Abstract
Viruses are important causes of acute and chronic diseases in humans. Newer viruses are still being discovered and those that are already known are being incriminated in the aetiology of clinical conditions with hitherto unknown causes. Apart from frequently causing infections in the general community, many types of viruses are also significant nosocomial pathogens. While it is generally agreed that we underestimate the proportion of nosocomial infections that are viral, due to a lack of routine monitoring, viruses easily account for more than 30% of the cases of hospital-acquired infections in many paediatric settings. Indeed, the relative importance of viruses in this respect is increasing due to a number of societal and demographic changes as well as alterations in healthcare practices. Safe vaccines against many common nosocomial viral agents are currently unavailable while there is also a virtual lack of effective and affordable chemotherapy against them. There is, therefore, renewed emphasis on preventive strategies by better understanding of the relative importance of various vehicles in the nosocomial spread of viruses and by infection control using microbicides. This, in turn, has stimulated considerable interest in the development of formulations that are not only safer but which also have demonstrated activity against major types of nosocomial viral pathogens. Further, much work is now underway to design better methods to assess the virucidal activity of microbicides used to decontaminate hands, reusable medical devices and environmental surfaces in critical areas of healthcare settings. It is anticipated that these approaches will result in reducing the health and economic impact of nosocomial infections due to viruses.
Keywords: Viruses, Nosocomial infections, Microbicides, Disinfection, Antisepsis, Virucides, Infection control
1. Introduction
Viruses are common pathogens in hospitals1., 2. and other healthcare settings.3 Even mild viral diseases can cause a significant burden on the economy and the healthcare system.4., 5. The true proportion of nosocomial infections due to viruses remains unknown because many hospitals, even in industrialized countries, do not have the resources for proper surveillance and differential laboratory diagnoses of infections acquired in hospitals. Dated estimates suggest that viruses may cause 5–32% of all nosocomial infections in the United States.6 In fact, ongoing changes in our societies,7 healthcare policies and practices in modern hospitals enhance our susceptibility and exposure to many types of pathogens including viruses. Whereas complete elimination of the transmission of viruses may not be attainable, proper and judicious use of microbicides has the potential to halt the spread of many such pathogens in hospitals and elsewhere. This aspect will be addressed here with particular emphasis on the activity of microbicides against surrogates for two types of nosocomial viral pathogens, namely, noroviruses and the severe acute respiratory syndrome (SARS) virus.
1.1. Environmental contamination with viruses
Unlike certain types of nosocomial bacterial and fungal pathogens, human pathogenic viruses are not a part of normal body flora, but are shed for varying periods only by those infected with them. Many types of viruses can remain viable for several hours on hands and, generally, much longer on environmental surfaces.8 Infectious virus particles have been isolated from naturally contaminated hands of caregivers and fomites.5 Since hands can donate or receive viruses during casual contact with animate and inanimate surfaces,9 proper and regular decontamination of hands by caregivers is crucial in preventing and controlling the spread of viral and other types of pathogen. Evidence from experimental as well as epidemiological studies indicates that environmental surfaces may also play a role in the nosocomial spread of respiratory and enteric nosocomial viral pathogens.8
The capacity of a given virus to spread from host-to-host is determined by, among other things, its ability to remain viable during transit to the susceptible host. Virus shedding generally begins prior to the onset of clinical symptoms and lasts for several days after recovery; chronic cases of hepatitis B, for example, can be life-long sources of the virus. The actual amount of virus discharge varies considerably depending on the type of infecting agent and the stage of the infection.
1.2. Virus survival on inanimate surfaces
The extent of virus survival on a surface or object is the key to its potential to act as a source of the virus. The most important contribution to survival is perhaps the protection afforded to the virus by the fluid in which it is discharged. Virus survival in the environment in general is inversely proportional to air temperature. Relative humidity (RH) also has a pronounced effect on virus survival and its effect is modulated by air temperature: Enveloped viruses usually survive better in dryer conditions and lower RH than can be tolerated by non-enveloped viruses.8., 10.
1.3. Virus transfer from contaminated to clean surfaces
Apart from direct contamination, both animate and inanimate surfaces can become contaminated indirectly through transfer of virus from other vehicles (Figure 1). Of these, deposition by settling of virus-containing aerosols or contact with contaminated fluids are obvious. Less obvious are the transfers, which may occur between different types of surfaces. Our laboratory-based studies on rotavirus transfer between animate-inanimate, animate-animate and inanimate-animate surfaces have shown that virus transfer: (a) readily occurs irrespective of the nature of donor and recipient surfaces, (b) was reduced with increase in the age of the inoculum, most likely due to greater loss of moisture, (c) was directly proportional to the amount of pressure applied during contact and (d) increased substantially when friction was applied during contact.11
Figure 1.

Direct and indirect vehicular spread of nosocomial viral infections.
1.4. Interrupting the spread of viruses
Figure 2 illustrates the many applications of microbicides in hospitals for controlling the environmental spread of pathogens including viruses. The use of such chemicals in infection control in general operates on the basic premise that the risk of acquiring a given infection is directly proportional to the amount of its causative agent in the vicinity and that proper use of microbicides lowers this risk by reducing the pathogen load. Microbicides can also interrupt pathogen transfer from contaminated to clean surfaces. In hospitals, viruses which lend themselves to interruption by proper microbicide use are those which spread mainly through hands, fomites, medical devices, and environmental surfaces.
Figure 2.
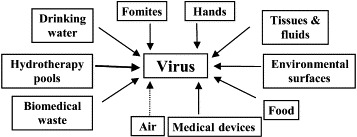
Uses of microbicides with nosocomial viral pathogens among the targets. The dotted line indicates lack of evidence for effective and safe decontamination of air by microbicides.
A major difficulty in halting virus transmission in hospitals is that most virus-infected individuals remain free of obvious disease symptoms and such silent cases generally are unrecognized while discharging viruses into their surroundings. Viruses discharged by them could cause serious disease in others, particularly those who are debilitated due to immunosuppression or other causes. Most hospitals have a pool of such highly susceptible individuals. Insufficient numbers of or inconvenient location of sinks and a lack of availability of skin-friendly products discourage compliance with handwashing by caregivers. While the use of non-aqueous liquids and gels for hygienic hand antisepsis has been a long-accepted practice in Europe,12., 13. such formulations are now meeting with increasing acceptance in North America as well.25 The use of such products has the potential to reduce handborne spread of viruses.14
Virus transfer from contaminated to clean surfaces can be interrupted through the use of microbicides.15 However, not all products are equally effective in this respect. In fact, the use of an ineffective product with a good surfactant can dislodge the virus from the surfaces without killing it, thus making it more readily available for transfer upon contact. In other words, the use of certain formulations for the decontamination of environmental surfaces may inadvertently enhance their potential in the direct or indirect spread of viruses.
1.5. Microbicide inactivation of the feline caliciviruses
Caliciviruses of humans (e.g. noroviruses), a frequent cause of nosocomial outbreaks of acute gastroenteritis, cannot be grown in the laboratory. Therefore, animal caliciviruses, such as the feline calicivirus (FCV), are commonly used as surrogates for the human ones.16., 17. We have recently assessed the activity of several environmental surface disinfectants against FCV using a quantitative carrier test.18 A soil load was used in all tests and water with a standard hardness of 400 ppm as CaCO3 was used to prepare use-dilutions of those formulations requiring dilution in water. All testing was carried out at 20 °C. The virucidal effectiveness criterion was arbitrarily set at a log10 reduction factor of ≥4 in the infectivity titre of the virus. As can be seen from Table I , all formulations tested met the effectiveness criterion but with a wide variation in contact time.
Table I.
Activity of selected microbicides against the feline calicivirus
| Germicide | Types and concentration of active compounds tested | Contact time (minutes) | Log10 reduction factor |
|---|---|---|---|
| Virox 5 (undiluted) | Accelerated H2O2—5000 ppm | 3 | >4.7 |
| Chlorine dioxide | 1000 ppm available chlorine | 1 | 4.5 |
| Coverage 256 (1:62 dilution) | Mixture of four quatsa—2470 ppm | 10 | 4.0 |
| Lysol Spray | 79% (V/V) ethanol+0.1% quat | 3 | >4.47 |
| Big Spray (undiluted) | Per 100 gram (ethanol—25.92 g; 2-propanol—11.50 g; polyhexanide—0.0054 g) | 3 | >4.7 |
| EcoTru (undiluted) | 0.2% PCMX | 30 | 4.12 |
| Ethanol | 75% Ethanol (V/V) | 10 | 4.7 |
Quaternary ammonium compounds.
The findings of the activity of domestic bleach (∼5% sodium hypochlorite) against FCV are given in Figure 3 . Bleach diluted to contain 1000 ppm of available chlorine reduced infectivity titre of FCV by a log10 factor nearly 4.5 in a contact time of only one minute; a further 2-fold dilution (500 ppm) required a contact time of 10 min to achieve the same level of reduction in virus activity. When the level of available chlorine was reduced to 100 ppm, there was virtually no reduction in the virus infectivity even at a contact time of 5 min.
Figure 3.



These findings show that FCV is somewhat less resistant to many microbicides than other nosocomial viral pathogens such as hepatitis A virus (HAC).19 FCV also does not survive on human hands and environmental surfaces (Sattar et al., unpublished data) as well as HAV and human rotaviruses.8 However, profuse diarrhoea and vomiting, which are the hallmarks of noroviral gastroenteritis, cause extensive contamination of surfaces and rapid and proper use of microbicides could reduce the environmental spread of such viruses. In case of airborne spread, the decontamination of hands and environmental surfaces alone is likely to be less effective in outbreak control.
1.6. Microbicide inactivation of a human coronavirus
The recent discovery of the SARS coronavirus and its ability to cause devastating nosocomial outbreaks has regenerated much interest in coronaviruses in general. The SARS virus can remain viable on environmental surfaces for a few hours.20 Whether it can be transmitted through environmental surfaces and hands in healthcare settings remains unknown. However, earlier studies with another human coronaviruses (229E) have found it to be somewhat more resistant to microbicides when compared to a human parainfluenzavirus.21 In these tests, 10 μL of the virus was suspended in mucin to give a final mucin concentration of 5 μg/mL. Each carrier (stainless steel disks of 1 cm diameter) with the dried virus inoculum was covered with 20 μL of test microbicide for 1 min at room temperature. The eluates were plaque assayed in L-132 cells and the arbitrary virucidal effectiveness criterion was a log10 reduction factor of ≥3 in virus infectivity.
Table II summarizes the data on coronavirucidal activity from the study.21 In spite of the fact that 229E is an enveloped virus, quaternary ammonium compound and a phenolic were unable to meet the product performance criterion in these tests. A 0.01% solution of domestic bleach (∼100 ppm available chlorine) also failed to achieve the desired level of virus inactivation. It should be noted that the contact time in these experiments was one minute, which more closely simulates the application of microbicides in the decontamination of environmental surfaces. Studies are now underway here to determine the activity of microbicides against the SARS virus itself.
Table II.
Microbicides and activity against human coronavirus 229E21
| Microbicide (concentration) | Use(s) | Activity |
|---|---|---|
| Sodium hypochlorite (0.01%) | Environmental surfaces | No |
| Iodophore (1% iodine) | Antisepsis | Yes |
| Ethanol (70% V/V) | Disinfectant/antiseptic | Yes |
| Alkaline glutaraldehyde (2%) | Medical devices | Yes |
| Quaternary ammonium (0.04%) | Environmental surfaces | No |
| Chlorhexidine gluconate (0.008%+cetrimide (0.08%) | Antisepsis | No |
| Triple phenolic (0.06%) | Environmental surfaces | No |
| Phenolic (5%)+sodium lauryl sulfate (0.06%) | Environmental surfaces | Yes |
| Chlorhexidine gluconate (0.008%)+cetrimide(0.08%)+ethanol (70%) | Antisepsis | Yes |
2. Concluding remarks
Viruses in general continue to exert a heavy toll on human health and the recognition of their impact as pathogens in most likely to increase further as ‘new’ viruses are being discovered continually and new roles for ‘old’ viruses are being identified.22 Relatively recent nosocomial outbreaks of noroviral gastroenteritis23 and SARS,24 which caused enormous health and economic impacts and major disruptions in health services, clearly illustrate how vulnerable our hospitals are to such infectious agents.
While viruses constitute important nosocomial pathogens, our understanding of the actual mechanism of spread of many viral infections remains weak. This makes it difficult to design and apply proper strategies to prevent and control nosocomial outbreaks of viral infections. Hand are universally recognized as vehicles for the spread of a number of viruses,25 but lack of compliance with handwashing and perhaps the use of ineffective handwash agents continue to undermine the full potential of infection control measures in this regard. The ease with which washed hands can pick up infectious viruses upon contact with contaminated environmental surfaces and objects suggests that the emphasis on handwashing should be combined with an awareness of the need for proper and frequent decontamination of those surfaces and objects that come in frequent contact with washed hands.
While pre-market evaluation of hygienic hand antiseptics is often carried out using bacteria only, laboratory-based testing shows many such products to be less effective against those viruses which are believed to spread via hands in healthcare settings.8 This reemphasizes the need for regularly testing such products against representative nosocomial viral pathogens and allowing suitable label claims for virucidal activity.26 At the same time, label claims against viruses such as human immunodeficiency viruses as well as hepatitis B and C viruses should be discouraged because hands of caregivers are not known to spread these otherwise important nosocomial pathogens.27 Standardized in vivo methods are new available to assess the activity of hygienic handwash and handrub agents against viruses.28
Acknowledgements
I am grateful to students and staff at CREM for their assistance in the generation of the data reported here. Ms S. Springthorpe of CREM kindly reviewed this manuscript. I also thank Dr G. Kampf and others at BODE Chemie for the opportunity to present this paper at the 7th International BODE Hygiene Days, held in May 2003.
Footnotes
Presented at the 7th International BODE Hygiene Days in Barcelona, May 15–18 2003.
References
- 1.Aitken C, Jeffries D.J. Nosocomial spread of viral disease. Clin Microbiol Rev. 2001;14:528–546. doi: 10.1128/CMR.14.3.528-546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meakins S.M, Adak G.K, Lopman B.A, O'Brien S.J. General outbreaks of infectious intestinal disease (IID) in hospitals, England and Wales, 1992–2000. J Hosp Infect. 2003;53:1–5. doi: 10.1053/jhin.2002.1326. [DOI] [PubMed] [Google Scholar]
- 3.Strausbaugh L.J, Sukumar S.R, Joseph C.L. Infectious disease outbreaks in nursing homes: an unappreciated hazard for frail elderly persons. Clin Infect Dis. 2003;36:870–876. doi: 10.1086/368197. [DOI] [PubMed] [Google Scholar]
- 4.Sartor C, Zandotti C, Romain F. Disruption of services in an internal medicine unit due to a nosocomial influenza outbreak. Infect Control Hosp Epidemiol. 2002;23:615–619. doi: 10.1086/501981. [DOI] [PubMed] [Google Scholar]
- 5.Piednoir E, Bureau-Chalot F, Merle C. Direct costs associated with a nosocomial outbreak of adenoviral conjunctivitis infection in a long-term care institution. Am J Infect Control. 2002;30:407–410. doi: 10.1067/mic.2002.125193. [DOI] [PubMed] [Google Scholar]
- 6.Valenti W.M, Menegus M.A, Hall C.B. Nosocomial viral infections. I. Epidemiology and significance. Infect Control. 1980;1:33–37. doi: 10.1017/s0195941700052371. [DOI] [PubMed] [Google Scholar]
- 7.Sattar S.A, Tetro J, Springthorpe V.S. Impact of changing societal trends on the spread of infectious diseases in American and Canadian homes. Am J Infect Control. 1999;27:S4–S21. doi: 10.1016/s0196-6553(99)70037-4. [DOI] [PubMed] [Google Scholar]
- 8.Sattar S.A, Springthorpe V.S. Transmission of viral infections through animate and inanimate surfaces and infection control through chemical disinfection. In: Hurst C.J, editor. Modeling disease transmission and its prevention by disinfection. Cambridge University Press; Cambridge, UK: 1996. pp. 224–257. [Google Scholar]
- 9.Ansari S.A, Sattar S.A, Springthorpe V.S. Rotavirus survival on human hands and transfer of infectious virus to animate and nonporous inanimate surfaces. J Clin Microbiol. 1988;26:1513–1518. doi: 10.1128/jcm.26.8.1513-1518.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ansari S.A, Springthorpe V.S, Sattar S.A. Survival and vehicular spread of human rotaviruses: possible relationship with seasonality of outbreaks. Rev Infect Dis. 1991;13:448–461. doi: 10.1093/clinids/13.3.448. [DOI] [PubMed] [Google Scholar]
- 11.Ansari S.A, Sattar S.A, Springthorpe V.S. Rotavirus survival on human hands and transfer of infectious virus to animate and non-porus inanimate surfaces. J Clin Microbiol. 1988;26:1513–1518. doi: 10.1128/jcm.26.8.1513-1518.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rotter M.L. Arguments for alcoholic hand disinfection. J Hosp Infect. 2001;48:S4–S8. doi: 10.1016/s0195-6701(01)90004-0. [DOI] [PubMed] [Google Scholar]
- 13.Kamp G, Ostermeyer C. Intra-laboratory reproducibility of the hand hygiene reference procedures of EN 1499 (hygienic handwash) and EN 1500 (hygienic hand disinfection) J Hosp Infect. 2002;52:219–224. doi: 10.1053/jhin.2002.1299. [DOI] [PubMed] [Google Scholar]
- 14.Sattar S.A, Abebe M, Bueti A, Jampani H, Newman J. Determination of the activity of an alcohol-based hand gel against human adeno-, and rotaviruses using the fingerpad method. Infect Control Hosp Epidemiol. 2000;21:516–519. doi: 10.1086/501796. [DOI] [PubMed] [Google Scholar]
- 15.Sattar S.A, Jacobsen H, Rahman H, Rubino J, Cusack T. Interruption of rotavirus spread through chemical disinfection. Infect Control Hosp Epidemiol. 1994;15:751–756. doi: 10.1086/646852. [DOI] [PubMed] [Google Scholar]
- 16.Doultree J.C, Druce J.D, Birch C.J. Inactivation of feline caliciviruses, a Norwalk virus surrogate. J Hosp Infect. 1999;41:51–57. doi: 10.1016/s0195-6701(99)90037-3. [DOI] [PubMed] [Google Scholar]
- 17.Gulati BR, Allwood PB, Hedberg CW, Goyal SM. Efficacy of commonly used disinfectants for the inactivation of caliciviruses on strawberry, lettuce, and a food-contact surface. J Food Prot 2001; 64: 1430–1434. [DOI] [PubMed]
- 18.Sattar S.A, Springthorpe V.S, Adegbunrin O. A disc-based quantitative carrier test method to assess the virucidal activity of chemical germicides. J Virol Methods. 2003;112:3–12. doi: 10.1016/s0166-0934(03)00192-7. [DOI] [PubMed] [Google Scholar]
- 19.Mbithi J.N, Springthorpe V.S, Sattar S.A. Chemical disinfection of hepatitis. A virus on environmental surfaces. Appl Environ Microbiol. 1990;56:3601–3604. doi: 10.1128/aem.56.11.3601-3604.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdullah A.S.M, Tomlison B, Cockram C.S, Thomas G.N. Lessons from the severe acute respiratory syndrome outbreak in Hong Kong. Emerg Infect Dis. 2003;9:1042–1045. doi: 10.3201/eid0909.030366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sattar S.A, Springthorpe V.S, Karim Y, Loro P. Chemical disinfection of non-porous inanimate surfaces experimentally contaminated with four human pathogenic viruses. Epidemiol Infect. 1989;102:493–505. doi: 10.1017/s0950268800030211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Microbial threats to health: emergence, detection, and response. Institute of Medicine: Washington, DC, 2003, 367. [PubMed]
- 23.Meakins S.M, Adak G.K, Lopman B.A, O'Brien S.J. General outbreaks of infectious intestinal diseases (IID) in hospitals, England and Wales, 1992–2000. J Hop Infect. 2003;53:1–5. doi: 10.1053/jhin.2002.1326. [DOI] [PubMed] [Google Scholar]
- 24.Maunder R, Hunter J, Vincent L. The immediate psychological and occupational impact of the 2003 SARS outbreak in a teaching hospital. Can Med Assoc J. 2003;168:1245–1251. [PMC free article] [PubMed] [Google Scholar]
- 25.Boyce J, Pittet D. Guideline for hand hygiene in health-care settings. Morb Mortal Wkly Rep. 2002;51(RR16):1–44. [Google Scholar]
- 26.Sattar S.A, Springthorpe V.S, Tetro J, Vashon B, Keswick B. Hygienic hand antiseptics: should they not have activity and label claims against viruses? Am J Infect Control. 2002;30:355–372. doi: 10.1067/mic.2002.124532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sattar S.A, Tetro J, Springthorpe V.S, Guilivi A. Preventing the spread of hepatitis B and C viruses: where are germicides relevant? Am J Infect Control. 2001;29:187–197. doi: 10.1067/mic.2001.114233. [DOI] [PubMed] [Google Scholar]
- 28.Ansari S.A, Sattar S.A. The need and methods for assessing the activity of topical agents against viruses. In: Paulson D, editor. Handbook of topical antimicrobials: industrial applications in consumer products and pharmaceuticals. Marcel Dekker; New York, NY: 2002. pp. 411–445. [Google Scholar]


