Abstract
Intracranial infection of C57BL/6 mice with mouse hepatitis virus (MHV) results in an acute encephalomyelitis followed by a demyelinating disease similar in pathology to the human disease multiple sclerosis (MS). T cells participate in both defense and disease progression following MHV infection. Expression of chemokine receptors on activated T cells is important in allowing these cells to traffic into and accumulate within the central nervous system (CNS) of MHV-infected mice. The present study evaluated the contributions of CCR5 to the activation and trafficking of virus-specific CD8+ T cells into the MHV-infected CNS mice. Comparable numbers of virus-specific CD8+ T cells derived from immunized CCR5+/+ or CCR5−/− mice were present within the CNS of MHV-infected RAG1−/− mice following adoptive transfer, indicating that CCR5 is not required for trafficking of these cells into the CNS. RAG1−/− recipients of CCR5−/−-derived CD8+ T cells exhibited a modest, yet significant (P ≤ 0.05), reduction in viral burden within the brain which correlated with increased CTL activity and IFN-γ expression. Histological analysis of RAG1−/− recipients of either CCR5+/+or CCR5−/−-derived CD8+ T cells revealed only focal areas of demyelination with no significant differences in white matter destruction. These data indicate that CCR5 signaling on CD8+ T cells modulates antiviral activities but is not essential for entry into the CNS.
Keywords: Chemokines, Chemokine receptors, Neuroinflammation, T cell, Virus
Introduction
Mouse hepatitis virus (MHV) is a single-strand, positive-sense RNA virus that is a member of the family Coronaviradae. Intracranial infection of susceptible mice with neurotropic strains of MHV reproducibly results in an acute encephalomyelitis followed by a chronic demyelinating disease similar in pathology to the human demyelinating disease multiple sclerosis (MS) Houtman and Fleming 1996, Lane and Buchmeier 1997. The acute stage of MHV infection is characterized by replication of virus in both neurons and glia and widespread distribution throughout the parenchyma. In contrast, during the chronic stage of disease, viral RNA and protein predominate within white matter tracts undergoing demyelination. The T cell response to MHV following infection of the CNS is crucial for optimal host defense and clearance of virus Lane et al 2000, Marten et al 2001, Williamson and Stohlman 1990. In addition, T cells have been implicated as important effector cells in amplifying the severity of demyelination in MHV-infected mice Houtman and Fleming 1996, Liu et al 2001a, Pewe et al 2002, Pewe and Perlman 2002, Wu et al 2000. Therefore, understanding the mechanisms by which these cells traffic and accumulate within the central nervous system (CNS) following MHV infection is important with regard to understanding both host defense and disease progression.
A robust expression of chemokines and chemokine receptors occurs within the CNS of MHV-infected mice during both acute and chronic stages of disease (Lane et al., 1998). These observations suggest a role for these molecules in both host defense and disease by attracting targeted populations of leukocytes into the CNS. Indeed, a series of recent studies from our laboratory using both chemokine receptor knock-out mice as well as neutralizing antibodies to select chemokines have clearly demonstrated a nonredundant and important role for chemokines and chemokine receptors in participating in clearance of virus from the brain as well as demyelination Chen et al 2001, Glass et al 2001, Lane et al 2000, Liu et al 2000, Liu et al 2001b. Given the important role for T cells and chemokine receptors in both host defense and disease development following MHV infection of the CNS, we have sought to better understand how these cells traffic into the CNS following viral infection. A recent report from our laboratory demonstrated that the CC chemokine receptor 5 (CCR5) is important for trafficking of virus-specific CD4+ T cells into the CNS of MHV-infected mice (Glass and Lane, 2003). Analysis of the chemokine receptor profile expressed by virus-specific CD4+ T cells obtained from CCR5−/− mice revealed reduced expression of various chemokine receptors, suggesting that CCR5 signaling regulates expression of additional chemokine receptors that participate in T cell trafficking and accumulation within the CNS of infected mice. To expand our understanding of how CCR5 regulates T cell activation and trafficking within the context of MHV infection of the CNS, CD8+ T cells specific for the immunodominant epitope present within the spike (S) glycoprotein spanning amino acids 510-518 (S510-518) were generated from either CCR5+/+ or CCR5−/− mice and the antiviral and migration activities analyzed. The results presented indicate that CCR5 signaling imparts a downstream effect on IFN-γ production and CTL activity but is not essential for trafficking into the CNS or the development of demyelination.
Results
Infiltration of virus-specific CD8+ T cells into the CNS of MHV-infected mice
T cells were isolated from the brains of MHV-infected CCR5+/+ and CCR5−/− mice at Days 7 and 12 p.i. and total numbers of CD8+ T cells specific for the S510-518 epitope were determined by intracellular cytokine staining. Such analysis revealed a >35% decrease in the number of S510-518 responsive CD8+ T cells in the CNS of CCR5−/− mice at Day 7 postinfection compared to CCR5+/+ mice as judged by IFN-γ secretion following S510-518 peptide stimulation (Figs. 1A and B). However, the number of S510-518 responsive cells within the CNS of CCR5−/− mice was >45% higher compared to CCR5+/+ mice at Day 12 p.i. (Figs. 1A and B). Analysis of total numbers of CD8+ T cells indicated a 40% decrease in numbers of cells isolated from the CNS of CCR5−/− mice at Day 7 p.i. compared to CCR5+/+ mice, which is consistent with earlier studies (Fig. 1C) (Glass et al., 2001). However, by Day 12 p.i., both groups of mice had similar numbers of CD8+ T cells in their CNS (Fig. 1C). These data suggested that CCR5 signaling modulates both antigen-specific and non-antigen-specific CD8+ T cell accumulation within the CNS of MHV-infected mice.
Fig. 1.

Flow analysis of CD8+ T cells within the CNS of MHV-infected mice. (A) Representative dot plot of flow cytometric analysis of CD8+ T cells infiltrating into the CNS following MHV-infection of CCR5+/+ and CCR5−/− mice. Intracellular cytokine staining for IFN-γ was performed to determine the frequency of S510-518-specific CD8+ T cells present in the brains of mice at Days 7 and 12 p.i. The average frequency of total and S510-518-specific CD8+ T cells are indicated in the bottom and top right panels, respectively. (B) Total numbers of S510-518-specific CD8+ T cells as determined by intracellular cytokine staining for IFN-γ. (C) Total numbers of CD8+ T cells present within the brains of CCR5+/+ and CCR5−/− at 7 and 12 days p.i. as determined by flow cytometry. Data presented in (A) are a representative plot of five individual mice per group at Day 7 and three individual mice per group at Day 12. Data in (B) and (C) are presented as average ± SEM and represent two separate experiments with a total of five mice in each group at Day 7 p.i. and three mice in each group at Day 12 p.i. *P ≤ 0.05 when compared to CCR5+/+ mice.
Adoptive transfer of S510-518-expanded CD8+ T cells
To further assess the contributions of CCR5 signaling to virus-specific CD8+ T cell migration into the CNS, S510-518-specific CD8+ T cells were obtained from immunized CCR5+/+ and CCR5−/− mice and adoptively transferred into MHV-infected RAG1−/− mice Chen et al 2001, Glass and Lane 2003. Recipient mice were injected intravenously (iv) with a total of 2.5 × 106 cells, sacrificed 9 days posttransfer (12 days p.i.), and brains were collected to determine viral titers and infiltration of CD8+ T cells into the CNS. Although neither recipient group of mice cleared virus, there was a twofold decrease (P ≤ 0.05) in the viral burden within the brains of mice that received CCR5−/−-derived CD8+ T cells compared to CCR5+/+-derived CD8+ T cells (Table 1). Interestingly there were comparable numbers of S510-518-specific CD8+ T cells present in the CNS of RAG1−/− recipients, suggesting an enhanced antiviral activity by CCR5−/−-derived CD8+ T cells (Table 1). The fact that similar numbers of S510-518-specific CD8+ T cells from both CCR5+/+ and CCR5−/− mice were present within the brains of infected recipient mice indicated that CCR5 is not required for entry of these cells into the CNS. Chemokine receptor gene expression on S510-518-expanded CD8+ T cells from CCR5+/+ and CCR5−/− mice was compared using semiquantitative RT-PCR analysis. As shown in Fig. 2, a similar chemokine receptor gene profile is detected in antigen-expanded CD8+ T cells from either CCR5+/+ or CCR5−/− mice. Transcripts for CCR1, CCR2, and CXCR3 are prominently expressed in both populations of cells, while CCR3 and CCR4 are not detected. We have previously demonstrated that CCR2 and CXCR3 signaling enhances T cell trafficking and accumulation within the CNS of MHV-infected mice Chen et al 2001, Liu et al 2000, Liu et al 2001b. Therefore, it is likely that S510-518-specific CD8+ T cells are able to utilize these receptors and gain access into the brains of mice.
Table 1.
CCR5 is not required for CNS entry of S510–518 specific CD8+ T cells
| Experimental condition | Posttransfer day | Brain titera, b (PFU/g) | S510-518 reactive CD8+ T Cells | CD45high/F480 | Demyelination |
|---|---|---|---|---|---|
| RAG1−/− | N/A | 2.7 × 105 ± 5.9 × 104 (7)c | 0 (3) | 5.8 × 104 ± 4.6 × 103 (3) | 0 (8) |
| CCR5+/+ (CD8) → RAG1−/− | 9 | 2.2 × 105 ± 1.8 × 104 (4) | 2.2 × 104 ± 6.2 × 103 (3) | 2.9 × 105 ± 6.8 × 103 (5) | 1.2 ± 0.3 (8) |
| CCR5−/− (CD8) → RAG1−/− | 9 | 1.1 × 105 ± 2.5 × 104 (6)d, e | 2.6 × 104 ± 2.2 × 103 (3) | 5.2 × 105 ± 3.9 × 103 (5)d | 0.8 ± 0.2 (8) |
All data are presented as mean±SEM.
Flow data are presented as total number of cells present within the gated population.
Parentheses indicate number of mice used.
P≤0.04 when compared to CCR5+/+ (CD8)→RAG 1−/−.
P≤0.01 when compared to RAG 1−/−.
Fig. 2.
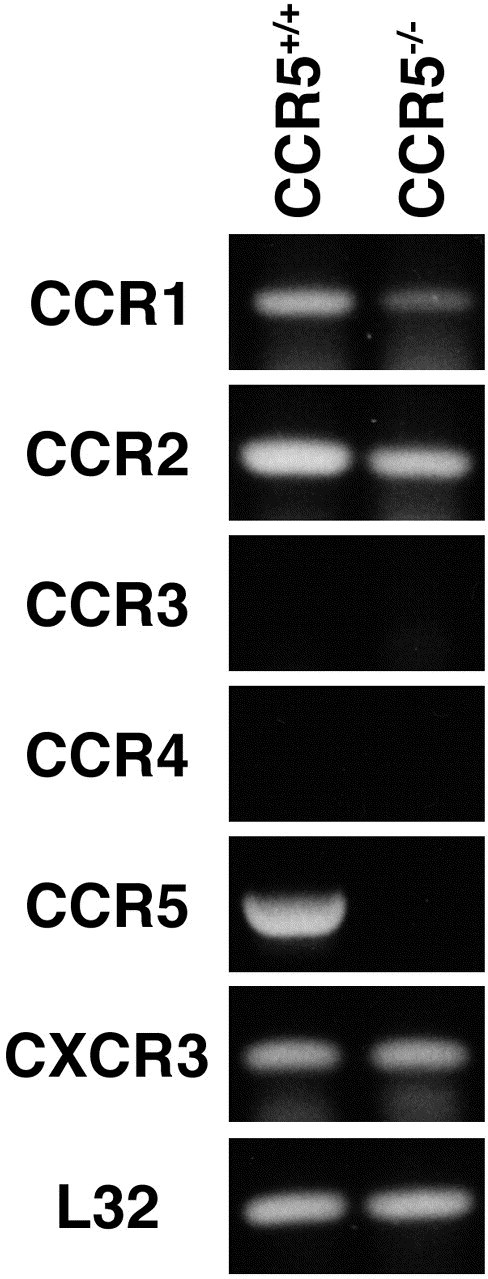
Equivalent chemokine receptor expression by CCR5+/+ and CCR5−/−-derived S510-518-specific CD8+ T cells. RT-PCR analysis of chemokine receptor expression by S510-518-expanded CD8+ T cells derived from CCR5+/+ or CCR5−/− mice. Similar levels of receptor transcript expression is seen from CCR5+/+ and CCR5−/−-derived CD8+ T cells with regards to CCR1, CCR2, and CXCR3. Expression of CCR3 or CCR4 is not found in either cell type.
Analysis of white matter damage
In addition to their important role in host defense, T cells also contribute to myelin destruction Lane et al 2000, Wu and Perlman 1999. One mechanism by which T cells amplify the severity of demyelination in MHV-infected mice is by attracting macrophages into the CNS (Lane et al., 2000). To determine whether S510-518-reactive CD8+ T cells contribute to demyelination, spinal cords were removed from RAG1−/− mice following adoptive transfer of either CCR5+/+ or CCR5−/−-derived CD8+ T cells and the severity of demyelination was determined by Luxol-fast blue staining. Such analysis revealed only focal areas of demyelination with accompanying cellular infiltration in RAG1−/− recipients of donor CD8+ T cells from either CCR5+/+ or CCR5−/− mice (Fig. 3). Although there was an approximate 45% increase in the number of macrophages (F480+/CD45high) present within the CNS of RAG1−/− recipients of CCR5−/− CD8+ T cells compared to CCR5+/+-derived CD8+ T cells, there was no significant difference in the severity of demyelination (Table 1).
Fig. 3.
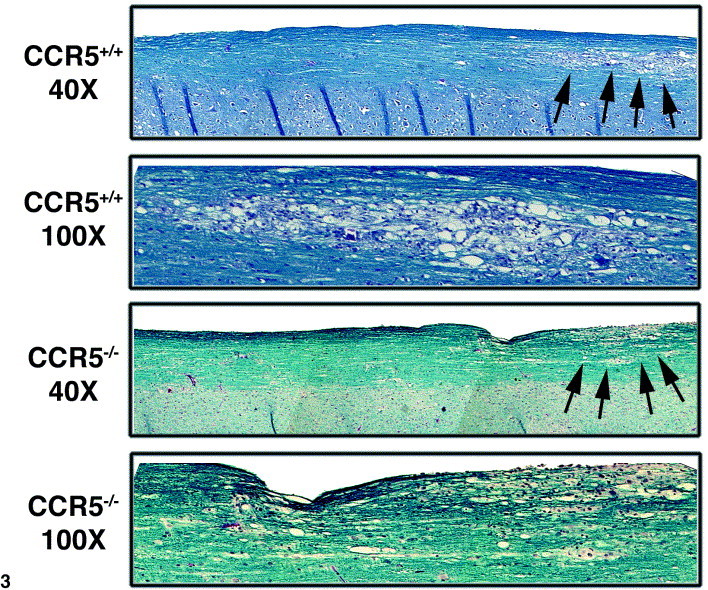
Representative Luxol fast blue staining of spinal cords removed from RAG1−/− recipients of CCR5+/+ and CCR5−/−-derived CD8+ T cells. RAG1−/− recipients of either CCR5+/+ and CCR5−/−-derived CD8+ T cells both display focal areas of cellular infiltration accompanied by demyelination (denoted by arrows). Each ×40 and ×100 panel was generated by photographing six fields of a single spinal cord at the indicated magnification and aligning images. Spinal cords presented are representative of at least four individual mice per group from two separate experiments.
IFN-γ production and cytolytic activity by CCR5−/− CD8+ T cells
To further investigate the contributions of CCR5 signaling to antiviral activities associated with CD8+ T cells, CCR5+/+ and CCR5−/− mice were immunized with MHV and spleen cells were exposed to the immunodominant CD8+ T cell epitope and IFN-γ production determined by ELISA. This analysis revealed a significant increase (P ≤ 0.05) in IFN-γ produced by splenocytes obtained from CCR5−/− mice compared to splenocytes obtained from CCR5+/+ mice (Fig. 4A). Moreover, intracellular cytokine staining of S510-518-expanded cells indicated CCR5−/−-derived CD8+ T cells produced increased levels of IFN-γ as compared to CCR5+/+-derived CD8+ T cells as measured by the MFI of IFN-γ staining (Fig. 4B). These data indicate that the absence of CCR5 signaling enhances IFN-γ production by CD8+ T cells following exposure to the immunodominant CD8+ T cell epitope in this model. To determine any differences in killing ability, a 51Cr-release CTL assay was employed. The results shown in Fig. 4C indicate that S510-518-specific CD8+ T cells from CCR5−/− mice displayed enhanced CTL activity when compared to antigen-specific CD8+ T cells obtained from CCR5+/+ mice.
Fig. 4.

Functional differences between S510-518-specific CD8+ T cells derived from CCR5+/+ and CCR5−/− mice. (A) ELISA measuring IFN-γ production from S510-518 peptide-stimulated splenocytes obtained from either sham or MHV-immunized splenocytes. Data presented are derived from two separate experiments with a total of eight mice in each group. All samples were performed in triplicate. *P ≤ 0.05 when compared to CCR5+/+ mice. (B) Intracellular staining for IFN-γ by CD8+ T cells following expansion in the presence of the S510-518 peptide. (C) Chromium release assay to measure CTL activity. CCR5−/−-derived CD8+ T cells exhibited increased CTL activity compared to CCR5+/+-derived CD8+ T cells. The differences are significant until the effector-to-target ratio reaches 1:5. Data presented are from two experiments with a total of five mice in each group.
Discussion
Activated CD8+ T cells increase expression of CCR5 in response to viral infection. For example, Nansen et al. (2002) have reported increased expression of chemokine receptors CCR2 and CCR5 on CD8+ T cells following infection with either lymphocytic choriomeningitis virus (LCMV) or vesicular stomatitis virus (VSV). Expression of these chemokine receptors by CD8+ T cells was associated with the ability of these cells to migrate to areas of viral infection, suggesting a functional role following infection with either of these viruses. Fukada et al. (2002) report that CCR5 is expressed on both effector and memory CD8+ T cells obtained from HIV-infected individuals. These cells were able to migrate in response to RANTES/CCL5, indicating a role in either host defense or immune-mediated pathogenesis by allowing these cells to traffic to areas of viral infection (Fukada et al., 2002). Further support for CCR5 in contributing to CD8+ T cell antiviral immunity comes from a report that demonstrated CTL clones expressed CCR5 and were able to migrate to areas of viral infection that contained the CCR5 ligands MIP-1α/CCL3 and MIP-1β/CCL4 following infusion into HIV-infected patients (Brodie et al., 2000). Therefore, the accumulated data indicate that CCR5 is expressed on activated CD8+ T cells following viral infection and the contributions of this receptor to either defense or disease appear to be dependent on the challenging virus.
MHV infection of the CNS results in expression of the CCR5 signaling ligands RANTES/CCL5 and MIP-1α/CCL3 Glass et al 2001, Lane et al 1998. Expression of both RANTES/CCL5 and CCR5 is important in contributing to demyelination following MHV infection by allowing macrophages to traffic into the CNS and participate in white matter destruction Glass et al 2001, Lane et al 2000. CCR5 is also expressed on activated T cells following viral infection and we have recently determined that signaling through this receptor enhances MHV-specific CD4+ T cell accumulation within the CNS presumably by responding to either RANTES/CCL5 or MIP-1α/CCL3 (Glass and Lane, 2003). The data presented in this article clearly indicate that CCR5 signaling is not required for CD8+ T cells to traffic to and/or accumulate within the CNS following MHV infection. Consistent with our earlier studies (Glass et al., 2001), there were decreased numbers of CD8+ T cells present within the CNS of MHV-infected CCR5−/− mice as compared to CCR5+/+ mice at Day 7, and there are increased numbers of CD8+ T cells at Day 12 p.i. This may be explained by an impaired ability to rapidly mount an adaptive immune response to virus during acute disease. However, at later stages of infection antigen-specific CD8+ T cells are able to accumulate within the CNS. RAG1−/− recipients of CCR5−/−-derived S510-518-specific CD8+ T cells displayed lower (P ≤ 0.05) viral titers in the brains compared to recipients of CD8+ T cells obtained from CCR5+/+ mice. These data are consistent with the demonstrated in vitro enhanced antiviral activities by CD8+ T cells derived from CCR5−/− mice. However, neither recipient group of mice was able to completely clear virus from the brain, which is consistent with recent reports that CD8+ T cells alone are not sufficient for elimination of MHV from the CNS Pewe and Perlman 2002, Stohlman et al 1998.
With regards to histological disease, emerging evidence indicates that CD8+ T cells can contribute to demyelination in MHV-infected mice Pewe and Perlman 2002, Wu et al 2000. The results presented in the current study support these earlier studies and demonstrate that adoptive transfer of MHV-specific CD8+ T cells from either CCR5+/+ or CCR5−/− mice into MHV-infected RAG1−/− mice can result in focal areas of demyelination with accompanying cellular infiltration (Pewe and Perlman, 2002). However, the overall extent of demyelination in either recipient group was markedly reduced as compared to RAG1−/− mice that received MHV-specific CD4+ T cells, which further supports a more prominent role for this T cell subset in contributing to MHV-induced demyelination Glass and Lane 2003, Lane et al 2000. An interesting observation was the demonstration of an increased numbers of macrophages present within the CNS of RAG1−/− recipients of CCR5−/−-derived CD8+ T cells as compared to recipients of CCR5+/+-derived CD8+ T cells. The underlying reasons for increased macrophage accumulation but only limited demyelination within the CNS of these mice are not entirely clear but may relate to altered expression of additional cytokines and/or chemokines that are necessary for macrophage activation and myelin destruction to occur.
In summary, these data highlight the nonredundant nature of chemokine receptor signaling as it relates to T cell activation and trafficking in response to MHV infection of the CNS. CCR5 signaling does not regulate IFN-γ expression by virus-specific CD4+ T cells but it is important for trafficking of these cells into the CNS of infected mice (Glass and Lane, 2003). In marked contrast, CCR5 regulates both IFN-γ and CTL activity by virus-specific CD8+ T cells, which correlates with both in vitro and in vivo antiviral activities. However, CCR5 signaling is not required for CD8+ T cell migration and infiltration into the CNS. Collectively, these data provide evidence that CCR5 signaling differentially regulates CD4+ and CD8+ T cell functional activities following viral infection. This is important when considering strategies for targeting this receptor in the treatment of human diseases.
Materials and methods
Virus and mice
MHV strain J2.2V-1 was kindly provided by Dr. John Fleming, University of Wisconsin, Madison, WI. Age-matched (5-7 weeks) CCR5+/+ and CCR5−/− mice (eighth generation backcrossed to C57BL/6, H-2b background, kindly provided by W. Kuziel, University of Texas, Austin, TX) were used for all experiments (Glass et al., 2001). PCR analysis of DNA obtained from tail preparations confirmed that CCR5 was not expressed in CCR5−/− mice Glass and Lane 2003, Glass et al 2001. Following anesthetization by inhalation of methoxyflurane (Pitman-Moore Inc., Washington Crossing, NJ), mice were injected intracranially (i.c.) with 1000 PFU of MHV suspended in 30 μL of sterile saline. Control (sham) animals were injected with 30 μL sterile saline alone. Animals were sacrificed at defined time points and brains and spinal cords were removed for analysis in studies described. One-half of each brain at each time point was used for plaque assay on the DBT astrocytoma cell line to determine viral burden Hirano et al 1978, Lane et al 1998. The remaining halves were either used for histological analysis, stored at −80°C for RNA isolation, or used for FACS analysis.
Generation of S510-518-specific CD8+ T cells
CCR5+/+ and CCR5−/− mice were immunized intraperitoneally (ip) with 2 × 105 PFU MHV and animals were sacrificed at Day 8 postimmunization. Spleens were harvested and a population of cells obtained through use of a magnetically labeled antibody specific for the CD4 antigen followed by passage over a magnetic column (Miltenyi Biotec, Auburn, CA). Cells separated with the CD4 antibody are considered CD8-enriched T cells, which was confirmed by flow cytometric analysis. The CD8-enriched population of cells was incubated in high-glucose DMEM containing 5 × 10−5 M β-mercaptoethanol and 10% FBS at a concentration of 5 × 106 cells/mL. Added was 5 μM of the CD8 immunodominant peptide corresponding to the viral spike (S) glycoprotein encompassing residues 510-518 (S510-518) (Castro and Perlman, 1995). Following 2 days of culture in the presence of peptide, T-stim (BD Biosciences, Bedford, MA) was added to the culture according to the manufacturer’s specifications and the cells were incubated an additional 4 days. Live cells were separated using Lympholyte-M (Cedarlane Laboratories Ltd., Ontario, Canada).
Intracellular cytokine staining
Intracellular staining for IFN-γ was performed on S510-518 peptide-expanded CD8+ T cells using a previously described procedure Chen et al 2001, Wu et al 2000. In brief, following a 6 h incubation at 37°C in medium containing GolgiStop (Cytofix/Cytoperm kit, Pharmingen, San Diego, CA), cells were washed and blocked with PBS containing 10% FBS and a 1:200 dilution of CD 16/32 (Pharmingen). Cells were then stained for surface antigens with either FITC-conjugated CD8 (Pharmingen) or Rat IgG-2b for 45 min at 4°C. Cells were fixed and permeabilized using the Cytofix/Cytoperm kit and stained for intracellular IFN-γ using PE-conjugated anti-IFN-γ (1:50; XMG1.2, Pharmingen) for 45 min at 4°C. Cells were analyzed on a FACStar (Becton–Dickinson, Mountain View, CA). Data are presented as the percentage of positive cells within the gated population.
ELISA
Supernatants from S510-518 peptide-expanded CCR5+/+ and CCR5−/− cells were collected following 48 h stimulation and IFN-γ production was determined through use of a Quantikine M Mouse IFN-γ Immunoassay kit (R&D Systems, Minneapolis, MN). Samples were measured in triplicate and IFN-γ concentrations were presented as picograms per milliliters.
Adoptive transfer
S510-518-expanded CD8+ T cells obtained from either CCR5+/+ or CCR5−/− mice were adoptively transferred (2.5 × 106 cells suspended in 100 μL sterile HBSS) via iv injection into the retro-orbital sinus of RAG1−/− mice 3 days following i.c. infection with 1000 PFU of MHV (Chen et al., 2001). Mice were sacrificed 9 days posttransfer (12 days p.i.) and brains and spinal cords were removed. One-half of the brains were used for flow analysis and the remaining half was used to determine viral titers. Spinal cords were removed and stained with Luxol-fast blue (LFB) to assess the severity of demyelination. Control animals included MHV-infected (i.c.) RAG1−/− mice receiving iv sterile HBSS.
T cell isolation and flow cytometry
Mononuclear cells were obtained from brains of experimental groups of mice at defined times postinfection (p.i.) and a single-cell suspension was obtained using a previously described protocol (Lane et al., 2000). Antibodies used for flow cytometry in these studies included FITC rat anti-mouse CD4, CD8 (Pharmingen, San Diego, CA), F4/80 (Serotec), and PE-conjugated rat anti-mouse CD45 (Pharmingen). In all cases, an isotype-matched FITC-conjugated antibody was used. Cells were incubated with antibodies for 45 min at 4°C, washed, and fixed in 1% paraformaldehyde. Following fixation, cells were analyzed on a FACStar (Becton–Dickinson). The data presented represent the number of positive cells present within the gated population.
Histology
Spinal cords were removed at 12 days p.i. and fixed by immersion overnight in 10% normal buffered Formalin (NBF) for paraffin. The severity of demyelination was determined by LFB staining of spinal cords and analyzed with a light microscope. LFB-stained spinal cord sections were coded and read blind by two investigators. Demyelination was scored as follows: 0, no demyelination; 1, mild inflammation accompanied by loss of myelin integrity; 2, moderate inflammation with increasing myelin damage; 3, numerous inflammatory lesions accompanied by significant increase in myelin stripping; and 4, intense areas of inflammation accompanied by numerous phagocytic cells engulfing myelin debris (Lane et al., 2000). An average of five spinal cords were scored per group with a minimum of six 10-μm sections per mouse investigated. Scores were averaged and presented as mean ± SEM.
CTL assay
Spleen-derived CD8+ T cells were analyzed for lytic activity following immunization of CCR5+/+ and CCR5−/− mice with 2.5 × 105 PFU of MHV. Splenocytes from infected mice were stimulated in vitro with 5 μM S510-518 peptide in RPMI 1640 supplemented with 2 mM glutamine, 1 mM sodium pyruvate, 5 × 10−2 M β-mercaptoethanol, nonessential amino acids, 10% FBS, and T-Stim (Becton–Dickinson, Bedford, MA) for 6 days before determination of lytic activity. CTL assays were performed with Na51CrO4 (New England Nuclear, Boston, MA)-labeled RMA-s (H-2b) target cells preincubated with 1 μM S510-518 peptide or 5 μM OVA Peptide (American Peptide Co., Sunnyvale, CA) as a control. Cr51 release was determined after 6 h of incubation. Specific lysis was defined as 100 × [(experimental release − spontaneous release)/(detergent release − spontaneous release)].
RT-PCR
Total RNA was extracted from S510-518-expanded CD8+ T cells using Trizol reagent (Gibco) and reverse-transcribed using the AMV reverse transcriptase system (Promega, Madison, WI). PCR amplification was performed on resulting cDNA for 30 cycles with specific primers for either CCR1 (forward, 5′ GAATTCGACAGCCAGGTTGAACAGGT; reverse, 5′ GCGGCCGCCTGCTGTAAGAGCCTTTGGG), CCR2 (forward, 5′ GAATTCGATTCCTGGAAGGTGGTCAA; reverse, 5′ GCGGCCGCATTCTCCACACCCTGTTTCG), CCR3 (forward, 5′ GAATTCTCCAAAAATGTGCTGTGGAA; reverse, 5′ GCGGCCGCGATCTTTCCTGCAGTCCTCG), CCR4 (forward, 5′ GAATTCAGAGAGGAGGCAGGAAGACC; reverse, 5′ GCGGCCGCAGAGGTCACAGACACCACCC), CCR5 (forward, 5′ GAATTCGTTCTCCTGTGGATCGGGTA; reverse, 5′ GCGGCCGCACACACTGCTGCCTAAACCC), CXCR3 (forward, 5′ GCGGCCGCAACTCTTCCATTGTGG; reverse, 5′ GAATTCAAGGCCCCTGCATAGAAGTT) or L32 (forward, 5′ AACCCAGAGGCATTGACAAC; reverse, 5′ AACGCTCAGCTCCTTGACAT). Amplification was performed on an automated Perkin–Elmer (Norwalk, CT) model 480 DNA thermocycler using the following profile: step 1, initial denaturation at 94°C for 45 s; step 2, annealing at 60°C for 45 s; and step 3, extension at 72°C for 2 min. Steps 1 to 3 were repeated 29 times for a total of 30 cycles and followed by a 7-min incubation at 72°C. The resulting amplicons were visualized by gel electrophoresis and were of the correct size corresponding to the specific primers used.
Statistical analysis
Statistically significant differences between groups of mice were determined by Student’s t test and P values of ≤0.05 were considered significant.
Acknowledgements
The authors gratefully acknowledge Edward Monuki (Department of Pathology, UCI) for assistance in histological evaluation of tissue sections. This work was supported by National Institutes of Health Grants NS37336 and NS41249 to T.E.L. W.G.G. was supported by National Institutes of Health Training Grant A107319-12.
References
- Brodie S.J., Patterson B.K., Lewinsohn D.A., Diem K., Spach D., Greenberg P.D., Riddell S.R., Corey L. HIV-specific cytotoxic T lymphocytes traffic to lymph nodes and localize at sites of HIV replication and cell death. J. Clin. Invest. 2000;105:1407–1417. doi: 10.1172/JCI8707. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Castro R.F., Perlman S. CD8+ T-cell epitopes within the surface glycoprotein of a neurotropic coronavirus and correlation with pathogenicity. J. Virol. 1995;69:8127–8131. doi: 10.1128/jvi.69.12.8127-8131.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B.P., Kuziel W.A., Lane T.E. Lack of CCR2 results in increased mortality and impaired leukocyte activation and trafficking following infection of the central nervous system with a neurotropic coronavirus. J. Immunol. 2001;167:4585–4592. doi: 10.4049/jimmunol.167.8.4585. [DOI] [PubMed] [Google Scholar]
- Fukada K., Sobao Y., Tomiyama H., Oka S., Takiguchi M. Functional expression of the chemokine receptor CCR5 on virus epitope-specific memory and effector CD8+ T cells. J. Immunol. 2002;168:2225–2232. doi: 10.4049/jimmunol.168.5.2225. [DOI] [PubMed] [Google Scholar]
- Glass W.G., Lane T.E. Functional expression of chemokine receptor CCR5 on CD4(+) T cells during virus-induced central nervous system disease. J. Virol. 2003;77:191–198. doi: 10.1128/JVI.77.1.191-198.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass W.G., Liu M.T., Kuziel W.A., Lane T.E. Reduced macrophage infiltration and demyelination in mice lacking the chemokine receptor CCR5 following infection with a neurotropic coronavirus. Virology. 2001;288:8–17. doi: 10.1006/viro.2001.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano N., Murakami T., Fujiwara K., Matsumoto M. Utility of mouse cell line DBT for propagation and assay of mouse hepatitis virus. Jpn. J. Exp. Med. 1978;48:71–75. [PubMed] [Google Scholar]
- Houtman J.J., Fleming J.O. Pathogenesis of mouse hepatitis virus-induced demyelination. J. Neurovirol. 1996;2:361–376. doi: 10.3109/13550289609146902. [DOI] [PubMed] [Google Scholar]
- Lane T.E., Asensio V.C., Yu N., Paoletti A.D., Campbell I.L., Buchmeier M.J. Dynamic regulation of alpha- and beta-chemokine expression in the central nervous system during mouse hepatitis virus-induced demyelinating disease. J. Immunol. 1998;160:970–978. [PubMed] [Google Scholar]
- Lane T.E., Buchmeier M.J. Murine coronavirus infection: a paradigm for virus-induced demyelinating disease. Trends Microbiol. 1997;5:9–14. doi: 10.1016/S0966-842X(97)81768-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane T.E., Liu M.T., Chen B.P., Asensio V.C., Samawi R.M., Paoletti A.D., Campbell I.L., Kunkel S.L., Fox H.S., Buchmeier M.J. A central role for CD4(+) T cells and RANTES in virus-induced central nervous system inflammation and demyelination. J. Virol. 2000;74:1415–1424. doi: 10.1128/jvi.74.3.1415-1424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.T., Armstrong D., Hamilton T.A., Lane T.E. Expression of Mig (monokine induced by interferon-gamma) is important in T lymphocyte recruitment and host defense following viral infection of the central nervous system. J. Immunol. 2001;166:1790–1795. doi: 10.4049/jimmunol.166.3.1790. [DOI] [PubMed] [Google Scholar]
- Liu M.T., Chen B.P., Oertel P., Buchmeier M.J., Armstrong D., Hamilton T.A., Lane T.E. The T cell chemoattractant IFN-inducible protein 10 is essential in host defense against viral-induced neurologic disease. J. Immunol. 2000;165:2327–2330. doi: 10.4049/jimmunol.165.5.2327. [DOI] [PubMed] [Google Scholar]
- Liu M.T., Chen B.P., Oertel P., Buchmeier M.J., Hamilton T.A., Armstrong D.A., Lane T.E. The CXC chemokines IP-10 and Mig are essential in host defense following infection with a neurotropic coronavirus. Adv. Exp. Med. Biol. 2001;494:323–327. doi: 10.1007/978-1-4615-1325-4_48. [DOI] [PubMed] [Google Scholar]
- Marten N.W., Stohlman S.A., Bergmann C.C. MHV infection of the CNS: mechanisms of immune-mediated control. Viral Immunol. 2001;14:1–18. doi: 10.1089/08828240151061329. [DOI] [PubMed] [Google Scholar]
- Nansen A., Christensen J.P., Andreasen S.O., Bartholdy C., Christensen J.E., Thomsen A.R. The role of CC chemokine receptor 5 in antiviral immunity. Blood. 2002;99:1237–1245. doi: 10.1182/blood.v99.4.1237. [DOI] [PubMed] [Google Scholar]
- Pewe L., Haring J., Perlman S. CD4 T-cell-mediated demyelination is increased in the absence of gamma interferon in mice infected with mouse hepatitis virus. J. Virol. 2002;76:7329–7333. doi: 10.1128/JVI.76.14.7329-7333.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pewe L., Perlman S. Cutting edge: CD8 T cell-mediated demyelination is IFN-gamma dependent in mice infected with a neurotropic coronavirus. J. Immunol. 2002;168:1547–1551. doi: 10.4049/jimmunol.168.4.1547. [DOI] [PubMed] [Google Scholar]
- Stohlman S.A., Bergmann C.C., Lin M.T., Cua D.J., Hinton D.R. CTL effector function within the central nervous system requires CD4+ T cells. J. Immunol. 1998;160:2896–2904. [PubMed] [Google Scholar]
- Williamson J.S., Stohlman S.A. Effective clearance of mouse hepatitis virus from the central nervous system requires both CD4+ and CD8+ T cells. J. Virol. 1990;64:4589–4592. doi: 10.1128/jvi.64.9.4589-4592.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G.F., Dandekar A.A., Pewe L., Perlman S. CD4 and CD8 T cells have redundant but not identical roles in virus-induced demyelination. J. Immunol. 2000;165:2278–2286. doi: 10.4049/jimmunol.165.4.2278. [DOI] [PubMed] [Google Scholar]
- Wu G.F., Perlman S. Macrophage infiltration, but not apoptosis, is correlated with immune-mediated demyelination following murine infection with a neurotropic coronavirus. J. Virol. 1999;73:8771–8780. doi: 10.1128/jvi.73.10.8771-8780.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


