Abstract
Recent research has shown that Coronavirus (CoV) replication depends on active immunophilin pathways. Here we demonstrate that the drug FK506 (Tacrolimus) inhibited strongly the growth of human coronaviruses SARS-CoV, HCoV-NL63 and HCoV-229E at low, non-cytotoxic concentrations in cell culture. As shown by plaque titration, qPCR, Luciferase- and green fluorescent protein (GFP) reporter gene expression, replication was diminished by several orders of magnitude. Knockdown of the cellular FK506-binding proteins FKBP1A and FKBP1B in CaCo2 cells prevented replication of HCoV-NL63, suggesting the requirement of these members of the immunophilin family for virus growth.
Abbreviations: EC50, 50% effective inhibitory concentration; RLU, relative light units
Keywords: SARS-CoV, HCoV-NL63, HCoV-229E, FK506, Tacrolimus, Immunophilins, FKBP1A (FKBP12), FKBP1B (FKBP12.6), Inhibition of viral replication
Coronaviruses cause severe diseases of the respiratory and gastrointestinal tract and the central nervous system in animals (Perlman and Netland, 2009). Infection of humans with HCoV-OC43 and HCoV-229E are known since the 1960s to be associated with respiratory tract i.e. common cold diseases. Severe Acute Respiratory Syndrome-Coronavirus (SARS-CoV) is the most aggressive human agent, causing the lung disease SARS (Drosten et al., 2003a). HCoV-NL63 and HCoV-HKU1 were discovered in 2004 and 2005, respectively (van der Hoek et al., 2004, Woo et al., 2005). They cause upper and lower respiratory tract infections including bronchiolitis and pneumonia especially in young children, immunocompromised patients and the elderly (van der Hoek, 2007).
Until now effective treatment of coronavirus infection in humans is unavailable (Stockman et al., 2006), even though inhibitors of coronavirus enzymes (reviewed by (Tong, 2009a, Tong, 2009b) and compounds inhibiting in vitro replication have been described (Kono et al., 2008, te Velthuis et al., 2010, Vincent et al., 2005). Coronaviruses represent the group of RNA viruses with the largest RNA genome known to date. Compared to other RNA viruses, the coronavirus polymerase has a rather low error frequency and may possess a RNA proof reading activity (Denison et al., 2011). However, the development of resistant mutants in the presence of drugs targeting coronaviral proteins remains a serious concern. Virus replication depends on a variety of host factors (de Haan and Rottier, 2006, Vogels et al., 2011, Zhang et al., 2010), which in turn represent potential antiviral targets. These might be more preferable targets than viral proteins as development of resistance is much less likely.
In a recent study we performed a genome-wide SARS-CoV yeast-two-hybrid interaction screen with human cDNA libraries identifying cyclophilins and FK506-binding proteins as interaction partners of SARS-CoV non-structural protein 1 (Nsp1). We identified Cyclosporin A (CsA) as a potent replication inhibitor of various human and animal CoVs (Pfefferle et al., 2011). Inhibition of SARS-CoV, HCoV-229E and, in addition, of Mouse Hepatitis Virus was also confirmed by de Wilde et al. (de Wilde et al., 2011). Cyclophilins and FK506-binding proteins are collectively referred to as immunophilins because they bind the immunosuppressive drugs CsA and FK506, respectively. Furthermore, they share peptidyl-prolyl isomerase (PPIase) domains and chaperone functions facilitating protein folding (Davis et al., 2010). CsA was recently shown to exert inhibitory effects on herpes simplex virus, vaccinia virus (Damaso and Keller, 1994), BK polyoma virus (Acott et al., 2008), human immunodeficiency virus 1 (HIV-1) (Briggs et al., 1999, Wainberg et al., 1988) and HCV (Fischer et al., 2010, Nakagawa et al., 2004, Watashi et al., 2003). Whereas cyclophilins were reported to be required for replication of HIV-1 (Franke and Luban, 1996), vesicular stomatitis virus (Bose et al., 2003) and HCV (Nakagawa et al., 2005), FK506-binding proteins were not found to play a role in the replication of HIV-1 (Briggs et al., 1999) and HCV (Nakagawa et al., 2004, Watashi et al., 2003). Orthopoxviruses are inhibited by FK506 (Reis et al., 2006).
FK506 was one of the first macrolides, discovered in 1984 in the bacterium Streptomyces tsukubaensis, with immunosuppressive activity. Although it is structurally unrelated to the cyclic undecapeptide CsA both molecules display similar properties of binding to the catalytic pocket of the PPIase domains of their cellular binding partners resulting in inhibition of their PPIase activities (Barik, 2006). Furthermore, the cyclophilin-CsA and FKBP-FK506 complexes bind to the cellular phosphatase calcineurin (CnA) which inhibits the dephosphorylation and activity of the nuclear factor of activated T-cells. The inhibition of this essential transcriptional regulator of important immune genes is the cause of immunosuppression. It is important to note that binding of the FKBP/FK506 complex to CnA, which is required for immunosuppression, and inhibition of PPIase activitiy, which is responsible for antiviral activity, are two independent mechanisms.
When studying the interaction of the SARS-CoV Nsp1 protein with cyclophilins A and B we discovered that replication of various coronaviruses is sensitive to CsA treatment including HCoV-NL63 and HCoV-229E (Pfefferle et al., 2011). Since Nsp1 also interacted with FK506-binding proteins FKBP1A and FKBP1B, we examined in this study whether FK506 inhibits replication of human coronaviruses. In a first experiment VeroFM cells were infected with SARS-CoV at a multiplicity of infection (MOI) of 0.0001 in the presence of increasing concentrations of CsA and FK506. Virus RNA concentrations were measured after 24 h in supernatant of infected cells by real-time RT-PCR (Drosten et al., 2003b). Titers were reduced 128,740-fold by 33 μM CsA, and 11,112 fold by 49 μM FK506, respectively. Fig. 1A shows the percentage of SARS-CoV inhibition under the influence of FK506, with an EC50 inhibitory concentration of about 6.9 μM. In Fig. 1B the corresponding log10 titer reductions are represented.
Fig. 1.
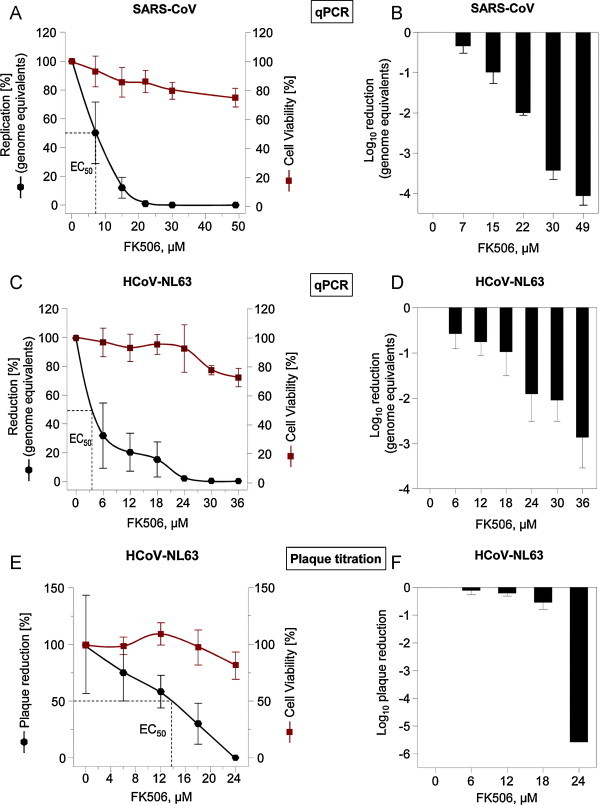
Effect of FK506 on SARS-CoV in VeroFM and on HCoV-NL63 replication in CaCo2 cells by qPCR (A–D) and plaque titration (E and F). Data shown are mean values of representative experiments performed in at least triplicates. Left Y-axes represent the percentage of reduction of virus replication in linear scale (A, C and E) and in log scale (B, D and F) at the indicated inhibitor concentrations, respectively. Percentage of cell viability with the mock-treated cells set to 100% are shown on the right Y-axes. FK506 concentrations used for each virus are given on the X-axis.
To examine whether FK506 exerts an inhibitory activity on other human coronaviruses, CaCo2 cells were infected with HCoV-NL63 at MOI = 0.004 (Herzog et al., 2008) in the presence of increasing inhibitor concentrations. After two days RNA from cell culture supernatant was harvested and viral RNA was extracted using the High Pure Viral Nucleic Acid Kit (Roche) and quantified by real-time PCR using Superscript™ III One-Step Quantitative System with ROX (Invitrogen) allowing reverse transcription, cDNA synthesis and PCR amplification in a single step. PCR primers used were NL-63RF2 for 5′-CTTCTGGTGACGCTAGTACAGCTTAT-3′ (genome position nt 14459–14484) and NL-63RR2rev 5′-AGACGTCGTTGTAGATCCCTAACAT-3′ (genome position nt 14573–14597) and NL-63 probe was 5′-FAM-CAGGTTGCTTAGTGTCCCATCAGATTCAT-TAMRA-3′ (genome position nt 14532–14560). The EC50 inhibitory concentration for HCoV-NL63 was about 5.1 μM and replication was completely inhibited at 36 μM FK506 (Fig. 1C), reflecting a 732-fold reduction of virus replication (Fig. 1D). At this concentration cell viability (CellTiterGlo, Promega) was still about 75%. Inhibition of virus growth in CaCo2 cells was also confirmed by plaque titration assay (Herzog et al., 2008) using serial virus dilutions in the presence of FK506 concentrations ranging from 0 μM to 24 μM. Fig. 1E and F shows percentage and log reductions of inhibition of virus replication, respectively. Under the conditions of the plaque assay involving virus growth for four days, the decrease of virus titers occurred in a more linear fashion with an EC50 of about 13.4 μM (E). Complete inhibition was observed at 24 μM with a reduction by more than five orders of magnitude (F).
HCoV-229E viruses expressing Renilla luciferase (Pfefferle et al., 2011) or GFP (Cervantes-Barragan et al., 2010) reporter genes were used to examine the inhibitory effect of FK506. HuH7 cells were infected with MOI = 0.1 and incubated for two days in the presence of increasing concentrations of FK506 in the culture medium. Viral replication was determined by measuring Renilla luciferase activity. Fig. 2A and B shows percent and Log reductions of virus growth, respectively. About 5.4 μM FK506 were found to be effective as an EC50 for HCoV-229E-luc. Cell viability was reduced to about 70% (at 24 μM) as compared to untreated cells, indicating that virus reduction was due to the drug but not to cell death. Fig. 2C and D shows a microscopic comparison of HCoV-229E-GFP infection under increasing concentrations of CsA and FK506. Reduction of virus infection was quantified by determining fluorescence intensity from microscope images of infected Huh7 cells. The pictures were analysed by using the software ImageJ 1.44 (Abramoff et al., 2004, Rasband, 1997). The integrated density for three different regions of interest was measured in each picture and the obtained averages were used to calculate correlates of virus replication efficiency. The determined EC50 was 12.2 μM for FK506 under these conditions. GFP fluorescence was inversely correlated to inhibitor concentration, with DAPI staining of cell nuclei indicating confluence of HuH7 cells and equivalent cell densities at all concentration steps. Virus growth was completely abolished between concentration of 8–12 μM CsA and 18–25 μM FK506. The latter correlates well with the inhibitory concentration determined for HCoV-229E-luc. For both HCoV-229E and HCoV-NL63, EC50 values were higher in plaque and GFP titrations as compared to direct replication assays by either RT-PCR or luciferase measurement. However, deviations between both assay formats were only by factors of 3.2 (HCoV-NL63) and 2.6 (HCoV-229E).
Fig. 2.
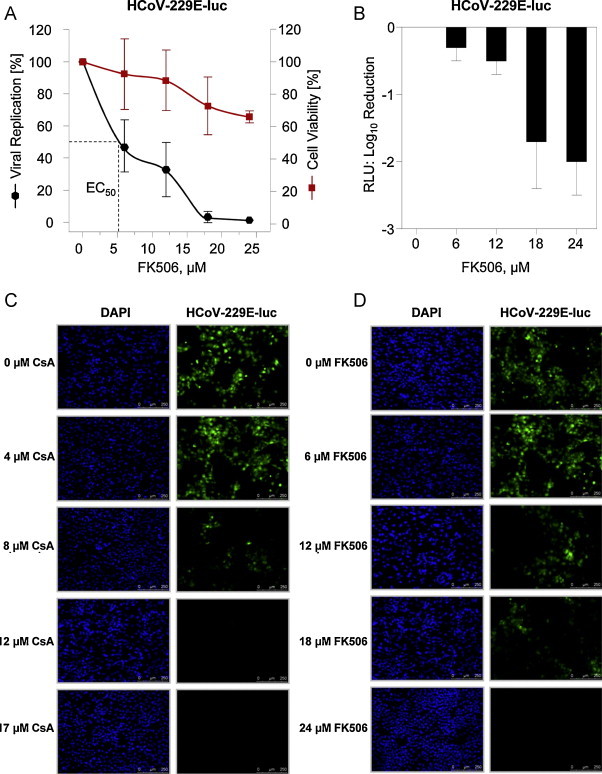
Effect of FK506 on HCoV-229E-LUC replication in HuH7 cells by Renilla activity measurement (A and B) and on HCoV-229E-GFP by quantification of GFP fluorescence (C and D). Data shown are mean values (RLU) of a representative experiment performed in triplicate. The left Y-axis represents the percentage of reduction of virus replication in linear scale (A) and in log scale (B), respectively, at the indicated inhibitor concentrations given on the X-axis. Percentage of cell viability with the mock-treated cells set to 100% are shown on the right Y-axis (A). Virus reduction values in log scale are given on the Y-axis of (B). Quantification of GFP fluorescence was used as a semiquantitative measure of virus reduction at the indicated inhibitor concentrations (C and D).
In order to examine whether the cellular FK506-binding proteins FKBP1A and FKBP1B are required for virus replication, CaCo2 knockdown cell lines were established using lentiviral expression of shRNA (Sirion GmbH, Martinsried, Germany). Cells were transduced at MOI 30 with MISSIONTM lentiviral non-target control, cyclophilin B (PPIB), FKBP1A, or FKBP1B shRNA lentiviruses. Sequences used for gene knockdown are listed in Table 1A. Stable shRNA-expressing cells were generated through 3 weeks of bulk-selection in 10–15 μg/ml puromycin-containing medium (DMEM + 10% FCS + 2 mM l-glutamine + 1 mM Na-pyruvate). The high puromycin concentration needed for selection of CaCo2 cells might result from an enhanced expression of MDR-1 (multi drug resistant protein 1 gene) depleting cells very efficiently from inhibitory drug molecules (Takara et al., 2002). From the bulk-selected knockdown and control cells mRNA expression was quantified by real-time RT-PCR. For reverse transcription 1 μg of total RNA was used. Amplification products were detected by SYBR I, and amplicon integrity was verified by melting point analysis. Cyclophilin A (PPIA) served as a reference gene against PPIB to determine the specificity of the knockdown. mRNA expression levels of PPIB, FKBP1A, and FKBP1B were then determined by real-time RT-PCR with primers listed in Table 1B. Fig. 3A shows PPIB, FKBP1A and FKBP1B mRNA expression in bulk-selected CaCo2 cells. In comparison to non-target controls a 98% and 96% knockdown was determined for PPIB and FKBP1A/1B, respectively.
Table 1.
| Particle set | Target | shRNA sequence (5′ → 3′) |
|---|---|---|
| (A) shRNA sequences used for lentiviral-based gene knockdown | ||
| Non-target control TRC1.5 Vector (pLKO.1-puro) |
CCGGCAACAAGATGAAGAGCACCAACTCGAGTTGGTGCTCTTCATCTTGTTGTTTTTG | |
| PPIB: SHVRS-NM_000942 | TRCN000049251 | CCGGGTTCTTCATCACGACAGTCAACTCGAGTTGACTGTCGTGATGAAGAACTTTTTG |
| FKBP1A: SHVRS-NM_054014 | TRCN0000005951 | CCGGGCCAAACTGACTATATCTCCACTCGAGTGGAGATATAGTCAGTTTGGCTTTTT |
| FKBP1B: SHVRS-NM_004116 | TRCN00000151663 | CCGGGAAGTTTGATTCATCCAGAGACTCGAGTCTCTGGATGAATCAAACTTCTTTTTTG |
| qRT-PCR primer | Sequence (5′ → 3′) |
|---|---|
| (B) Sequences of primers used for quantification of gene knockdown | |
| hPPIB_F | CTCTCCGAACGCAACATGAAG |
| hPPIB_R | ACCTTGACGGTGACTTTGGG |
| h_FKBP1A_F | CACTACACCGGGATGCTTGAA |
| h_FKBP1A_R | TTCTTCCCAGCCTCGGATCA |
| h_FKBPB_F | GCAGGAAGGAACTCAAGGTG |
| h_FKBP1B_R | AGCAACTTGGGCAGAGAGAA |
Fig. 3.
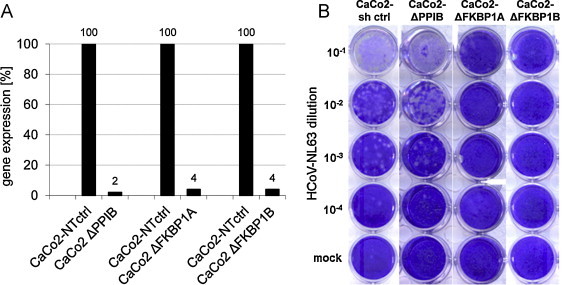
Growth of HCoV-NL63 on CaCo2-ΔPPIB, -ΔFKBP1A, -ΔFKBP1B knockdown mutant cells. Knockdown of gene expression was analysed by qPCR (A). Virus growth was tested by plaque titration on the knockdown cells (B).
Plaque titration showed that HCoV-NL63 grew to similar titers in CaCo2 wild type (not shown), CaCo2 shRNA control and CaCo2-ΔPPIB knockdown cells indicating that expression of PPIB was not necessary for viral growth. Conversely, knockdown of FKBP1ΔA/B did not result in any plaque formation, identifying FKBP1A/B products as key proteins required for viral replication. Similarly, real-time PCR amplification of RNA genomes in the supernatant of the HCoV-NL63-infected cell lines showed significantly reduced titers in the CaCo2-ΔFKBP1A knockdown cells as compared to CaCo2sh and CaCo2-ΔPPIB knockdown cells (not shown), confirming the results of the plaque titrations.
In summary, FK506 inhibits the replication of SARS-CoV, HCoV-NL63 and HCoV-229E at non-toxic, low-micromolar concentrations with a reduction of virus titers by several orders of magnitude to undetectable levels. In a comparative study of the effect of CsA and FK506 on HIV-1, the former inhibited virus replication completely (IC50: 1–2 μg/ml) whereas latter had an inhibitory effect on chronically but not on newly infected cells [up to 10 μg/ml FK506 (Briggs et al., 1999)]. Also in the case of HCV, FK506 had no effect on the replication of replicon RNA up to concentrations of 3 μg/ml (Watashi et al., 2003). Considering the higher drug concentrations of CsA and FK506 needed for inhibition of coronavirus replication it can be speculated that also HIV-1 and HCV might be inhibited by higher FK506 concentrations. It also remains to be clarified why the low nanomolar affinities of FKBP1A/B to FK506 do not reflect the EC50 inhibitory concentrations of coronaviruses which reside in the low micromolar range. This might be explained by a stronger affinity of coronavirus proteins to the cellular proteins as compared to FK506.
Lack of growth of HCoV-NL63 in stable CaCo2-ΔFKBP1A and -ΔFKBP1B, but not -ΔPPIB knockdown mutants indicates the requirement of FKBP1A and FKBP1B for HCoV-NL63 replication. As the two proteins share about 83% homology on the amino acid level (not shown) they do not substitute for each other upon individual knockdown. A common structural feature of FKBP proteins is the PPIase domain, also called FK506-binding domain. Its peptidylprolyl cis/trans isomerase activity influences cellular pathways by binding to cellular proteins (Kang et al., 2008). These activities are common to many FKBP family members containing a PPIase domain and separate chaperone functions can be discriminated from them (Barik, 2006).
The two less pathogenic coronaviruses HCoV-NL63 and HCoV-229E and the highly pathogenic SARS-CoV are sensitive to CsA and FK506, indicating the involvement of cyclophilins and FK506-binding proteins in viral replication. However, it is not clear what functions of these cellular proteins are required for virus replication. For SARS-CoV we have shown that Nsp1 binds to cyclophilins and to FKBP1A and FKBP1B by Y2H and Lumier assay (Pfefferle et al., 2011). Also, Nsp1 proteins of HCoV-NL63 and -229E interact with FKBP1A and FKBP1B in Y2H (not shown). The mechanism and function of these interactions have to be analysed in more detail. Furthermore it has to be clarified whether other viral proteins are involved in the relation of coronaviruses and immunophilins.
Acknowledgements
This work was supported by the “Bundesministerium fuer Bildung und Forschung” of the German Government (Zoonosis Network, Consortium on ecology and pathogenesis of SARS, project code 01KI1005A,F; http://www.gesundheitsforschung-bmbf.de/de/1721.php#SARS). We are grateful to Julia Schöpf and Nicole Senninger for technical help. We also thank Drs. C. Thirion and M. Salomon (SIRION, Martinsried, Germany) for production of the CaCo2 knockdown cell lines via lentiviral technology. We greatly appreciate the provision of HuH7 Lunet cells by Ralf Bartenschlager (Heidelberg, Germany).
References
- Abramoff M.D., Magalhaes P.J., Ram S.J. Image processing with ImageJ. Biophotonics Int. 2004;11(7):36–42. [Google Scholar]
- Acott P.D., O’Regan P.A., Lee S.H., Crocker J.F. In vitro effect of cyclosporin A on primary and chronic BK polyoma virus infection in Vero E6 cells. Transpl. Infect. Dis. 2008;10(6):385–390. doi: 10.1111/j.1399-3062.2008.00330.x. [DOI] [PubMed] [Google Scholar]
- Barik S. Immunophilins: for the love of proteins. Cell. Mol. Life Sci. 2006;63(24):2889–2900. doi: 10.1007/s00018-006-6215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S., Mathur M., Bates P., Joshi N., Banerjee A.K. Requirement for cyclophilin A for the replication of vesicular stomatitis virus New Jersey serotype. J. Gen. Virol. 2003;84(Pt (7)):1687–1699. doi: 10.1099/vir.0.19074-0. [DOI] [PubMed] [Google Scholar]
- Briggs C.J., Ott D.E., Coren L.V., Oroszlan S., Tozser J. Comparison of the effect of FK506 and cyclosporin A on virus production in H9 cells chronically and newly infected by HIV-1. Arch. Virol. 1999;144(11):2151–2160. doi: 10.1007/s007050050629. [DOI] [PubMed] [Google Scholar]
- Cervantes-Barragan L., Zust R., Maier R., Sierro S., Janda J., Levy F., Speiser D., Romero P., Rohrlich P.S., Ludewig B., Thiel V. Dendritic cell-specific antigen delivery by coronavirus vaccine vectors induces long-lasting protective antiviral and antitumor immunity. mBio. 2010;1(4) doi: 10.1128/mBio.00171-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaso C.R., Keller S.J. Cyclosporin A inhibits vaccinia virus replication in vitro. Arch. Virol. 1994;134(3–4):303–319. doi: 10.1007/BF01310569. [DOI] [PubMed] [Google Scholar]
- Davis T.L., Walker J.R., Campagna-Slater V., Finerty P.J., Paramanathan R., Bernstein G., MacKenzie F., Tempel W., Ouyang H., Lee W.H., Eisenmesser E.Z., Dhe-Paganon S. Structural and biochemical characterization of the human cyclophilin family of peptidyl-prolyl isomerases. PLoS Biol. 2010;8(7):e1000439. doi: 10.1371/journal.pbio.1000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan C.A., Rottier P.J. Hosting the severe acute respiratory syndrome coronavirus: specific cell factors required for infection. Cell. Microbiol. 2006;8(8):1211–1218. doi: 10.1111/j.1462-5822.2006.00744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wilde A.H., Zevenhoven-Dobbe J.C., van der Meer Y., Thiel V., Narayanan K., Makino S., Snijder E.J., van Hemert M.J. Cyclosporin A inhibits the replication of diverse coronaviruses. J. Gen. Virol. 2011;92(Pt 11):2542–2548. doi: 10.1099/vir.0.034983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison M.R., Graham R.L., Donaldson E.F., Eckerle L.D., Baric R.S. Coronaviruses: an RNA proofreading machine regulates replication fidelity and diversity. RNA Biol. 2011;8(2):270–279. doi: 10.4161/rna.8.2.15013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Gunther S., Preiser W., van der W.S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguiere A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Muller S., Rickerts V., Sturmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Drosten C., Preiser W., Gunther S., Schmitz H., Doerr H.W. Severe acute respiratory syndrome: identification of the etiological agent. Trends Mol. Med. 2003;9(8):325–327. doi: 10.1016/S1471-4914(03)00133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer G., Gallay P., Hopkins S. Cyclophilin inhibitors for the treatment of HCV infection. Curr. Opin. Invest. Drugs. 2010;11(8):911–918. [PubMed] [Google Scholar]
- Franke E.K., Luban J. Inhibition of HIV-1 replication by cyclosporine A or related compounds correlates with the ability to disrupt the Gag-cyclophilin A interaction. Virology. 1996;222(1):279–282. doi: 10.1006/viro.1996.0421. [DOI] [PubMed] [Google Scholar]
- Herzog P., Drosten C., Muller M.A. Plaque assay for human coronavirus NL63 using human colon carcinoma cells. Virol. J. 2008;5:138. doi: 10.1186/1743-422X-5-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C.B., Hong Y., Dhe-Paganon S., Yoon H.S. FKBP family proteins: immunophilins with versatile biological functions. Neurosignals. 2008;16(4):318–325. doi: 10.1159/000123041. [DOI] [PubMed] [Google Scholar]
- Kono M., Tatsumi K., Imai A.M., Saito K., Kuriyama T., Shirasawa H. Inhibition of human coronavirus 229E infection in human epithelial lung cells (L132) by chloroquine: involvement of p38 MAPK and ERK. Antiviral Res. 2008;77(2):150–152. doi: 10.1016/j.antiviral.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M., Sakamoto N., Enomoto N., Tanabe Y., Kanazawa N., Koyama T., Kurosaki M., Maekawa S., Yamashiro T., Chen C.H., Itsui Y., Kakinuma S., Watanabe M. Specific inhibition of hepatitis C virus replication by cyclosporin A. Biochem. Biophys. Res. Commun. 2004;313(1):42–47. doi: 10.1016/j.bbrc.2003.11.080. [DOI] [PubMed] [Google Scholar]
- Nakagawa M., Sakamoto N., Tanabe Y., Koyama T., Itsui Y., Takeda Y., Chen C.H., Kakinuma S., Oooka S., Maekawa S., Enomoto N., Watanabe M. Suppression of hepatitis C virus replication by cyclosporin a is mediated by blockade of cyclophilins. Gastroenterology. 2005;129(3):1031–1041. doi: 10.1053/j.gastro.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat. Rev. Microbiol. 2009;7(6):439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferle S., Schopf J., Kogl M., Friedel C.C., Muller M.A., Carbajo-Lozoya J., Stellberger T., von Dall’Armi E., Herzog P., Kallies S., Niemeyer D., Ditt V., Kuri T., Zust R., Pumpor K., Hilgenfeld R., Schwarz F., Zimmer R., Steffen I., Weber F., Thiel V., Herrler G., Thiel H.J., Schwegmann-Wessels C., Pohlmann S., Haas J., Drosten C., von Brunn A. The SARS-coronavirus–host interactome: identification of cyclophilins as target for pan-coronavirus inhibitors. PLoS Pathog. 2011;7(10):e1002331. doi: 10.1371/journal.ppat.1002331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband W.S. U.S. National Institutes of Health; Bethesda, Maryland, USA: 1997–2011. ImageJ. http://imagej.nih.gov/ij/ [Google Scholar]
- Reis S.A., Moussatche N., Damaso C.R. FK506, a secondary metabolite produced by Streptomyces, presents a novel antiviral activity against Orthopoxvirus infection in cell culture. J. Appl. Microbiol. 2006;100(6):1373–1380. doi: 10.1111/j.1365-2672.2006.02855.x. [DOI] [PubMed] [Google Scholar]
- Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3(9):e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takara K., Tsujimoto M., Ohnishi N., Yokoyama T. Digoxin up-regulates MDR1 in human colon carcinoma Caco-2 cells. Biochem. Biophys. Res. Commun. 2002;292(1):190–194. doi: 10.1006/bbrc.2002.6619. [DOI] [PubMed] [Google Scholar]
- te Velthuis A.J., van den Worm S.H., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6(11):e1001176. doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong T.R. Therapies for coronaviruses. Part 2. Inhibitors of intracellular life cycle. Expert Opin. Ther. Pat. 2009;19(4):415–431. doi: 10.1517/13543770802600698. [DOI] [PubMed] [Google Scholar]
- Tong T.R. Therapies for coronaviruses. Part I of II. Viral entry inhibitors. Expert Opin. Ther. Pat. 2009;19(3):357–367. doi: 10.1517/13543770802609384. [DOI] [PubMed] [Google Scholar]
- van der Hoek L. Human coronaviruses: what do they cause? Antiviral Therapy. 2007;12(4 (Pt B)):651–658. [PubMed] [Google Scholar]
- van der Hoek L., Pyrc K., Jebbink M.F., Vermeulen-Oost W., Berkhout R.J., Wolthers K.C., Wertheim-van Dillen P.M., Kaandorp J., Spaargaren J., Berkhout B. Identification of a new human coronavirus. Nat. Med. 2004;10(4):368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., Seidah N.G., Nichol S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels M.W., van Balkom B.W., Kaloyanova D.V., Batenburg J.J., Heck A.J., Helms J.B., Rottier P.J., de Haan C.A. Identification of host factors involved in coronavirus replication by quantitative proteomics analysis. Proteomics. 2011;11(1):64–80. doi: 10.1002/pmic.201000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainberg M.A., Dascal A., Blain N., Fitz-Gibbon L., Boulerice F., Numazaki K., Tremblay M. The effect of cyclosporine A on infection of susceptible cells by human immunodeficiency virus type 1. Blood. 1988;72(6):1904–1910. [PubMed] [Google Scholar]
- Watashi K., Hijikata M., Hosaka M., Yamaji M., Shimotohno K. Cyclosporin A suppresses replication of hepatitis C virus genome in cultured hepatocytes. Hepatology. 2003;38(5):1282–1288. doi: 10.1053/jhep.2003.50449. [DOI] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Chu C.M., Chan K.H., Tsoi H.W., Huang Y., Wong B.H., Poon R.W., Cai J.J., Luk W.K., Poon L.L., Wong S.S., Guan Y., Peiris J.S., Yuen K.Y. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J. Virol. 2005;79(2):884–895. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Zhang Z.P., Zhang X.E., Lin F.S., Ge F. Quantitative proteomics analysis reveals BAG3 as a potential target to suppress severe acute respiratory syndrome coronavirus replication. J. Virol. 2010;84(12):6050–6059. doi: 10.1128/JVI.00213-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


