Abstract
Freezing of gait, defined as sudden and usually brief episodes of inability to produce effective stepping, often results in falls, and is both disabling and common in parkinsonism. In this narrative review, sprung from the 2nd International workshop on Freezing of Gait in Leuven, we summarize the latest insights into clinical and methodological challenges for assessing freezing of gait. We also highlight the role of emerging wearable technology to improve the management of this debilitating symptom.
Keywords: Freezing of gait, wearable sensors, clinical assessment, technology
INTRODUCTION
Freezing of gait (FOG) is among the most disabling symptoms in Parkinson’s disease (PD), as it reduces mobility and frequently results in falls and fall-related injuries1. During an episode of FOG, patients experience a feeling as if their feet are being glued to the floor. These episodes are typically provoked during turning (particularly narrow turns in tight quarters), gait initiation, when walking through narrow passages such as doorways or when performing a concomitant task when walking.2, 3 More recently, it has become clear that anxiety is also a common provocative factor for FOG4, 5.
Different behavioural phenotypes of FOG have been described. One phenotype classification is based on the type of leg movements: 1) FOG with alternating trembling of the legs with a frequency of 3–8 Hz, 2) FOG during which patients make very small shuffling steps, and 3) akinetic FOG when no movements of the legs are observed6. Another phenotype classification is based on the possible underlying neurobiological underpinnings and their related triggering events: 1) motor impairments (eg difficulties to turn on the spot), 2) increased levels of anxiety (eg more freezing when in a hurry) and 3) attentional impairments (eg difficulties performing a cognitive dual task when walking)7. It is yet unclear whether these different manifestations of FOG share the same underlying mechanism.
Despite its common and disabling features in everyday circumstances, FOG is surprisingly difficult to assess in daily clinical practice. This has several reasons. First, FOG disappears when patients shift from an automatic control of gait towards a more goal-directed one8, 9. In other words, gait improves when patients consciously focus on walking, and this is what typically happens when the gait pattern is being examined by a clinician. Second, the typically well-lit and broad hospital corridors without obstacles – where patients are often assessed – are in many ways ideal for NOT provoking FOG, as the phenomenon is commonly provoked in narrow spaces and while turning, or in dimly lit environmnets where patients cannot use visual feedback to compensate10. Third, patients are typically ‘ON’ dopaminergic medication when they visit the clinician, while FOG occurs more often (and is more severe) when the dopaminergic medication has worn off6.
These difficulties to assess FOG are problematic as optimal management depends on valid and reliable assessment3. Assessment involves the very identification of FOG (i.e. the presence or absence of FOG), and also aims to map its’ severity. Reliable assessment of FOG is needed to initiate or change treatment (e.g. to start or increase the dosage of levodopa, or to initiate a referral to a specialised physiotherapist) and to evaluate its effect. Furthermore, the future evaluation of novel symptomatic therapies will demand reliable and objective outcomes.
In this narrative review, we will elaborate on the current state-of-the-art assessment of FOG for clinical and research purposes, as discussed in a specific session during the workshop. Moreover, we will highlight how wearable technology might improve the assessment in the near future, and what challenges need to be overcome to move these novel technologies from the research setting into daily clinical practice.
ASSESSING THE PRESENCE AND SEVERITY OF FOG IN CLINICAL PRACTICE
Presence of FOG
The presence of FOG can be ‘diagnosed’ in a subjective or objective manner. Subjective diagnosis implies that patients are asked whether they sometimes experience the episodic feeling of their feet being glued to the floor, or whether they are episodically unable to move forward3. Hower, such questions are not infallible: not all patients adequately respond to the questions (a commom mistake is that patients deny having FOG, and just inform their clinician about their seemingly spontaneous falling events during turning). It helps to show examples of FOG, for example using the video footage that accompanies the New-Freezing of Gait Questionnaire (N-FOGQ)11. Including the spouse or other immediate caregiver in the interview is mandatory. Importantly, not every gait initiation problem reflects FOG, and is recognised as such. Further, functional (or psychogenic) episodes with gait blocks have also been reported12. Therefore, it is important to diagnose FOG in an objective manner, which currently requires observation of FOG by a trained clinician.
To evaluate FOG fully, it needs to provoked so the typical phenotype can be observed by the physician, but this is challenging for the reasons described above. What complicates matters further is the fact that the available time in daily clinical practice is usually limited13. Hence, tests should ideally be sensitive, but at the same time not time-consuming. Several FOG-provoking-tasks have been described, with rapid 360 degrees turns in place being the most sensitive one14. Importantly, these full turns need to be performed in both directions (because FOG can be very asymmetrical and be present only or predominantly when turning in one direction), likely at fast speed and repeated at least once when negative. Dual-tasking and walking with short rapid steps are less sensitive in provoking FOG compared to such full and rapid turns14, 15. However, when full rapid turns do not yield FOG, addition of these tasks can increase the sensitivity.
Severity of FOG
Like its presence, the severity of FOG can be assessed in a subjective and objective manner. The N-FOGQ is a useful and comprehensive tool to subjectively assess the severity of FOG and its impact on activities of daily living and quality of life11. The N-FOGQ evaluates the frequency of occurrence, and the duration of freezing episodes during gait initiation and turning. In addition, it is well validated and used worldwide11, 16, although the magnitude of the clinically important change has not been well established yet16, 17. A disadvantage of the N-FOGQ is the fact that other provoking circumstances of FOG (such as dual-tasking) are not mapped, and that daytime fluctuations (also in relation to medication intake) are not evaluated. These limitations are addressed in the recently introduced ‘Characterizing Freezing of Gait questionnaire (C-FOG), which showed internal consistency and construct validity in group of 41 PD patients7. Further validation – in a larger group of patients and ideally in a multicentre study – is needed in coming years.
To subjectively document the occurrence of FOG and daytime fluctuations in more detail, diaries can be helpful tools to assess the frequency, duration, and provoking circumstance of FOG18. The use of a diary also has disadvantages; it is not suitable for patients with marked cognitive impairments (which is common in patients with FOG), and it is nearly impossible to record all FOG-episodes in those with severe FOG. Diary use may also wear-off with time19.
Assessing the severity of FOG in an objective way is challenging. A FOG-score has been developed in an attempt to objectively assess the severity of FOG in clinical settings20. It evaluates the occurrence of FOG during four possible triggering circumstances (gait initiation, turning clockwise and counter clockwise and walking through a doorway). These tasks are performed with and without two types of dual tasks. During every triggering circumstance, the gait pattern is rated on a four-point scale (0 when no FOG occurs, 1 in the presence of festination or FOG with shuffling steps, 2 in the presence of akinetic FOG or FOG with alternating trembling of the legs, and 3 when the examiner needs to interfere to overcome the episode of FOG). The FOG-score takes approximately 15 minutes to administer. Although the FOG-score is likely the best way to objectify the severity of FOG at the current time, it also has several disadvantages. For example, the psychometric properties are not well established 16, 21, freezing during straight line walking is not evaluated (while this is reasonably the most severe form of FOG), and the duration of FOG episodes is not considered. And again, daytime fluctuations are not mapped.
Lastly, scoring from actual video recordings22 or computer-generated animations23 (derived from inertial sensors) showed moderate reliability across 10 experts raters for the number of FOG episodes (intraclass correlation coefficient 0.63) and high reliability for the percent time frozen (intraclass correlation coefficient 0.73) in a rather small sample of 10 people with PD while performing a Timed-Up and Go test22. On the one hand, the ability of clinical observers to quantify FOG from actual or computer-generated animations provides a potential approach for validation of novel methods to identify FOG outside of the clinic. On the other hand, it may constitute a burden on movement disorders specialists and may suffer from the abovementioned bias.
To map daytime fluctuations, and to prevent a shift from automatic to goal-directed gait control interfering with the test results, objective assessment of FOG should ideally take place during natural activities in the home or community setting. Currently, objective assessment in the home setting is not commercially available yet, but recent developments in wearable technology might enable this in the near future.
ASSESSING THE PRESENCE AND SEVERITY OF FOG WITH WEARABLE SENSORS
In research laboratory settings, multiple studies have objectively characterized FOG using instruments available in traditional gait laboratories, such as motion analysis to measure body kinematics24, foot switches to measure foot contact, 25, 26 force plates27 to measure forces under the feet or surface EMG28 to characterize lower limb muscle activity. More recently, wearable inertial sensors have been used to characterize episodes of FOG29–32. The possibility of detecting deterioration of gait pattern prior to FOG, and assessing FOG severity in daily life with wearable technology has emerged as a valid, potential tool33, 34. Being able to predict an upcoming FOG episode – by analyzing changes in the quality of gait – is particularly interesting from possible therapeutics perspective, because this would potentially allow for a timely coupling to an intervention to maintain or improve the gait quality and thereby prevent the FOG episode from happening. An additional advantage is that the intervention – such as sensory cues – do not have to be presented continuously to the patient, which may go at the expense of compliance, and which carries the risk of habituation to the cues. Wearable inertial sensors, typically embedding a tri-axial accelerometer, gyroscope and magnetometers, have been used increasingly in the past decade to objectively measure static and dynamic balance in PD, walking and postural transitions both in the laboratory and only very recently in the home settings35.
Regarding the algorithm used to characterize occurrence and severity of FOG, Hausdorff et al. and Moore et al. pioneered the use of leg movement frequency to quantify the typical trembling of the legs during FOG with wearable sensors (foot switches26 or accelerometers31). This approach takes advantage of the fact that ‘high-frequency’ components of movement have been associated with the leg ‘trembling’ phenotype of FOG (at frequencies much higher than that associated with regular gait itself). Therefore, by placing wearable accelerometers on the shins, a frequency ratio can be calculated as the square of the total power of the signal in the 3–8 Hz band, defined as “freezing band” divided by the square of the total power in the 0.5–3 Hz band, defined as “movement band”, see Figure 1.
Figure 1.

Representative examples of the right and left angular velocity and antero-posterior acceleration signals in a subject with PD experiencing freezing. A moving freezing ratio index was calculated as the ratio between high frequency (3–8Hz) and low frequency (0.5–3Hz) from the antero-posterior acceleration.
This algorithm, when applied in the laboratory, can reliably identify the occurrence (start and end) of freezing episodes and measure their duration under controlled conditions such as when initiating gait, turning, approaching an obstacle31 or during a Timed-Up and Go test22. It is based on the assumption that when the frequency ratio exceeds a certain threshold, a FOG episode is considered to have occured. Specifically, 78% of the freezing episodes were detected using a global “one size fits all” threshold for the frequency ratio, and the freezing detection reached 89% when the threshold was calibrated for each subject by accounting for the mean and standard deviation of the frequency of each subjects’ standing31. However, this approach was originally described for offline data analysis and not real-time FOG detection, and, even when correcting for each individual’s standing frequency, is prone to false positives when subjects are at rest or are performing other motor tasks. Furthermore, this approach does not classify any akinetic freezing, because none of the subjects experienced it in the studies reported so far. Akinetic freezing is likely be difficult to detect using the frequency ratio because the typical signature (trembling) is by definition missing. Algorithms can be taight to see that the patient is not moving, but whether this is a voluntary stop or an involuntary stop due to FOG may well be very difficult – if not impossible – to decide.
To overcome some of the latter limitations, different groups have modified the approach to either detect the occurrence and duration of FOG episodes32, 36–41 or to measure overall FOG severity42, 43. Specifically, the introduction of a second threshold, detecting whether relevant motion was present when the frequency ratio was supposedly identifying a FOG episode, significantly improved the algorithm performance to detect FOG occurrence,36 still keeping the computational cost of the algorithm low (low amount of resources necessary to run it) which is essential for online FOG detection. However, these methods seem to work better with relatively long temporal windows (optimal at 4.5s36), and therefore, brief FOG episodes might be missed. More recently, other studies utilizing machine learning approaches have been applied to identify the best set of features to identify a FOG episode from sensors worn on the waist at home or on the shins in the laboratory environment 44–47. Despite the higher sensitivity in detecting the occurrence of even shorter FOG episodes compared to the previous method (an accuracy above 90% was achieved), these approaches may require a higher computational cost (high amount of resources necessary to run the algorithm), requiring up to about a minute from the occurrence of the episode to its detection44.
A recent review summarized the aforementioned studies and pointed out that the achieved sensitivity in detecting FOG occurrence, under controlled (laboratory settings) and uncontrolled (home setting) conditions ranges from 73 to 100%, the specificity ranged from 67 to 100%, and the accuracy ranged from 68% to 96%34. Specifically, among the different sensors used, accelerometers were the most widely used, either as a single sensor or in combination with gyroscopes or magnetometers34. The location used mostly was the shin in 66% of the studies, followed by the waist (33%; see Figure 2).
Figure 2.

Summary of the major sources of variability on the accuracy of FOG detection with wearable sensors.
It is important to note that only a minority of studies were performed in the home setting (only two of the 18 papers in the review), and overall, in both controlled and uncontrolled settings, the validity of such approaches varied considerably (accuracy 68% to 96%). Of the two home-based studies, the most promising one used one sensor on the belt, achieving in the best case 91.7% sensitivity and 87.4% specificity using a machine learning approach trained on 21 patients with PD46. However, this approach has not been validated in a new population, so the performance of this algorithm in another group of people with PD remains unclear. Evaluation of overfitting and validation on a different cohort is of paramount importance when using machine learning approaches that can fit a specific set of data very well, but may fail on a new set48, 49. An additional short report measured the amount of FOG and its variability using a mixed approach (frequency of right and left foot accelerations and correlation of right and left foor angular velocities) with two wearable sensors on the feet worn for seven days at home50. Results showed that quantitative measures of FOG were associated to the perceived FOG, assessed by the N-FOGQ, in a sample of fourteen subjects with FOG50.
Figure 2 summarizes the main sources of variability on accuracy of FOG detection. Specifically, a large amount of variability can be explained by the differences in utilized protocol and sensors (see Figure 3 for sensors placement), with most consistent results when sensors are located on the shins. Another source is the difference in algorithm used, in fact there is clearly a variation across the original Moore Freezing ratio, a Freezing ratio adapted to an individual subject, or one derived from machine learning approaches, which whilst potentially offering better accuracy in detecting FOG may come with temporal delay because of its’ higher computational cost.
Figure 3.
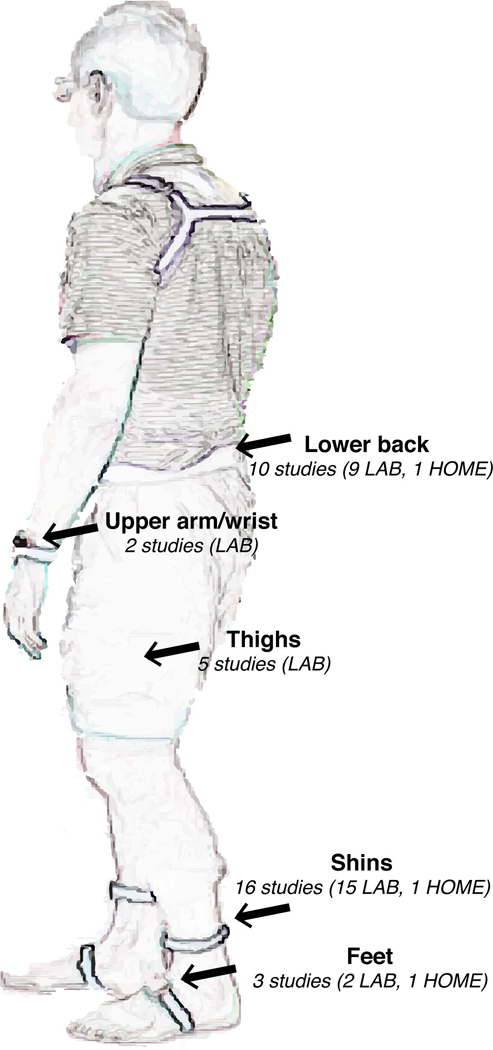
Summary of body position locations used in studying detecting FOG with wearable inertial sensors in the laboratory and in the home settings.
Medication status and heterogeneity of FOG in the measured group are poorly investigated and can represent a source of variability, in fact the majority of subjects tend to experience more freezing when OFF medication and among the subjects included in the same study there may be different subtypes of FOG patients7.
Lastly, an important limitation, common to all the cited studies, is the relatively small sample size, with N varying from 1 to 25, no approach developed in one cohort and tested in another one, and the fact that psychometric properties, apart from validity and reliability, are not fully established (for instance the clinical important change).
There is always a trade-off between optimal sensitivity and specificity on the one hand, and signal processing operations requiring significant time and computational resources on the other hand. When data analysis is done offline, computational resources do not respresent an obstacle. However, when portable devices must be able to detect FOG in real time (for example, because this could trigger a connected cueing device aimed at overcoming the freeze), then processing must be done immediately by high-accuracy algorithms. This processing time may well introduce significant delays in FOG detection. Moreover, the required computational resources could negatively affect power consumption and hence reduce the wear time. This coulbd be a real concern as adoption by patients will diminish if the detection device has to be recharged very frequently.
Are we ready for home monitoring of FOG?
It is important to consider that continuous monitoring of mobility performance during daily life is a relatively new field in general. In fact, only recently, few groups have started to focus on the quality of mobility in PD during daily life, using wearable, light-weight inertial sensors positioned on different body segments51–58. Due to the recent progress in wearable technology, novel measures calculated from both accelerometers and gyroscopes have enabled a more detailed analysis of gait bouts and turning behaviour over a week of continuous recording, as well as analysis of patterns of accumulated activity. Recent studies suggest that the quantity of walking and the quantity of turning (mean number of turns/30min) at home is similar among freezers and non-freezers 55, 57. However, the quality of walking and turning, such as variability and consistency may be different among freezers versus non-freezers with PD55, 57.
As previously mentioned, only few studies have evaluated the presence of FOG in the home environment with wearable sensors. Larger validation studies need to be carried out to identify a valid and reliable measure of FOG as it occurs at home. As walking at home during daily task performance is variable by nature and characterised by short walking bouts59; and validation of freezing detection algorithms has been conducted mainly in relatively controlled circumstances, we reccommend to adapt and validate these algorithm for home use. For example, validation at home could rely on simultaneuous recording of video (camera mounted on the waist to record feet movements) and the wearable used to detect FOG. An interesting question is whether these sensors should be body-worn (e.g. a smartwatch), or perhaps be more loosely attached (e.g. a smartphone), or even incorporated into the patient’s house (e.g. wall-mounted video cameras).
A potential avenue for future development from these findings is the use of smartphones for FOG monitoring at home. Indeed, smartphones are nowadays equipped with accelerometers and gyroscopes, with internet connectivity and they won’t require the patient to wear another device. So far, this avenue is more theoretical, as no studies have been carried out in the home, but some evidence exists demonstrating its feasibility in a laboratory setting60. New challenges arise here, for example the obvious question where the smart phone should be carried in order to perform optimally. Men will likely carry the smartphone in their pockets, but this is less straightforward for women who may carry the phone in their purse. It is as yet unclear how this might affect the accuracy of detecting freezing and other gait parameters. It is important to note that a second process of validation will also require independent confirmation in new cohorts, independent from those where the algorithm was originally developed.
Long-term compliance is a generic problem in the field of wearable sensors, and this will have to be addressed here as well. Finally, further studies are needed to associate the amount of freezing in daily life and its impact on the quality of mobility, on the rate of falls and on the overall physical activities at home.
How wearables could be of further help?
It is well known that FOG is responsive to sensory cues (eg. visual, auditory, tactile)61–64. Ideally, cues should not be delivered continuously, but only when needed, in an on-demand manner. With wearable technology, it could be possible to sense a sudden deterioration in gait pattern that would anticipate a FOG episode and then deliver an immediate cue at that time, to prevent the occurrence of FOG. This has been referred to as intelligent cueing, as recently described in a comprehensive review65.
In order for this online cueing modality to work, a “pre-freezing phase” needs to be characterized reliably. A few studies have investigated the presence of a pre-FOG phase in the laboratory using different apporaches, including motion analysis systems24, surface electromyography28, electrocardiography66 and, more recently, with mobile imaging such as ambulatory EEG67–69, fNIRS70 and wearable inertial sensors71. Nieuwboer et al. found signs of deterioration of the gait pattern as early as 3 steps prior to a FOG episodes, reflected by a decreased stride length and increased or stable cadence72. Similarly, surface EMG activity of the tibialis anterior and gastrocnemius medialis deteriorated in the steps prior to a FOG episode28.
Only few reports have characterized the pre-FOG phase with inertial sensors66, 71. The results were promising, particularly in a study of 11 subjects with PD and FOG where information was combined from accelerometers and gyroscopes. This approach of pre-FOG identification was based on a progressive deterioration of the gait pattern leading to a FOG episode. Briefly, a classifier was built from the three best features in discriminating gait from pre-FOG (considered as the 2 seconds prior to an arrest, as identified from annotated videos), reaching a mean sensitivity and specificity of 83% and 67% in detecting pre-FOG episodes71. However, this model might not work well when walking is not preceding FOG, i.e. for gait initiation, or when the gait pattern is changing anyway, i.e. prior to a turn. Future work must address these situations and verify this promising approach in a new and larger set of freezing episodes. Future work must address these situations and verify this promising approach in a new and larger set of freezing episodes.
Overall, it is clear that wearable sensors have the potential to provide important information on impairments of overall mobility at home in people who experience FOG and can quantify the percentage of time spent freezing during a whole day. Once the validity and reliability of the FOG measures at home is established, these data could be used in clinical decision making, and in on-demand cueing. Additionaly, these derived by wearbles could be used in future pharmacological or rehabilitation trials evaluating the impact of different interventions on FOG in daily life.
Conclusion
The summarized research findings on the measurement of FOG, both in the laboratory and more importantly at home, must be appropriately translated to both clinicians, therapists, caregivers, and patients, with the ultimate aim of improving the management of this debilitating symptom. However, the route to readily achieve this knowledge transfer is not fully clear at this point, although it should follow, where possible, the guidelines recently proposed by the MDS Task Force on Technology73. Focused meetings aiming to determine which kind of information would be most beneficial for clinicians and therapists could be a start. Standardization procedures for protocol and sensors to use in the laboratory/clinic and sharing collected data available across centers, specifically for the data collected at home, would be of tremendous help to validate the most promising approaches to detect FOG.
Acknowledgments
Financial Disclosure for the past year from the date of submission
Dr. Mancini has received grant support from the NIH (R00 078492, R44 AG056012–02) and the Medical Research Foundation of Oregon.
Prof. Bloem currently serves as Associate Editor for the Journal of Parkinson’s disease, serves on the editorial of Practical Neurology and Digital Biomarkers, has received honoraria from serving on the scientific advisory board for Abbvie, Biogen, UCB and Walk with Path, has received fees for speaking at conferences from AbbVie, Zambon and Bial. He has received grant support from: Netherlands Organization for Scientific Research, Stichting Parkinson Fonds, Michael J Fox Foundation, Parkinson Vereniging, Parkinson’s Foundation, Hersenstichting Nederland, Verily Life Sciences, Horizon 2020, Topsector Life Sciences and Health, UCB, and Abbvie. He serves as consultant for Biogen, AbbVie, UCB, and Walk with Path.
Dr. Horak has financial interest in ADPM, a company with commercial interests in technology for movement disoders.This conflict has been reviewed and managed by OHSU. She has received grant support from NIH, DoD, and Medtronic. She serves as consultant for Biogen and Sanofi.
Dr. Lewis has received grant support from an NHMRC-ARC Dementia Fellowship (#1110414).
Dr. Nieuwboer has received grant support from the European Union, Research Funds Flanders, KU Leuven BOF Research Funds, Jacques and Gloria Gossweiler Foundation, Kind Baudouin Foundation and the Michael J Fox Foundation.
Dr. Nonnekes has received grant support from ZonMW Veni (grant 16.196.022) and Michael J Fox Foundation.
Footnotes
Financial Disclosures:
The authors declare no relevant financial disclosures or conflicts of interest with regard to this work.
REFERENCES
- 1.Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011;10(8):734–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okuma Y Practical approach to freezing of gait in Parkinson’s disease. Pract Neurol. 2014;14(4):222–30. [DOI] [PubMed] [Google Scholar]
- 3.Nonnekes J, Snijders AH, Nutt JG, Deuschl G, Giladi N, Bloem BR. Freezing of gait: a practical approach to management. Lancet Neurol. 2015;14(7):768–78. [DOI] [PubMed] [Google Scholar]
- 4.Ehgoetz Martens KA, Ellard CG, Almeida QJ. Does anxiety cause freezing of gait in Parkinson’s disease? PLoS One. 2014;9(9):e106561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehgoetz Martens KA, Lukasik EL, Georgiades MJ, Gilat M, Hall JM, Walton CC, et al. Predicting the onset of freezing of gait: A longitudinal study. Mov Disord. 2018;33(1):128–35. [DOI] [PubMed] [Google Scholar]
- 6.Schaafsma JD, Balash Y, Gurevich T, Bartels AL, Hausdorff JM, Giladi N. Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson’s disease. Eur J Neurol. 2003;10(4):391–8. [DOI] [PubMed] [Google Scholar]
- 7.Ehgoetz Martens KA, Shine JM, Walton CC, Georgiades MJ, Gilat M, Hall JM, et al. Evidence for subtypes of freezing of gait in Parkinson’s disease. Mov Disord. 2018;33(7):1174–8. [DOI] [PubMed] [Google Scholar]
- 8.Hallett M The intrinsic and extrinsic aspects of freezing of gait. Mov Disord. 2008;23 Suppl 2:S439–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, Bergman H, et al. Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nat Rev Neurosci. 2010;11(11):760–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barthel C, Mallia E, Debu B, Bloem BR, Ferraye MU. The Practicalities of Assessing Freezing of Gait. J Parkinsons Dis. 2016;6(4):667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nieuwboer A, Rochester L, Herman T, Vandenberghe W, Emil GE, Thomaes T, et al. Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson’s disease and their carers. Gait Posture. 2009;30(4):459–63. [DOI] [PubMed] [Google Scholar]
- 12.Bloem BR, van Balken IMF, Nonnekes J. Functional freezing. Eur J Neurol. 2017;24(12):e91–e2. [DOI] [PubMed] [Google Scholar]
- 13.van Dijsseldonk K, Wang Y, van Wezel R, Bloem BR, Nonnekes J. Provoking Freezing of Gait in Clinical Practice: Turning in Place is More Effective than Stepping in Place. J Parkinsons Dis. 2018;8(2):363–5. [DOI] [PubMed] [Google Scholar]
- 14.Snijders AH, Haaxma CA, Hagen YJ, Munneke M, Bloem BR. Freezer or non-freezer: clinical assessment of freezing of gait. Parkinsonism Relat Disord. 2012;18(2):149–54. [DOI] [PubMed] [Google Scholar]
- 15.Nonnekes J, Janssen AM, Mensink SH, Oude Nijhuis LB, Bloem BR, Snijders AH. Short rapid steps to provoke freezing of gait in Parkinson’s disease. J Neurol. 2014;261(9):1763–7. [DOI] [PubMed] [Google Scholar]
- 16.Bloem BR, Marinus J, Almeida Q, Dibble L, Nieuwboer A, Post B, et al. Measurement instruments to assess posture, gait, and balance in Parkinson’s disease: Critique and recommendations. Mov Disord. 2016;31(9):1342–55. [DOI] [PubMed] [Google Scholar]
- 17.Martin T, Weatherall M, Anderson TJ, MacAskill MR. A Randomized Controlled Feasibility Trial of a Specific Cueing Program for Falls Management in Persons With Parkinson Disease and Freezing of Gait. J Neurol Phys Ther. 2015;39(3):179–84. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery GK, Reynolds NC. Compliance, Reliability, and Validity of Self-Monitoring for Physical Disturbances of Parkinsons-Disease - the Parkinsons Symptom Diary. Journal of Nervous and Mental Disease. 1990;178(10):636–41. [DOI] [PubMed] [Google Scholar]
- 19.Hunter H, Rochester L, Morris R, Lord S. Longitudinal falls data in Parkinson’s disease: feasibility of fall diaries and effect of attrition. Disabil Rehabil. 2018;40(19):2236–41. [DOI] [PubMed] [Google Scholar]
- 20.Ziegler K, Schroeteler F, Ceballos-Baumann AO, Fietzek UM. A New Rating Instrument to Assess Festination and Freezing Gait in Parkinsonian Patients. Movement Disorders. 2010;25(8):1012–8. [DOI] [PubMed] [Google Scholar]
- 21.Fietzek U, Schulz S, Ziegler K, Ceballos-Baumann AO. The FOG score detects relevant changes of gait freezing for patients and experts. Mov Disord, 2018. [Google Scholar]
- 22.Morris TR, Cho C, Dilda V, Shine JM, Naismith SL, Lewis SJ, et al. A comparison of clinical and objective measures of freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(5):572–7. [DOI] [PubMed] [Google Scholar]
- 23.Morris TR, Cho C, Dilda V, Shine JM, Naismith SL, Lewis SJ, et al. Clinical assessment of freezing of gait in Parkinson’s disease from computer-generated animation. Gait Posture. 2013. [DOI] [PubMed] [Google Scholar]
- 24.Delval A, Snijders AH, Weerdesteyn V, Duysens JE, Defebvre L, Giladi N, et al. Objective detection of subtle freezing of gait episodes in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2010;25(11):1684–93. [DOI] [PubMed] [Google Scholar]
- 25.Hausdorff JM, Schaafsma JD, Balash Y, Bartels AL, Gurevich T, Giladi N. Impaired regulation of stride variability in Parkinson’s disease subjects with freezing of gait. Exp Brain Res. 2003;149(2):187–94. [DOI] [PubMed] [Google Scholar]
- 26.Hausdorff JM, Balash Y, Giladi N. Time series analysis of leg movements during freezing of gait in Parkinson’s disease: Akinesia, rhyme or reason? Physica A: Statistical Mechanics and its Applications 2003;321:565–70. [Google Scholar]
- 27.Nantel J, de Solages C, Bronte-Stewart H. Repetitive stepping in place identifies and measures freezing episodes in subjects with Parkinson’s disease. Gait Posture. 2011;34(3):329–33. [DOI] [PubMed] [Google Scholar]
- 28.Nieuwboer A, Dom R, De Weerdt W, Desloovere K, Janssens L, Stijn V. Electromyographic profiles of gait prior to onset of freezing episodes in patients with Parkinson’s disease. Brain. 2004;127(Pt 7):1650–60. [DOI] [PubMed] [Google Scholar]
- 29.Moore O, Peretz C, Giladi N. Freezing of gait affects quality of life of peoples with Parkinson’s disease beyond its relationships with mobility and gait. Movement disorders : official journal of the Movement Disorder Society. 2007;22(15):2192–5. [DOI] [PubMed] [Google Scholar]
- 30.Moore ST, Dilda V, Hakim B, Macdougall HG. Validation of 24-hour ambulatory gait assessment in Parkinson’s disease with simultaneous video observation. Biomedical engineering online. 2011;10:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore ST, MacDougall HG, Ondo WG. Ambulatory monitoring of freezing of gait in Parkinson’s disease. Journal of neuroscience methods. 2008;167(2):340–8. [DOI] [PubMed] [Google Scholar]
- 32.Zach H, Janssen AM, Snijders AH, Delval A, Ferraye MU, Auff E, et al. Identifying freezing of gait in Parkinson’s disease during freezing provoking tasks using waist-mounted accelerometry. Parkinsonism Relat Disord. 2015;21(11):1362–6. [DOI] [PubMed] [Google Scholar]
- 33.Horak F, King L, Mancini M. Role of body-worn movement monitor technology for balance and gait rehabilitation. Phys Ther. 2015;95(3):461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva de Lima AL, Evers LJW, Hahn T, Bataille L, Hamilton JL, Little MA, et al. Freezing of gait and fall detection in Parkinson’s disease using wearable sensors: a systematic review. J Neurol. 2017;264(8):1642–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Del Din S, Godfrey A, Mazza C, Lord S, Rochester L. Free-living monitoring of Parkinson’s disease: Lessons from the field. Mov Disord. 2016;31(9):1293–313. [DOI] [PubMed] [Google Scholar]
- 36.Bachlin M, Plotnik M, Roggen D, Maidan I, Hausdorff JM, Giladi N, et al. Wearable assistant for Parkinson’s disease patients with the freezing of gait symptom. IEEE transactions on information technology in biomedicine : a publication of the IEEE Engineering in Medicine and Biology Society 2010;14(2):436–46. [DOI] [PubMed] [Google Scholar]
- 37.Cole BT, Roy SH, Nawab SH. Detecting freezing-of-gait during unscripted and unconstrained activity. Conf Proc IEEE Eng Med Biol Soc 2011;2011:5649–52. [DOI] [PubMed] [Google Scholar]
- 38.Moore ST, Yungher DA, Morris TR, Dilda V, MacDougall HG, Shine JM, et al. Autonomous identification of freezing of gait in Parkinson’s disease from lower-body segmental accelerometry. J Neuroeng Rehabil. 2013;10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Capecci M, Pepa L, Verdini F, Ceravolo MG. A smartphone-based architecture to detect and quantify freezing of gait in Parkinson’s disease. Gait Posture. 2016;50:28–33. [DOI] [PubMed] [Google Scholar]
- 40.Pham TT, Moore ST, Lewis SJG, Nguyen DN, Dutkiewicz E, Fuglevand AJ, et al. Freezing of Gait Detection in Parkinson’s Disease: A Subject-Independent Detector Using Anomaly Scores. IEEE Trans Biomed Eng. 2017;64(11):2719–28. [DOI] [PubMed] [Google Scholar]
- 41.Rezvanian S, Lockhart TE. Towards Real-Time Detection of Freezing of Gait Using Wavelet Transform on Wireless Accelerometer Data. Sensors (Basel). 2016;16(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mancini M, Priest KC, Nutt JG, Horak FB. Quantifying freezing of gait in Parkinson’s disease during the instrumented timed up and go test. Conf Proc IEEE Eng Med Biol Soc 2012;2012:1198–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mancini M, Smulders K, Cohen RG, Horak FB, Giladi N, Nutt JG. The Clinical Significance Of Freezing While Turning in Parkinson’s Disease. Neuroscience. 2016;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahlrichs C, Sama A, Lawo M, Cabestany J, Rodriguez-Martin D, Perez-Lopez C, et al. Detecting freezing of gait with a tri-axial accelerometer in Parkinson’s disease patients. Med Biol Eng Comput. 2016;54(1):223–33. [DOI] [PubMed] [Google Scholar]
- 45.Djuric-Jovicic MD, Jovicic NS, Radovanovic SM, Stankovic ID, Popovic MB, Kostic VS. Automatic identification and classification of freezing of gait episodes in Parkinson’s disease patients. IEEE Trans Neural Syst Rehabil Eng. 2014;22(3):685–94. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez-Martin D, Sama A, Perez-Lopez C, Catala A, Moreno Arostegui JM, Cabestany J, et al. Home detection of freezing of gait using support vector machines through a single waist-worn triaxial accelerometer. PLoS One. 2017;12(2):e0171764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tripoliti EE, Tzallas AT, Tsipouras MG, Rigas G, Bougia P, Leontiou M, et al. Automatic detection of freezing of gait events in patients with Parkinson’s disease. Comput Methods Programs Biomed. 2013;110(1):12–26. [DOI] [PubMed] [Google Scholar]
- 48.Ramdhani RA, Khojandi A, Shylo O, Kopell BH. Optimizing Clinical Assessments in Parkinson’s Disease Through the Use of Wearable Sensors and Data Driven Modeling. Front Comput Neurosci. 2018;12:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rush B, Celi LA, Stone DJ. Applying machine learning to continuously monitored physiological data. J Clin Monit Comput. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mancini M, Curtze C, Stuart S, El-Gohary M, McNames James, et al. The Impact Of Freezing Of Gait On Balance Perception And Mobility In Community-Living With Parkinson’S Disease. Conf Proc IEEE Eng Med Biol Soc 2018;2018:3040–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Del Din S, Godfrey A, Galna B, Lord S, Rochester L. Free-living gait characteristics in ageing and Parkinson’s disease: impact of environment and ambulatory bout length. J Neuroeng Rehabil. 2016;13(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Del Din S, Godfrey A, Rochester L. Validation of an accelerometer to quantify a comprehensive battery of gait characteristics in healthy older adults and Parkinson’s disease: toward clinical and at home use. IEEE J Biomed Health Inform. 2015. [DOI] [PubMed] [Google Scholar]
- 53.El-Gohary M, Pearson S, McNames J, Mancini M, Horak F, Mellone S, et al. Continuous monitoring of turning in patients with movement disability. Sensors (Basel). 2013;14(1):356–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mancini M, El-Gohary M, Pearson S, McNames J, Schlueter H, Nutt JG, et al. Continuous monitoring of turning in Parkinson’s disease: Rehabilitation potential. Neurorehabilitation 2015;37(1):3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mancini M, Weiss A, Herman T, Hausdorff JM. Turn Around Freezing: Community-Living Turning Behavior in People with Parkinson’s Disease. Frontiers in Neurology. 2018;9(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weiss A, Brozgol M, Dorfman M, Herman T, Shema S, Giladi N, et al. Does the evaluation of gait quality during daily life provide insight into fall risk? A novel approach using 3-day accelerometer recordings. Neurorehabil Neural Repair. 2013;27(8):742–52. [DOI] [PubMed] [Google Scholar]
- 57.Weiss A, Herman T, Giladi N, Hausdorff JM. New evidence for gait abnormalities among Parkinson’s disease patients who suffer from freezing of gait: insights using a body-fixed sensor worn for 3 days. J Neural Transm (Vienna). 2015;122(3):403–10. [DOI] [PubMed] [Google Scholar]
- 58.Weiss A, Herman T, Giladi N, Hausdorff JM. Objective assessment of fall risk in Parkinson’s disease using a body-fixed sensor worn for 3 days. PLoS One. 2014;9(5):e96675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Galperin I, Hillel I, Del Din S, Bekkers EMJ, Nieuwboer A, Abbruzzese G, et al. Associations between daily-living physical activity and laboratory-based assessments of motor severity in patients with falls and Parkinson’s disease. Parkinsonism Relat Disord. 2019. [DOI] [PubMed] [Google Scholar]
- 60.Kim HB, Lee HJ, Lee WW, Kim SK, Jeon HS, Park HY, et al. Validation of Freezing-of-Gait Monitoring Using Smartphone. Telemed J E Health. 2018;24(11):899–907. [DOI] [PubMed] [Google Scholar]
- 61.Barthel C, Nonnekes J, van Helvert M, Haan R, Janssen A, Delval A, et al. The laser shoes: A new ambulatory device to alleviate freezing of gait in Parkinson disease. Neurology. 2018;90(2):e164–e71. [DOI] [PubMed] [Google Scholar]
- 62.Fietzek UM, Schroeteler FE, Ziegler K, Zwosta J, Ceballos-Baumann AO. Randomized cross-over trial to investigate the efficacy of a two-week physiotherapy programme with repetitive exercises of cueing to reduce the severity of freezing of gait in patients with Parkinson’s disease. Clin Rehabil. 2014;28(9):902–11. [DOI] [PubMed] [Google Scholar]
- 63.Nieuwboer A, Kwakkel G, Rochester L, Jones D, van Wegen E, Willems AM, et al. Cueing training in the home improves gait-related mobility in Parkinson’s disease: the RESCUE trial. Journal of neurology, neurosurgery, and psychiatry. 2007;78(2):134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nonnekes J, Ruzicka E, Nieuwboer A, Hallett M, Fasano A, Bloem BR. Compensation Strategies for Gait Impairments in Parkinson Disease: A Review. JAMA Neurol. 2019. [DOI] [PubMed] [Google Scholar]
- 65.Ginis P, Nackaerts E, Nieuwboer A, Heremans E. Cueing for people with Parkinson’s disease with freezing of gait: A narrative review of the state-of-the-art and novel perspectives. Ann Phys Rehabil Med. 2018;61(6):407–13. [DOI] [PubMed] [Google Scholar]
- 66.Mazilu S, Calatroni A, Gazit E, Mirelman A, Hausdorff JM, Troster G. Prediction of Freezing of Gait in Parkinson’s From Physiological Wearables: An Exploratory Study. IEEE J Biomed Health Inform. 2015;19(6):1843–54. [DOI] [PubMed] [Google Scholar]
- 67.Handojoseno AM, Shine JM, Nguyen TN, Tran Y, Lewis SJ, Nguyen HT. Analysis and Prediction of the Freezing of Gait Using EEG Brain Dynamics. IEEE Trans Neural Syst Rehabil Eng. 2015;23(5):887–96. [DOI] [PubMed] [Google Scholar]
- 68.Handojoseno AMA, Naik GR, Gilat M, Shine JM, Nguyen TN, Ly QT, et al. Prediction of Freezing of Gait in Patients with Parkinson’s Disease Using EEG Signals. Stud Health Technol Inform. 2018;246:124–31. [PubMed] [Google Scholar]
- 69.Shine JM, Handojoseno AM, Nguyen TN, Tran Y, Naismith SL, Nguyen H, et al. Abnormal patterns of theta frequency oscillations during the temporal evolution of freezing of gait in Parkinson’s disease. Clin Neurophysiol. 2014;125(3):569–76. [DOI] [PubMed] [Google Scholar]
- 70.Maidan I, Bernad-Elazari H, Gazit E, Giladi N, Hausdorff JM, Mirelman A. Changes in oxygenated hemoglobin link freezing of gait to frontal activation in patients with Parkinson disease: an fNIRS study of transient motor-cognitive failures. J Neurol. 2015;262(4):899–908. [DOI] [PubMed] [Google Scholar]
- 71.Palmerini L, Rocchi L, Mazilu S, Gazit E, Hausdorff JM, Chiari L. Identification of Characteristic Motor Patterns Preceding Freezing of Gait in Parkinson’s Disease Using Wearable Sensors. Front Neurol. 2017;8:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nieuwboer A, Dom R, De Weerdt W, Desloovere K, Fieuws S, Broens-Kaucsik E. Abnormalities of the spatiotemporal characteristics of gait at the onset of freezing in Parkinson’s disease. Mov Disord. 2001;16(6):1066–75. [DOI] [PubMed] [Google Scholar]
- 73.Espay AJ, Hausdorff JM, Sanchez-Ferro A, Klucken J, Merola A, Bonato P, et al. A roadmap for implementation of patient-centered digital outcome measures in Parkinson’s disease obtained using mobile health technologies. Mov Disord. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]


