection facilitates measles virus entry into the brain although vasculitis is absent [15].
The neuron is often the main, if not the only target cell, of most neurotropic viruses, including JE virus [16], TBE virus [24], West Nile virus [30], rabies [42], measles, herpesviruses and enterovirus 71 [83]. Neuronal infection most likely leads to viral cytolysis and cell damage in various critical parts of the CNS leading to an encephalitic syndrome. Hence, significant pathological changes such as viral inclusions and virus localisation/detection by various means are expected to be found in relation to the neuron. Since viral inclusions can be found in many other viral encephalitides, it is not that helpful for the diagnosis of henipavirus encephalitis. Compared to endothelium and neurons, glial and epithelial cells are rarely involved. This may be related to the density of ephrin B2 and B3 recently found to be the receptors for henipavirus although this has not been studied [4, 57, 58]. A recent paper suggests that the blood vessel may also be a target in JE, but this has yet to be confirmed [25]. Macrophages are the main infected cells in subacute HIV encephalitis [22].
IHC is very useful to detect viral antigens to confirm the diagnosis of henipavirus infection and indeed in numerous other viral encephalitides as well. Both polyclonal and monoclonal antibodies to NiV and HeV have been produced and many of them are cross reactive and sensitive enough to detect both infections [53, 77, 87, 89]. Unfortunately, most antibodies suitable for IHC are proprietary and not commercially available. Needless to say, a good knowledge of target cells is needed to assess the IHC assay, and in the case of henipavirus, viral antigen localisation in neurons and blood vessels are useful for diagnosis. If available, ISH which detects viral RNA is also a useful adjunct to tissue diagnosis of henipavirus infection [84].
One of the most interesting complications of acute henipavirus infection is relapsing encephalitis. Fortunately, it is relatively rare and not uniformly fatal. The presence of viral inclusions, nucleocapsids, antigens and RNA confirms relapsing henipavirus encephalitis as a recurrent infection rather than post-infectious encephalitis [74]. It is assumed that if recurrent viruses were from extra-CNS sites, then viraemia (and vasculitis) have to occur to enable virus to enter the CNS similar to acute henipavirus encephalitis. Hence, the absence of vasculitis and extra CNS organ involvement indirectly suggests reactivation of latent viral foci from within the CNS, introduced during the acute infection. Vasculitis-induced thrombosis, ischaemia and microinfarction do not appear to play a role in contrast to acute henipavirus encephalitis.
The risk factors for relapsing henipavirus encephalitis are unknown. Clinically, relapsing henipavirus encephalitis does share some similarities with SSPE. However, the latter typically sets in several years after the acute infection and may be more often fatal. Nonetheless, like relapsing henipavirus encephalitis, SSPE is not invariably fatal and recurrences have been reported [12, 17, 74]. Virus genomic mutations reported in SSPE is one possible mechanism for henipaviruses to remain latent and escape the immune response [6]. So far, no viral mutations have been found in relapsing henipavirus encephalitis [82]. Measles virus is well known to cause immune suppression [29] and being paramyxoviruses themselves, henipaviruses may similarly cause immune suppression that might impact on the development of relapsing encephalitis. Further investigations are much needed to unravel the pathogenesis of relapsing henipavirus infection.
Conclusion
It is apparent that NiV and HeV, both from the same Henipavirus genus, share many common clinicopathological features suggesting that the pathogenesis of the human diseases, respectively, are essentially the same.
The outbreaks of henipavirus infections in Asia and Australia are prime examples of emerging zoonotic infectious diseases that continue to occur worldwide, many of them associated with severe epidemic encephalitis. As far as newly emerging or novel viruses are concerned, one of the most important natural host is the bat. As many as 40 viruses have recently been isolated from bat species in as many years, though not all have been shown to be pathogenic to humans. These include Ebola virus, Australian bat lyssavirus, SARS coronavirus, Menangle virus, Tioman virus, henipaviruses [5, 18, 88]. Because of the worldwide range of bats and their ability to fly to large areas of human habitat, they are very effective for virus dissemination under the right conditions. In the case of henipaviruses, the range of pteropid bats includes Southeast Asia, China, Japan, Oceania, the Indian subcontinent, Australia and Africa, and there is evidence of bat infection in many of these countries [13, 19, 33, 34, 37, 39, 45, 46, 61, 68, 78]. Hence, future henipavirus outbreaks can be expected in these regions.
Needless to say, the role of pathologists in understanding the pathology and pathogenesis of emerging viral encephalitides is critical. The relative lack of interest in these infections among pathologists especially in more developed countries is perhaps not surprising, as they are often regarded as tropical or “third world” diseases. However, with increasing air travel and ability of viruses to emerge in previously unaffected areas, e.g. West Nile virus in North America, it is quite clear that greater efforts should be made to study these diseases and to improve diagnostic capabilities. Further understanding of the pathology and pathogenesis of emerging epidemic viral encephalitides should continue to contribute significantly to the development of therapeutic strategies and vaccines.
References
- 1.Abdel-Ghafar AN, Chotpitayasunondh T, Gao Z, et al. Update on avian influenza A (H5N1) virus infection in humans. N Engl J Med. 2008;358:261–273. doi: 10.1056/NEJMra0707279. [DOI] [PubMed] [Google Scholar]
- 2.Ali R, Mounts AW, Parashar UD, et al. Nipah virus among military personnel involved in pig culling during an outbreak of encephalitis in Malaysia, 1998–1999. Emerg Infect Dis. 2001;7:759–761. doi: 10.3201/eid0704.010433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bautista E, Chotpitayasunondh T, Gao Z, et al. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 2010;362:1708–1719. doi: 10.1056/NEJMra1000449. [DOI] [PubMed] [Google Scholar]
- 4.Bonaparte MI, Dimitrov AS, Bossart KN, et al. Ephrin-B2 is a functional receptor for Hendra virus and Nipah virus. Proc Natl Acad Sci USA. 2005;102:10652–10657. doi: 10.1073/pnas.0504887102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T. Bats: important reservoir hosts of emerging viruses. Clin Microbiol Rev. 2006;19:531–545. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cattaneo R, Schmid A, Spielhofer P, et al. Mutated and hypermutated genes of persistent measles viruses which caused lethal human brain diseases. Virology. 1989;173:415–425. doi: 10.1016/0042-6822(89)90554-0. [DOI] [PubMed] [Google Scholar]
- 7.CDC (1999) Outbreak of Hendra-like virus - Malaysia and Singapore, 1998–1999. MMWR Morb Mortal Wkly Rep 48:265–269 [PubMed]
- 8.CDC Update: outbreak of Nipah virus—Malaysia and Singapore, 1999. MMWR Morb Mortal Wkly Rep. 1999;48:335–337. [PubMed] [Google Scholar]
- 9.CDC West Nile virus update—United States, January 1-November 13, 2007. MMWR Morb Mortal Wkly Rep. 2007;56:1191–1192. [PubMed] [Google Scholar]
- 10.Chew MH, Arguin PM, Shay DK, et al. Risk factors for Nipah virus infection among abattoir workers in Singapore. J Infect Dis. 2000;181:1760–1763. doi: 10.1086/315443. [DOI] [PubMed] [Google Scholar]
- 11.Chong HT, Kunjapan SR, Thayaparan T, et al. Nipah encephalitis outbreak in Malaysia, clinical features in patients from Seremban. Neurol J Southeast Asia. 2000;5:61–67. doi: 10.1017/s0317167100001785. [DOI] [PubMed] [Google Scholar]
- 12.Chong HT, Tan CT. Relapsed and late-onset Nipah encephalitis, a report of three cases. Neurol J Southeast Asia. 2003;8:109–112. [Google Scholar]
- 13.Chua KB, Koh CL, Hooi PS, et al. Isolation of Nipah virus from Malaysian island flying-foxes. Microbes Infect. 2002;4:145–151. doi: 10.1016/S1286-4579(01)01522-2. [DOI] [PubMed] [Google Scholar]
- 14.Chua KB, Lam SK, Goh KJ, et al. The presence of Nipah virus in respiratory secretions and urine of patients during an outbreak of Nipah virus encephalitis in Malaysia. J Infect. 2001;42:40–43. doi: 10.1053/jinf.2000.0782. [DOI] [PubMed] [Google Scholar]
- 15.Cosby SL, Brankin B. Measles virus infection of cerebral endothelial cells and effect on their adhesive properties. Vet Microbiol. 1995;44:135–139. doi: 10.1016/0378-1135(95)00006-V. [DOI] [PubMed] [Google Scholar]
- 16.Desai A, Shankar SK, Ravi V, Chandramuki A, Gouri-Devi M. Japanese encephalitis virus antigen in the human brain and its topographic distribution. Acta Neuropathol. 1995;89:368–373. doi: 10.1007/BF00309631. [DOI] [PubMed] [Google Scholar]
- 17.Donner M, Waltimo J, Porras J, Forsius H, Saukkonen AL. Subacute sclerosing panencephalitis as a cause of chronic dementia and relapsing brain disorder. J Neurol Neurosurg Psychiatry. 1972;35:180–185. doi: 10.1136/jnnp.35.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eaton BT. Introduction to current focus on Hendra and Nipah viruses. Microbes Infect. 2001;3:277–278. doi: 10.1016/S1286-4579(01)01380-6. [DOI] [PubMed] [Google Scholar]
- 19.Epstein JH, Prakash V, Smith CS, et al. Henipavirus infection in fruit bats (Pteropus giganteus), India. Emerg Infect Dis. 2008;14:1309–1311. doi: 10.3201/eid1408.071492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esiri MM, Kennedy PGE (1997) Viral diseases. In: Graham D, Lantos P (eds) Greenfield’s neuropathology. Arnold, London, pp 3–63
- 21.Field H, Schaaf K, Kung N, et al. Hendra virus outbreak with novel clinical features, Australia. Emerg Infect Dis. 2010;16:338–340. doi: 10.3201/eid1602.090780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frosch MP, Anthony DC, De Girolami U (2005) The central nervous system. In: Kumar V, Abbas AK, Fausto N (eds) Robbins and Cotran pathologic basis of disease, chap 28. Elsevier, Amsterdam, pp 1347–1418
- 23.Fukumoto S, Kinjo M, Hokamura K, Tanaka K. Subarachnoid hemorrhage and granulomatous angiitis of the basilar artery: demonstration of the varicella-zoster-virus in the basilar artery lesions. Stroke. 1986;17:1024–1028. doi: 10.1161/01.str.17.5.1024. [DOI] [PubMed] [Google Scholar]
- 24.Gelpi E, Preusser M, Garzuly F, Holzmann H, Heinz FX, Budka H. Visualization of Central European tick-borne encephalitis infection in fatal human cases. J Neuropathol Exp Neurol. 2005;64:506–512. doi: 10.1093/jnen/64.6.506. [DOI] [PubMed] [Google Scholar]
- 25.German AC, Myint KS, Mai NT, et al. A preliminary neuropathological study of Japanese encephalitis in humans and a mouse model. Trans R Soc Trop Med Hyg. 2006;100:135–145. doi: 10.1016/j.trstmh.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Ghosh D, Basu A. Japanese encephalitis- a pathological and clinical perspective. PLoS Negl Trop Dis. 2009;3:e437. doi: 10.1371/journal.pntd.0000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goh KJ, Tan CT, Chew NK, et al. Clinical features of Nipah virus encephalitis among pig farmers in Malaysia. N Engl J Med. 2000;342:1229–1235. doi: 10.1056/NEJM200004273421701. [DOI] [PubMed] [Google Scholar]
- 28.Goldsmith CS, Whistler T, Rollin PE, et al. Elucidation of Nipah virus morphogenesis and replication using ultrastructural and molecular approaches. Virus Res. 2002;92:89–98. doi: 10.1016/S0168-1702(02)00323-4. [DOI] [PubMed] [Google Scholar]
- 29.Griffin DE, Bellini WJ (1996) Measles virus. In: Fields B, Knipe D, Howley P, Chanock R, Melnick J, Monath T et al. (eds) Field’s virology. Lippincott-Raven, Philadelphia, pp 1267–1312
- 30.Guarner J, Shieh WJ, Hunter S, et al. Clinicopathological study and laboratory diagnosis of 23 cases with West Nile virus encephalomyelitis. Hum Pathol. 2004;35:983–990. doi: 10.1016/j.humpath.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Gurley ES, Montgomery JM, Hossain MJ, et al. Person-to-person transmission of Nipah virus in a Bangladeshi community. Emerg Infect Dis. 2007;13:1031–1037. doi: 10.3201/eid1307.061128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gyure KA. West Nile virus infections. J Neuropathol Exp Neurol. 2009;68:1053–1060. doi: 10.1097/NEN.0b013e3181b88114. [DOI] [PubMed] [Google Scholar]
- 33.Halpin K, Young PL, Field H, Mackenzie JS. Newly discovered viruses of flying foxes. Vet Microbiol. 1999;68:83–87. doi: 10.1016/S0378-1135(99)00063-2. [DOI] [PubMed] [Google Scholar]
- 34.Halpin K, Young PL, Field HE, Mackenzie JS. Isolation of Hendra virus from pteropid bats: a natural reservoir of Hendra virus. J Gen Virol. 2000;81:1927–1932. doi: 10.1099/0022-1317-81-8-1927. [DOI] [PubMed] [Google Scholar]
- 35.Hanna JN, McBride WJ, Brookes DL, et al. Hendra virus infection in a veterinarian. Med J Aust. 2006;185:562–564. doi: 10.5694/j.1326-5377.2006.tb00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harit AK, Ichhpujani RL, Gupta S, et al. Nipah/Hendra virus outbreak in Siliguri, West Bengal, India in 2001. Indian J Med Res. 2006;123:553–560. [PubMed] [Google Scholar]
- 37.Hayman DTS, Suu-Ire R, Breed AC, et al. Evidence of Henipavirus infection in West African fruit bats. PloS One. 2008;3:e2739. doi: 10.1371/journal.pone.0002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hossain MJ, Gurley ES, Montgomery JM, et al. Clinical presentation of Nipah virus infection in Bangladesh. Clin Infect Dis. 2008;46:977–984. doi: 10.1086/529147. [DOI] [PubMed] [Google Scholar]
- 39.Hsu VP, Hossain MJ, Parashar UD, et al. Nipah virus encephalitis emergence, Bangladesh. Emerg Infect Dis. 2004;10:2082–2087. doi: 10.3201/eid1012.040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang CC, Liu CC, Chang YC, Chen CY, Wang ST, Yeh TF. Neurologic complications in children with enterovirus 71 infection. N Engl J Med. 1999;341:936–942. doi: 10.1056/NEJM199909233411302. [DOI] [PubMed] [Google Scholar]
- 41.Hyatt A, Zaki SR, Goldsmith CS, Wise TG, Hengstberger SG. Ultrastructure of Hendra virus and Nipah virus within cultured cells and host animals. Microbes Infect. 2001;3:297–306. doi: 10.1016/S1286-4579(01)01383-1. [DOI] [PubMed] [Google Scholar]
- 42.Jogai S, Radotra BD, Banerjee AK. Immunohistochemical study of human rabies. Neuropathology. 2000;20:197–203. doi: 10.1046/j.1440-1789.2000.00332.x. [DOI] [PubMed] [Google Scholar]
- 43.Kleinschmidt-DeMasters BK, Gilden DH. Varicella-Zoster virus infections of the nervous system: clinical and pathologic correlates. Arch Pathol Lab Med. 2001;125:770–780. doi: 10.5858/2001-125-0770-VZVIOT. [DOI] [PubMed] [Google Scholar]
- 44.Lanciotti RS, Roehrig JT, Deubel V, et al. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 1999;286:2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- 45.Lehle C, Razafitrimo G, Razainirina J, et al. Henipavirus and Tioman virus antibodies in pteropodid bats, Madagascar. Emerg Infect Dis. 2007;13:159–161. doi: 10.3201/eid1301.060791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Wang J, Hickey AC, et al. Antibodies to Nipah or Nipah-like viruses in bats, China. Emerg Infect Dis. 2008;14:1974–1976. doi: 10.3201/eid1412.080359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim CC, Sitoh YY, Hui F, et al. Nipah viral encephalitis or Japanese encephalitis? MR findings in a new zoonotic disease. AJNR Am J Neuroradiol. 2000;21:455–461. [PMC free article] [PubMed] [Google Scholar]
- 48.Lindsey NP, Staples JE, Lehman JA, Fisher M. Surveillance for human West Nile virus disease—United States, 1999–2008. MMWR Surveill Summ. 2010;59:1–17. [PubMed] [Google Scholar]
- 49.Luby SP, Hossain MJ, Gurley ES, et al. Recurrent zoonotic transmission of Nipah virus into humans, Bangladesh, 2001–2007. Emerg Infect Dis. 2009;15:1229–1235. doi: 10.3201/eid1508.081237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luby SP, Rahman M, Hossain MJ, et al. Foodborne transmission of Nipah virus, Bangladesh. Emerg Infect Dis. 2006;12:1888–1894. doi: 10.3201/eid1212.060732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mansfield KL, Johnson N, Phipps LP, Stephenson JR, Fooks AR, Solomon T. Tick-borne encephalitis virus—a review of an emerging zoonosis. J Gen Virol. 2009;90:1781–1794. doi: 10.1099/vir.0.011437-0. [DOI] [PubMed] [Google Scholar]
- 52.McCormack JG, Allworth AM, Selvey LA, Selleck PW. Transmissibility of horses to humans of a noval paramyxovirus, Equine Morbillivirus (EMV) J Infect. 1999;38:22–23. doi: 10.1016/S0163-4453(99)90023-3. [DOI] [PubMed] [Google Scholar]
- 53.Middleton D, Westbury HA, Morrissy CJ, et al. Experimental Nipah virus infection in pigs and cats. J Comp Path. 2002;126:124–136. doi: 10.1053/jcpa.2001.0532. [DOI] [PubMed] [Google Scholar]
- 54.Mohd Nor MN, Gan CH, Ong BL. Nipah virus infection of pigs in peninsular Malaysia. Rev Sci Tech. 2000;19:160–165. doi: 10.20506/rst.19.1.1202. [DOI] [PubMed] [Google Scholar]
- 55.Mounts AW, Kaur H, Parashar UD, et al. A cohort study of health care workers to assess nosocomial transmissibility of Nipah virus, Malaysia, 1999. J Infect Dis. 2001;183:810–813. doi: 10.1086/318822. [DOI] [PubMed] [Google Scholar]
- 56.Murray K, Selleck P, Hooper P, et al. A morbillivirus that caused fatal disease in horses and humans. Science. 1995;268:94–97. doi: 10.1126/science.7701348. [DOI] [PubMed] [Google Scholar]
- 57.Negrete OA, Levroney EL, Aguilar HC, et al. Ephrin B2 is the entry receptor for Nipah virus, an emergent deadly paramyxovirus. Nature. 2005;436:401–405. doi: 10.1038/nature03838. [DOI] [PubMed] [Google Scholar]
- 58.Negrete OA, Wolf MC, Aguilar HC, et al. Two key residues in ephrinB3 are critical for its use as an alternative receptor for Nipah virus. Plos Pathog. 2006;2(2):e7. doi: 10.1371/journal.ppat.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ng BY, Lim CC, Yeoh A, Lee WL. Neuropsychiatric sequelae of Nipah virus encephalitis. J Neuropsychiatry Clin Neurosci. 2004;16:500–504. doi: 10.1176/jnp.16.4.500. [DOI] [PubMed] [Google Scholar]
- 60.O’Sullivan JD, Allworth AM, Paterson DL, et al. Fatal encephalitis due to novel paramyxovirus transmitted from horses. Lancet. 1997;349:93–95. doi: 10.1016/S0140-6736(96)06162-4. [DOI] [PubMed] [Google Scholar]
- 61.Olson JG, Rupprecht C, Rollin PE, et al. Antibodies to Nipah-like virus in bats (Pteropus lylei), Cambodia. Emerg Infect Dis. 2002;8:987–988. doi: 10.3201/eid0809.010515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parashar UD, Sunn LM, Ong F, et al. Case–control study of risk factors for human infection with the new zoonotic paramyxovirus, Nipah virus, during a 1998–1999 outbreak of severe encephalitis in Malaysia. J Infect Dis. 2000;181:1755–1759. doi: 10.1086/315457. [DOI] [PubMed] [Google Scholar]
- 63.Paton NI, Leo YS, Zaki SR, et al. Outbreak of Nipah-virus infection among abattoir workers in Singapore. Lancet. 1999;354:1253–1256. doi: 10.1016/S0140-6736(99)04379-2. [DOI] [PubMed] [Google Scholar]
- 64.Playford EG, McCall B, Smith G, et al. Human Hendra virus encephalitis associated with equine outbreak, Australia, 2008. Emerg Infect Dis. 2010;16:219–223. doi: 10.3201/eid1602.090552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Premalatha GD, Lye MS, Arokiasamy J, et al. Assessment of Nipah virus transmission among pork sellers in Seremban, Malaysia. Southeast Asian J Trop Med Public Health. 2000;31:307–309. [PubMed] [Google Scholar]
- 66.Quddus R, Alam S, Majumdar MA, et al. A report of 4 patients with Nipah encephalitis from Rajbari district, Bangladesh in the January 2004 outbreak. Neurology Asia. 2004;9:33–37. [Google Scholar]
- 67.Ramasundram V, Tan CT, Chua KB, et al. Kinetics of IgM and IgG seroconversion in Nipah virus infection. Neurol J Southeast Asia. 2000;5:23–28. [Google Scholar]
- 68.Reynes JM, Counor D, Ong S, et al. Nipah Virus in Lyle’s Flying Foxes, Cambodia. Emerg Infect Dis. 2005;11:1042–1046. doi: 10.3201/eid1107.041350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sahani M, Parashar U, Ali R, et al. Nipah virus infection among abbatoir workers in Malaysia, 1998–1999. Int J Epidemiol. 2001;30:1017–1020. doi: 10.1093/ije/30.5.1017. [DOI] [PubMed] [Google Scholar]
- 70.Sarji SA, Abdullah BJJ, Goh KJ, Tan CT, Wong KT. Magnetic resonance imaging features of Nipah encephalitis. AJR. 2000;175:437–442. doi: 10.2214/ajr.175.2.1750437. [DOI] [PubMed] [Google Scholar]
- 71.Schmitt JA, Dietzmann K, Muller U, Krause P. Granulomatous vasculitis-an uncommon manifestation of herpes simplex infection of the central nervous system. Zentralbl Pathol. 1992;138:298–302. [PubMed] [Google Scholar]
- 72.Selvey LA, Wells RM, McCormack JG, et al. Infection of humans and horses by a newly described morbillivirus. Med J Aust. 1995;162:642–645. doi: 10.5694/j.1326-5377.1995.tb126050.x. [DOI] [PubMed] [Google Scholar]
- 73.Somer T, Finegold SM. Vasculitidies associated with infection, immunization, and antimicrobial drugs. Clin Infect Dis. 1995;20:1010–1036. doi: 10.1093/clinids/20.4.1010. [DOI] [PubMed] [Google Scholar]
- 74.Tan CT, Goh KJ, Wong KT, et al. Relapsed and late-onset Nipah encephalitis. Ann Neurol. 2002;51:703–708. doi: 10.1002/ana.10212. [DOI] [PubMed] [Google Scholar]
- 75.Tan CT, Tan KS. Nosocomial transmissibility of Nipah virus. J Infect Dis. 2001;184:1367. doi: 10.1086/323996. [DOI] [PubMed] [Google Scholar]
- 76.Tan KS, Sarji SA, Tan CT, et al. Patients with asymptomatic Nipah virus infection may have abnormal cerebral MR imaging. Neurol J Southeast Asia. 2000;5:69–73. [Google Scholar]
- 77.Tanimura N, Imada T, Kashiwazaki Y, et al. Reactivity of anti-Nipah virus monoclonal antibodies to formalin-fixed, paraffin embedded lung tissues from experimental Nipah and Hendra virus infections. J Vet Med Sci. 2004;66:1263–1266. doi: 10.1292/jvms.66.1263. [DOI] [PubMed] [Google Scholar]
- 78.Wacharapluesadee S, Lumlertdache B, Boongird K, et al. Bat Nipah virus, Thailand. Emerg Infect Dis. 2005;11:1949–1951. doi: 10.3201/eid1112.050613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Walker DH, Dumler JS (1997) Rickettsial infections. In: Connor D, Chandler F, Schwartz D, Manz H, Lack E (eds) Pathology of infectious diseases, Appleton and Lange, Stamford, pp 789–799
- 80.Williamson M, Hooper P, Selleck P, et al. Transmission studies of Hendra virus (equine morbillivirus) in fruit bats, horses and cats. Aust Vet J. 1998;76:813–818. doi: 10.1111/j.1751-0813.1998.tb12335.x. [DOI] [PubMed] [Google Scholar]
- 81.Wong KT. Emerging and re-emerging epidemic encephalitis: a tale of two viruses. Neuropathol Appl Neurobiol. 2000;26:313–318. doi: 10.1046/j.1365-2990.2000.00256.x. [DOI] [PubMed] [Google Scholar]
- 82.Wong KT. Nipah and Hendra viruses: recent advances in pathogenesis. Future Virol. 2010;5:129–131. doi: 10.2217/fvl.10.7. [DOI] [Google Scholar]
- 83.Wong KT, Badmanthan M, Ong KC, et al. The distribution of inflammation and virus in human Enterovirus 71 encephalomyelitis suggest possible viral spread by neural pathways. J Neuropathol Exp Neurol. 2008;67:162–169. doi: 10.1097/nen.0b013e318163a990. [DOI] [PubMed] [Google Scholar]
- 84.Wong KT, Grosjean I, Brisson C, et al. A golden hamster model for human acute Nipah virus infection. Am J Pathol. 2003;163:2127–2137. doi: 10.1016/S0002-9440(10)63569-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wong KT, Robertson T, Ong BB, et al. Human Hendra virus infection causes acute and relapsing encephalitis. Neuropathol Appl Neurobiol. 2009;35:296–305. doi: 10.1111/j.1365-2990.2008.00991.x. [DOI] [PubMed] [Google Scholar]
- 86.Wong KT, Shieh W-J, Zaki SR, Tan CT. Nipah virus infection, an emerging paramyxoviral zoonosis. Springer Semin Immunopathol. 2002;24:215–228. doi: 10.1007/s00281-002-0106-y. [DOI] [PubMed] [Google Scholar]
- 87.Wong KT, Shieh WJ, Kumar S, et al. Nipah virus infection: pathology and pathogenesis of an emerging paramyxoviral zoonosis. Am J Pathol. 2002;161:2153–2167. doi: 10.1016/S0002-9440(10)64493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wong S, Lau S, Woo P, Yuen KY. Bats as continuing source of emerging infections in humans. Rev Med Virol. 2007;17:67–91. doi: 10.1002/rmv.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xiao C, Liu J, Jiang Y, et al. Monoclonal antibodies against the nucleocapsid proteins of henipaviruses: production, epitope mapping and application to immunohistochemistry. Arch Virol. 2008;153:273–281. doi: 10.1007/s00705-007-1079-x. [DOI] [PubMed] [Google Scholar]
- 90.Yang F, Ren L, Xiong Z, et al. Enterovirus 71 outbreak in the People’s Republic of China in 2008. J Clin Microbiol. 2009;47:2351–2352. doi: 10.1128/JCM.00563-09. [DOI] [PMC free article] [PubMed] [Google Scholar]


