Abstract
MCP-1/CCL2 plays a critical role in monocyte recruitment into sites of immune responses and cancer. However, the role of other MCPs remains unclear. In this study, we generated a novel MCP-1-deficient (designated as MCP-1Δ/Δ) mouse model by deleting a 2.3-kb DNA fragment from the mouse genome using the Cre/loxP system. MCP-1 was not produced by LPS-activated MCP-1Δ/Δ macrophages; however, the production of MCP-3, coded by the immediate downstream gene, was significantly increased. In contrast, macrophages from another mouse line with a neo-gene cassette in intron 2 produced a significantly lower level of MCP-1 and MCP-3. Decreased MCP-3 production was also detected in previously generated MCP-1-deficient mice in which a neo-gene cassette was inserted in exon 2 (designated as MCP-1 knockout (KO)). Altered MCP-1 and/or MCP-3 production was also observed in vivo in each mouse model in response to i.p. injection of thioglycolate or zymosan. The up- and down-regulation of MCP-3 production in MCP-1Δ/Δ and MCP-1 KO mice, respectively, provided us with a unique opportunity to evaluate the role for MCP-3. Despite the increased MCP-3 production in MCP-1Δ/Δ mice, thioglycolate- or zymosan-induced monocyte/macrophage accumulation was still reduced by ~50% compared with wild-type mice, similar to the reduction detected in MCP-1 KO mice. Thus, up-regulated MCP-3 production did not compensate for the loss of MCP-1, and MCP-3 appears to be a less effective mediator of monocyte recruitment than MCP-1. Our results also indicate the presence of other mediators regulating the recruitment of monocytes in these models.
Recruitment of blood monocytes into inflammatory sites is a critical step for host defense and is regulated by local production of chemoattractants in response to noxious stimuli. MCPs, including MCP-1/CCL2, MCP-2/CCL8, MCP-3/CCL7, and MCP-4/CCL13 in human or MCP-5/CCL12 in mice, are structurally related chemokines with in vitro monocyte chemotactic activity (1), and the genes coding for these MCPs are clustered on human chromosome 17q11.2–12 or mouse chromosome 11B5-C (2). Thus, MCPs form a subgroup in the CC-chemokine branch of the chemokine family, which all interact with the CCR2 receptor.
Among MCPs, MCP-1 was the first to be identified based on its potent in vitro monocyte chemotactic activity (3, 4) and is the best studied molecule. The gene for MCP-1 was subsequently disrupted in mice (designated as MCP-1 knockout (KO)4 mice in this study) (5), and studies of these mice provided strong evidence that this chemokine plays a critical, nonredundant role in the recruitment of monocytes into sites of inflammatory responses and contributes to the development of many diseases, including atherosclerosis, pulmonary fibrosis, and multiple sclerosis (6).
In contrast to MCP-1, study of the other MCPs has been limited, and their role in inflammation and disease process remains unclear. Recently, mice lacking MCP-3 or MCP-5 or MCP-2 plus MCP-5 have been generated. These additional mouse models provided evidence that MCP-3 plays a role in monocyte mobilization from bone marrow and recruitment to inflammatory sites by interacting with CCR2, one of the receptors with which MCP-3 has been shown to interact (7).
MCP-1 is produced by a variety of cell types, including macrophages, fibroblasts, epithelial cells, and endothelial cells (8). To determine the cell source of MCP-1 in specific diseases, we have been developing cell type-specific, conditional MCP-1-deficient mice using the Cre-loxP system. During the process, we obtained systemic MCP-1-deficient mice (designated as MCP-1Δ/Δ mice in the present work) in which the 2.3-kb fragment of the MCP-1 gene, including the 5′-flanking region, exon 1, intron 1, and exon 2 of the MCP-1 gene, is deleted. Unexpectedly, we found MCP-3 production was markedly up-regulated in vitro in LPS-activated MCP-1Δ/Δ peritoneal macrophages and in vivo in response to i.p. injection by thioglycolate (TG) or zymosan A. In contrast, the production of MCP-3 was found to be down-regulated in previously generated MCP-1 KO mice both in vitro and in vivo. This provided us with a unique opportunity to evaluate the role for MCP-3 in the recruitment of monocytes in the absence of MCP-1 in TG- or zymosan A-induced peritonitis model.
Materials and Methods
Reagents
RPMI 1640 and TRIzol reagent were from Invitrogen. FCS was from Hy-Clone. Anti-MCP-3 goat IgG and normal goat IgG were from R&D Systems. The neutralization dose 50 for this Ab was ~15–30 μg/ml in the presence of 1 μg/ml MCP-3 in vitro, according to the provider. TG was from Difco Laboratories. LPS and zymosan A were from Sigma-Aldrich. LPS was dissolved in PBS at 0.5 mg/ml. Zymosan A was suspended in PBS, boiled for 1 h, rinsed with PBS three times, and resuspended in PBS at 1 mg/ml. The NF-κB inhibitor caffeic acid phenethyl ester (CAPE) (9) was purchased from Calbiochem and dissolved in DMSO at 25 mg/ml and diluted by RPMI 1640 containing 10% FCS. A total of 10 μg/ml was optimal to inhibit MCP-1 production by mouse macrophages (data not shown). [α−32P]dCTP was from Amersham.
Mice
MCP-1 KO mice on a C57BL/6 background (5) were purchased from The Jackson Laboratory. CCR2 KO mice on a C57BL/6 background (10) were obtained from Z. Howard (National Cancer Institute, Frederick, MD). C57BL/6 mice were purchased from Charles River Laboratories. The protocols of this study were approved by the National Cancer Institute Animal Care and Use Committee.
Generation of MCP-1Δ/Δ mice
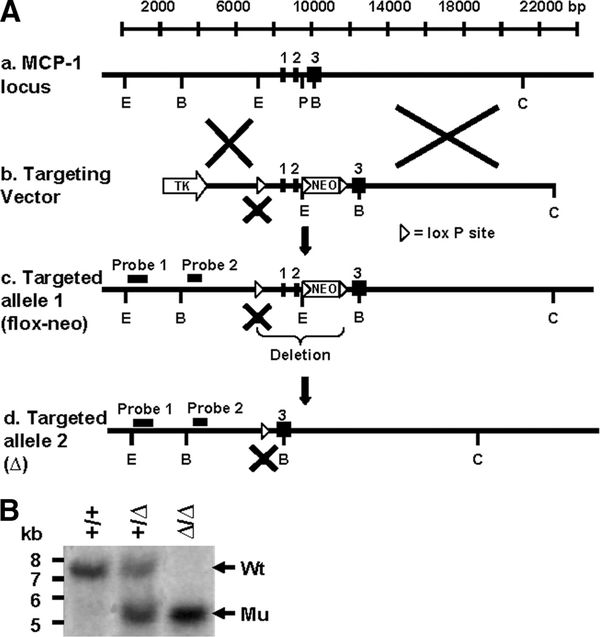
A murine genomic clone containing the MCP-1 gene was isolated from a 129/SVJ mouse λ phage library (Stratagene) and subcloned into pBluescript vector (Stratagene). A loxP-neo-loxP cassette was inserted in a PstI site upstream of exon 3 for G418 selection, and a second loxP site was inserted in an EcoRI site upstream of exon 1, destroying the EcoRI site. A thymidine kinase gene was inserted into the multiple cloning site of the targeting vector for negative selection against random insertion (Fig. 1A). The vector was linearized with ClaI and electroporated into strain 129-derived mouse embryonic stem (ES) cells. ES cell clones, which had undergone homologous recombination with the targeting vector, were selected on G418 (250 μg/ml) and gancyclovir (2 μM)-containing medium, using standard procedures. EcoRI- or BamHI-digested DNA from ES cell clones was analyzed by Southern blot analysis, using two external probes, located upstream of the 5′ loxP site, which detected a 7.2-kb EcoRI fragment of the MCP-1 wild-type (WT) allele, a 9.5-kb fragment of the MCP-1 flox-neo allele, a 7.5-kb BamHI fragment of the MCP-1 WT allele, and a 9.3-kb BamHI fragment of the MCP-1 flox-neo allele (data not shown).
FIGURE 1.
Strategy for targeted deletion of the mouse MCP-1 gene. A, Targeting of the murine MCP-1 locus. a, WT MCP-1 gene locus showing three exons and long 5′ and 3′ noncoding regions. b, MCP-1 targeting vector, in which loxP-neo-loxP cassette was inserted into intron 2 and a single loxP site replaced an EcoRI site in the 5′ flanking region. c, MCP-1 flox-neo allele in the mutant mice. d, MCP-1-deleted (Δ) allele after crossing to EIIaCre mice. Representative restriction endonuclease-digesting sites are shown. E, EcoRI; B, BamHI; P, PstI; C, ClaI. B, A 5′ external probe was used to detect BamHI fragments of 7.5 kb (Wt) and 5.5 kb (Δ allele) by Southern blot analysis.
The targeted 129/SVJ ES cells were injected into the blastocysts of C57BL/6 mice, and chimeras were selected that showed high chimeric rates and bred to C57BL/6 mice to achieve the germline transmission of the MCP-1 mutated allele. The pups were genotyped by Southern blot analysis of EcoRI- or BamHI-digested tail DNA, using the external probes and strategy described above. Hetrozygous mice with the mutated allele (targeted allele 1) were intercrossed to generate homozygous mice (MCP-1neo/neo), which were subsequently crossed to EIIaCre mice to obtain mice lacking exons 1 and 2 of the MCP-1 gene (Fig. 1A, targeted allele 2), according to the method described by Holzenberger et al. (11). Offspring of the mice were genotyped by Southern blot analysis of BamHI-digested tail DNA, using the probe described above, which detected a 7.5-kb BamHI fragment for the MCP-1 WT allele and a 5.5-kb BamHI fragment for the MCP-1-deleted allele (Fig. 1B) or by PCR (data not shown). For PCR, two primer sets were used, amplifying a 780-bp fragment of the WT allele or 250 bp of the mutated allele. Primer sequences used are as follows: F-Lox1, 5′-GATACCTGAGTGGAAGACTC-3′ and R-Int2, 5′-GTCAGCACAGACCTCTCTC-3′ (for the WT allele); F-Exon1, 5′-CGTGTTGGCTCAGCCAG-3′ and R-Exon2, 5′-TGGGGCGTTAACTGCAT-3′ (for the mutated allele).
Induction of TG- or zymosan A-induced peritonitis
Eight- to 12-wk-old mice were i.p. injected with 1 ml of sterile, 3% TG broth or 0.5 ml of 400 μg/ml zymosan A. To neutralize MCP-3 activity, 10 or 25 μg of goat anti-mouse MCP-3 IgG or goat IgG in 100 μl of PBS was injected together with TG. The mice were sacrificed at several time points, and peritoneal exudate cells (PEC) were harvested by peritoneal lavage, using 5 ml of cold PBS. The concentration of cells in each exudate was counted using hemocytometers under microscope. Cells were applied to microscope slides, using a cytospin centrifuge (Thermo Shandon), and stained with Diff-Quick (Baxter Healthcare), and differential cell counts were obtained by morphological analysis. The number of macrophages was calculated, using the total cell number and the percentage of macrophages in the same sample. When MCP-1Δ/Δ mice with a mixed genetic background were used, littermates were used as control. MCP-1Δ/Δ mice that had been backcrossed onto C57BL/6 background for nine generations were also used in some experiments.
Quantification of chemokine concentration
The concentrations of MCP-1, eotaxin, and MCP-3 were measured in the Lymphokine Testing Laboratory (Clinical Services Program, SAIC-Frederick, National Cancer Institute), with an ELISA kit specific for mouse MCP-1, eotaxin (R&D Systems), and MCP-3 (Bender MedSystems), respectively. The concentrations of mouse MIP-2, KC, MCP-5, and MIP-1α were measured by the SearchLight Proteome Array (Pierce).
Southern blotting
Southern blot analysis was performed as described in a 1% agarose gel with 10 μg of endonuclease-digested DNA per lane (12). Filters were hybridized at 42°C overnight in 50% formamide, 5× SSC, 2.5× Denhardt’s solution, 50 μg/ml sheared-denatured salmon sperm DNA, 1% SDS, and 2 × 106 dpm/ml 32P-labeled probe. Filters were washed twice with 2× SSC, 1% SDS, at 42°C for 15 min and 0.2× SSC, 1% SDS at 60°C for 30 min before autoradiographic exposure.
Northern blotting
Northern blot analysis was performed, as described, in 1.2% agarose gels containing formaldehyde (12). Filters were hybridized at 42°C overnight in 50% formamide, 5× SSC, 5× Denhardt’s solution, 50 μg/ml sheared-denatured salmon sperm DNA, 1% SDS, and l × 106 dpm/ml 32P-labeled cDNA probe. Filters were washed twice with 2× SSC, 0.5% SDS at room temperature for 15 min and 0.1× SSC, 0.5% SDS at 60°C for 30 min before autoradiographic exposure. The cloning of mouse MCP-1 cDNA was previously reported (13). Mouse MCP-3 and MIP-2 cDNA were cloned by RT-PCR using a cDNA library derived from LPS-activated ANA-1 mouse macrophage cell line cells. The primer pairs used were as follows: mouse MCP-3 forward, 5′-ATGAGGATCTCTGCCACGCTTC-3′ and reverse, 5′-TCAAGGCTTTGGAGTTGGGG-3′; mouse MIP-2 forward, 5′-ACACTTCAGCCTAGCGCCATG-3′ and reverse, 5′-TCAGTTAGCCTTGCCTTTGTTC-3′. MCP-1Δ/Δ mice showed no obvious developmental defects and were born at the expected Mendelian ratio.
Statistical analysis
Statistical analysis was performed by one-way ANOVA, followed by the Bonferroni multiple comparison test, using the GraphPad Prism, version 4 (GraphPad). A value of p < 0.05 was considered to be statistically significant.
Results
Deletion of a 2.3-kb genomic DNA from the MCP-1 locus up-regulates the macrophage production of MCP-3 and MCP-5
Macrophages are one of the major MCP-1-producing cells. To confirm the absence of MCP-1 production in MCP-1Δ/Δ mice, we obtained 96-h TG-induced PEC, of which 80–90% were macrophages, from MCP-1+/+, MCP-1+/Δ, or MCP-1Δ/Δ mice. Approximately 5 × 106 cells were seeded in 24-well plates and incubated for 30–60 min at 37°C. After removing nonadherent cells by gentle wash, the remaining macrophages were activated with 100 ng/ml LPS in vitro for 24 h, and the concentration of MCP-1 in the culture supernatants was measured by ELISA. As shown in Fig. 2A, ~4400 pg/ml MCP-1 was detected in the culture supernatants of MCP-1+/+ macrophages. The MCP-1 concentration was significantly reduced by 60% in the culture supernatants of MCP-1+/Δ macrophages, compared with that of MCP-1+/+ macrophages. MCP-1 was undetectable in the culture supernatants of MCP-1Δ/Δ macrophages.
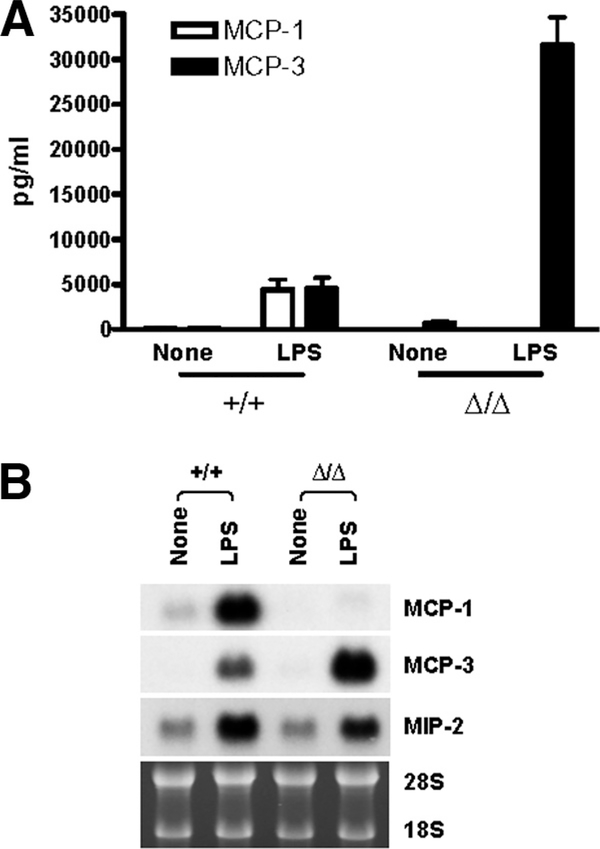
FIGURE 2.
Up-regulated MCP-3 production by LPS-activated, MCP-1Δ/Δ, TG-induced peritoneal macrophages in vitro. A, Five million PEC obtained from MCP-1+/+, MCP-1+/Δ, or MCP-1Δ/Δ mice 4 days after i.p. TG injection were seeded in 24-well plates, and adherent cells were incubated for 24 h in the presence or absence of 100 ng/ml LPS; the concentration of MCP-1 and MCP-3 in the cell-free supernatants was measured by ELISA specific for MCP-1 and MCP-3, respectively. Data are presented as mean ± SD obtained with cells from three to five mice. B, Five million PEC obtained from MCP-1+/+ or MCP-1Δ/Δ mice 4 days after i.p. TG injection were seeded in 24-well plates. Adherent cells were pretreated with 10 μg/ml CAPE or DMSO alone for 30 min, and then incubated for an additional 24 h in the presence or absence of 100 ng/ml LPS. The concentration of MCP-1 and MCP-3 in the culture supernatants was measured by ELISA specific for MCP-1 and MCP-3, respectively. Data are presented as mean ± SD obtained with cells from three mice.
The mouse MCP-3 gene resides ~8 kb downstream of the MCP-1 gene, and the deletion of the 2.3-kb fragment of the MCP-1 gene could affect the expression and subsequent production of MCP-3. We therefore measured the concentration of MCP-3 in the same culture supernatants (Fig. 2A). In the culture supernatants of MCP-1+/+ macrophages, ~5000 pg/ml MCP-3 was detected. Unexpectedly, the concentration of MCP-3 in the culture supernatants of MCP-1+/Δ or MCP-1Δ/Δ macrophages was ~6-fold higher than that of MCP-1+/+ macrophages.
LPS induces the transcription of many genes, including the MCP-1 gene, by activating NF-κB. To examine whether increased MCP-3 production was due to the activation of NF-κB, we pretreated MCP-1+/+ or MCP-1Δ/Δ macrophages with the NF-κB inhibitor CAPE, stimulated them with LPS, and measured MCP-1 concentration in the culture supernatants. As shown in Fig. 2B, CAPE pretreatment almost completely inhibited the production of both MCP-1 and MCP-3 by LPS-activated MCP-1+/+ macrophages, indicating that the transcription of the MCP-3 gene is also regulated by NF-κB. CAPE also dramatically inhibited the production of MCP-3 by MCP-1Δ/Δ macrophages. These results supported the hypothesis that the increased MCP-3 production by MCP-1Δ/Δ macrophages was due to the up-regulated transcription of the MCP-3 gene caused by the deletion of the MCP-1 gene.
To obtain additional evidence supporting our hypothesis, we examined the release and expression of MCP-1 and MCP-3 by resident macrophages that were not conditioned by mediators produced during the peritonitis. As shown in Fig. 3A, the culture supernatants of LPS-activated resident MCP-1+/+ macrophages contained ~5000 pg/ml MCP-1 and MCP-3. In contrast, the culture supernatants of LPS-activated resident MCP-1Δ/Δ macrophages contained no MCP-1 and a ~6-fold higher level of MCP-3. We next examined the expression of MCP-1 and MCP-3 mRNA in LPS-activated resident macrophages by Northern blotting. The level of MCP-3 mRNA was higher in resident MCP-1Δ/Δ macrophages than in MCP-1+/+ macrophages (Fig. 3B). The expression of MIP-2 mRNA was not significantly altered. These results further supported our hypothesis that the deletion of the MCP-1 gene directly affected the expression of MCP-3.
FIGURE 3.
Up-regulated MCP-3 production by LPS-activated, MCP-1Δ/Δ, peritoneal resident macrophages in vitro. A, Five million resident peritoneal cells obtained from MCP-1+/+ or MCP-1Δ/Δ mice were incubated for 24 h in the presence or absence of 100 ng/ml LPS, and the concentration of MCP-1 and MCP-3 in the culture supernatants was measured by ELISA specific for MCP-1 and MCP-3, respectively. Data are presented as mean ± SD obtained with cells from six mice. B, Resident peritoneal cells obtained from three MCP-1+/+ or MCP-1Δ/Δ mice were incubated at 5 × 106/ml for 6 h in the presence or absence of 100 ng/ml LPS. Total RNA was extracted, and 10 μg was used to evaluate the expression of MCP-1, MCP-3, and MIP-2 mRNA by Northern blotting. To indicate equal loading of each RNA sample, the image of the ethidium bromide-stained gel is also presented. Representative data of two individual experiments with similar results are shown.
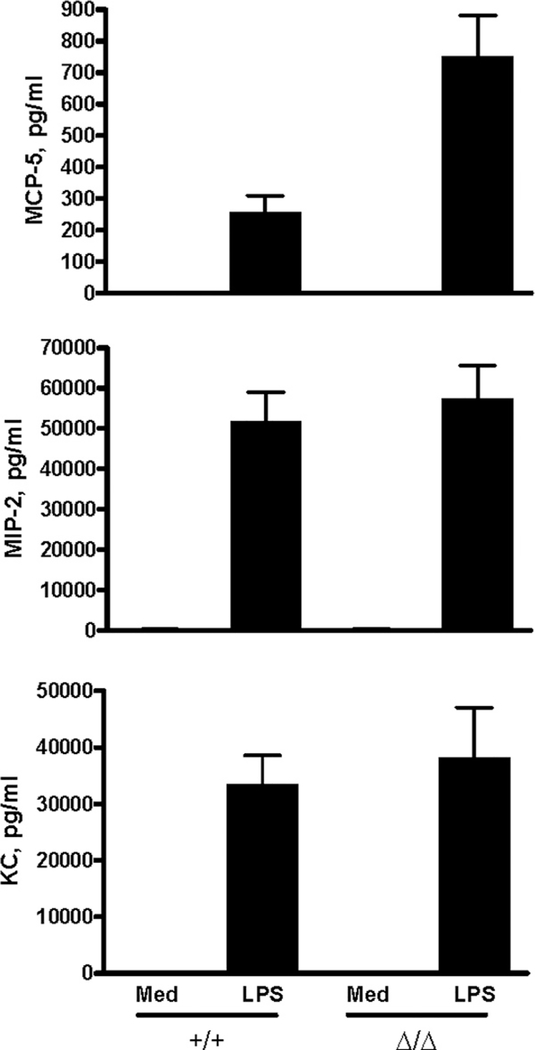
To examine the effect of the 2.3-kb DNA deletion on the expression of further downstream genes in this locus, we evaluated the production of eotaxin and MCP-5. Eotaxin was not detectable in the culture supernatants of either MCP-1+/+ or MCP-1Δ/Δ macrophages. However, MCP-5 concentration in the culture supernatants of MCP-1Δ/Δ macrophages was higher than that of MCP-1+/+ macrophages (Fig. 4). These results strongly suggested that the deletion of the 2.3-kb fragment of the MCP-1 gene positively regulated the transcription of the downstream MCP-3 and MCP-5 genes. It should be noted, however, that the concentration of MCP-5 was 40- to 70-fold lower than that of MCP-3. The production of MIP-2 and KC was not altered in MCP-1Δ/Δ macrophages. In addition to LPS-activated peritoneal macrophages, Con A-activated spleen cells from MCP-1Δ/Δ mice produced a higher level of MCP-3 than those from MCP-1+/+ mice (data not shown), indicating that increased production of MCP-3 was not limited to LPS-activated macrophages.
FIGURE 4.
Up-regulated MCP-5 production by LPS-activated, MCP-1Δ/Δ, TG-induced peritoneal macrophages in vitro. Five million PEC obtained from MCP-1+/+ or MCP-1Δ/Δ mice 4 days after i.p. TG injection were seeded in 24-well plates. Adherent cells were incubated for 24 h in the presence or absence of 100 ng/ml LPS, and the concentration of MIP-2, KC, and MCP-5 in the culture supernatants was measured. Data are presented as mean ± SD obtained with cells from three mice.
Down-regulation of MCP-1 and MCP-3 production by an insertion of a neo-gene cassette inside the MCP-1 gene
We next asked whether insertion of a neo-gene cassette inside the MCP-1 gene locus can also affect the expression of MCP-1 and/or MCP-3. To answer the question, we evaluated the production of MCP-1 and MCP-3 by macrophages from MCP-1neo/neo mice in which a neo-gene cassette was inserted into intron 2 of the MCP-1 gene (see Fig. 1A, targeted allele 1). As shown in Fig. 5A, the concentrations of MCP-1 and MCP-3 in the culture supernatants of resident MCP-1neo/neo macrophages were much lower compared with those of MCP-1+/+ macrophages, indicating that the insertion of a neo-gene cassette down-regulated the expression of both MCP-1 and MCP-3. In a previously generated MCP-1 KO mouse model (5), the MCP-1 gene was disrupted by the insertion of a neo-gene cassette into exon 2, very close to the position where a neo-gene cassette was inserted in our MCP-1neo/neo mice, suggesting that the production of MCP-3 might be reduced in MCP-1 KO mice. Therefore, we measured the concentration of MCP-1 and MCP-3 in the culture supernatants of resident MCP-1 KO macrophages. As expected, there was no MCP-1 in the culture supernatants. Interestingly, the concentration of MCP-3 was dramatically reduced in the culture supernatants of LPS-activated, resident MCP-1 KO macrophages in comparison with those of WT macrophages (Fig. 5B). In addition to MCP-3, the concentration of MCP-5 was also reduced (Fig. 5C). By Northern blotting, we found a significantly lower level of MCP-3 mRNA in MCP-1 KO macrophages (Fig. 5D). In contrast, a similar level of MIP-2 mRNA was detected in macrophages from both MCP-1 KO and WT control mice (Fig. 5D).
FIGURE 5.
Down-regulation of MCP-1 and MCP-3 production by the insertion of a neo cassette in the MCP-1 gene locus. A, Five million resident peritoneal cells obtained from MCP-1+/+ or MCP-1neo/neo mice were incubated for 24 h in the presence or absence of 100 ng/ml LPS, and the concentration of MCP-1 and MCP-3 in the culture supernatants was measured by ELISA specific for MCP-1 and MCP-3, respectively. Data are presented as mean ± SD obtained with cells from three to five mice. B, Five million resident peritoneal cells obtained from WT C57BL/6 or MCP-1 KO mice were incubated for 24 h in the presence or absence of 100 ng/ml LPS, and the concentration of MCP-1 and MCP-3 in the culture supernatants was measured by ELISA specific for MCP-1 and MCP-3, respectively. Data are presented as mean ± SD obtained with cells from three mice. C, Five million resident peritoneal cells obtained from WT C57BL/6 or MCP-1 KO mice were incubated for 24 h in the presence or absence of 100 ng/ml LPS, and the concentration of MCP-5 in the culture supernatants was measured. Data are presented as mean ± SD obtained with cells from three mice. D, Resident peritoneal cells obtained from three WT C57BL/6 or MCP-1Δ/Δ mice were incubated at 5 × 106/ml for 6 h in the presence or absence of 100 ng/ml LPS. Total RNA was extracted, and 10 μg was used to evaluate the expression of MCP-1, MCP-3, and MIP-2 mRNA by Northern blotting. To indicate equal loading of each RNA sample, the image of the ethidium bromide-stained gel is also presented. Representative data of two individual experiments with similar results are shown.
MCP-3 concentration is increased in the peritoneal exudates of MCP-1Δ/Δ mice in response to i.p. injection of TG
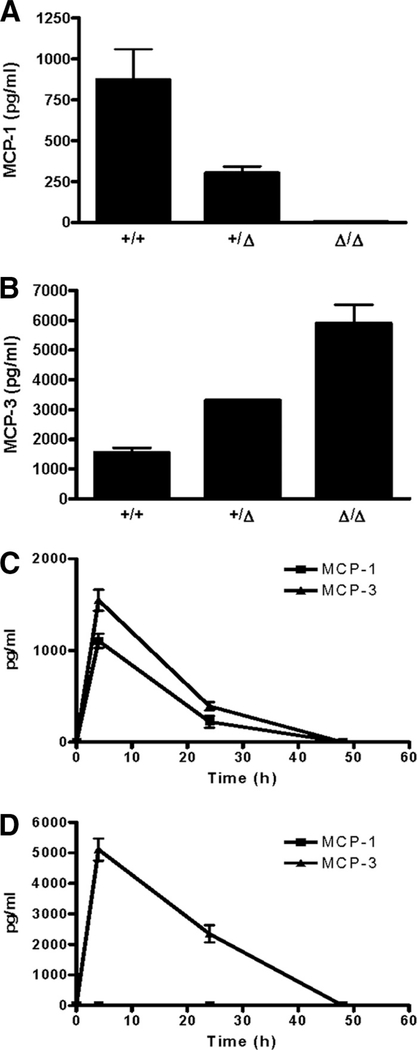
Next, using a TG-induced peritonitis model, we examined whether the production of MCP-3 is up-regulated in MCP-1Δ/Δ mice in vivo in response to proinflammatory stimulation. The production of MCP-1 and its kinetics have been well characterized in this model (14). As shown in Fig. 6A, ~850 pg/ml MCP-1 was detected in the peritoneal fluids of MCP-1+/+ mice 4 h after TG injection. The concentration of MCP-1 was significantly reduced (~300 pg/ml) in the peritoneal fluids of MCP-1+/− mice. As expected, MCP-1 was not detectable in the peritoneal fluids of MCP-1Δ/Δ mice. In the peritoneal fluids of MCP-1+/+ mice, ~1600 pg/ml MCP-3 was detected. Consistent with our in vitro results, the concentration of MCP-3 in the peritoneal fluids of MCP-1+/Δ and MCP-1Δ/Δ mice was markedly higher compared with MCP-1+/+ mice. A ~4-fold higher level of MCP-3 was detected in the peritoneal fluids of MCP-1Δ/Δ mice, compared with MCP-1+/+ mice.
FIGURE 6.
Elevated MCP-3 production in MCP-1Δ/Δ mice in response to i.p. injection of TG. One milliliter of 3% TG was i.p. injected into MCP-1+/+, MCP-1+/Δ, or MCP-1Δ/Δ mice. Peritoneal fluids were obtained, and the concentration of MCP-1 and MCP-3 was measured by ELISA specific for MCP-1 and MCP-3, respectively. A, MCP-1 in 4-h peritoneal fluids. Data are presented as mean ± SD obtained from three to four mice. B, MCP-3 in 4-h peritoneal fluids. Data are presented as mean ± SD obtained from three mice. C, Kinetics of MCP-1 and MCP-3 production in MCP-1+/+ mice. Data are presented as mean ± SD obtained from three to six mice. D, Kinetics of MCP-1 and MCP-3 production in MCP-1Δ/Δ mice. Data are presented as mean ± SD obtained from four to seven mice.
We then measured the concentration of MCP-1 and MCP-3 in the peritoneal exudates obtained at later time points (24 and 48 h). As shown in Fig. 6C, the kinetics of MCP-1 and MCP-3 concentration in the peritoneal fluids of TG-injected MCP-1+/+ mice were very similar. The concentration of both chemokines decreased at 24 h and was below the detection limit by 48 h. In the peritoneal fluids of MCP-1Δ/Δ mice, a significant amount of MCP-3 was still detectable at 24 h, but MCP-3 was no longer detectable at 48 h (Fig. 6D). Thus, the level of MCP-3 production was up-regulated in vivo in MCP-1Δ/Δ mice in response to TG injection, but the kinetics was not affected.
We also evaluated the in vivo production of MCP-1 and MCP-3 in MCP-1neo/neo, MCP-1 KO, and CCR2 KO mice. As shown in Fig. 7, the concentrations of both MCP-1 and MCP-3 were markedly reduced in the peritoneal fluids of MCP-1neo/neo mice at 4 h. The concentration of MCP-3 was reduced also in MCP-1 KO mice. These results were consistent with the results obtained in vitro. The concentrations of both MCP-1 and MCP-3 were significantly higher in the peritoneal fluids of CCR2 mice, most likely due to the decreased CCR2-dependent clearance of these chemokines, as previously reported for MCP-1 (15).
FIGURE 7.
Down-regulated MCP-3 production in MCP-1neo/neo and MCP-1 KO mice in response to i.p. injection of TG. One milliliter of 3% TG was i.p. injected into MCP-1+/+, MCP-1neo/neo, WT C57BL/6, MCP-1 KO, and CCR2 KO mice. Peritoneal fluids were obtained 4 h after TG injection, and the concentration of MCP-1 and MCP-3 was measured by ELISA specific for MCP-1 and MCP-3, respectively. Data are presented as mean ± SD obtained from three to four mice. ∗, p < 0.01; ∗∗, p < 0.001.
Up-regulated MCP-3 production does not compensate for the loss of MCP-1 in TG- or zymosan-induced peritonitis
As shown above, the production of MCP-3 was increased in our MCP-1Δ/Δ mice in response to i.p. injection of TG, whereas the production of MCP-3 was reduced in MCP-1 KO mice. Thus, these two mouse models provided a unique opportunity to evaluate the in vivo role for MCP-3 in the recruitment of monocytes. As shown in Fig. 8A, ~2.3 × 107 macrophages accumulated at 96 h in the peritoneal cavities of TG-injected MCP-1+/+ mice. The number of macrophages at this time point was ~6-fold higher than that of resident macrophages (~4 × 106 macrophages). In contrast, in MCP-1Δ/Δ and MCP-1 KO mice, the increase in macrophage number was ~3-fold, only 50% of that observed in MCP-1+/+ mice. CCR2 KO mice had the lowest number of macrophages. To further evaluate the role of MCP-3 in the accumulation of macrophages in MCP-1Δ/Δ mice, up to 25 μg of anti-MCP-3 goat IgG, considered sufficient to neutralize MCP-3 produced in response to TG injection, or normal goat IgG was administered together with TG. There was no effect by the coadministration of anti-MCP-3 IgG (data not shown).
FIGURE 8.
Reduced TG-induced macrophage accumulation in MCP-1Δ/Δ and MCP-1 KO mice. A, Accumulation of macrophages 96 h after TG injection. The number of macrophages in each peritoneal cavity was calculated by multiplying total cell number by the percentage of macrophages. The number of resident macrophages is indicated by a broken line. The level of MCP-1 and MCP-3 expression in each mouse strain is also indicated. B, Accumulation of macrophages 24 h after TG injection. The number of macrophages in each peritoneal cavity was calculated by multiplying total cell number by the percentage of macrophages. MCP-1Δ/Δ mice were backcrossed onto C57BL/6 background for nine generations. C, The percentage of neutrophils in the peritoneal exudate cells 24 h after TG injection. MCP-1Δ/Δ mice were backcrossed onto C57BL/6 background for nine generations. ∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001.
Because the concentration of MCP-1 and MCP-3 peaked at 4 h, we examined the infiltration of monocytes at 24 h. The number of macrophages increased in response to TG injection in all strains; however, the number of macrophages that accumulated in MCP-1Δ/Δ and MCP-1 KO mice was significantly lower than that in WT mice (Fig. 8B). The number of accumulating macrophages in MCP-1 KO mice was not statistically significant compared with that in MCP-1Δ/Δ mice. In contrast to macrophages, a higher percentage of neutrophils was consistently present in the exudates of MCP-1Δ/Δ and MCP-1 KO mice compared with WT mice (Fig. 8C). The numbers of total infiltrating cells were not significantly different among three strains because of the increased number of neutrophils in MCP-1Δ/Δ and MCP-1 KO mice (data not shown).
We next used a zymosan A-induced peritonitis model. Zymosan A is a component of yeast cell wall and is reported to activate cells via interaction with TLR2 (16); therefore, this model is more physiologically relevant. We first stimulated peritoneal resident cells with zymosan A in vitro and evaluated the production of MCP-1. As shown in Fig. 9A, peritoneal cells of MCP-1Δ/Δ mice released 3-fold higher level of MCP-3 compared with cells of WT mice. In contrast, cells of MCP-1 KO mice released a very low level of MCP-3. These results are consistent with those obtained by stimulation with the TLR4 ligand LPS (Figs. 3A and 5B).
FIGURE 9.
Reduced macrophage accumulation in MCP-1Δ/Δ and MCP-1 KO mice in response to zymosan A injection. A, Resident peritoneal cells (2 × 106/ml) of WT, MCP-1Δ/Δ, or MCP-1 KO mice were incubated in the presence of 20 μg/ml zymosan A for 24 h, and the concentration of MCP-1 or MCP-3 in the culture supernatants was quantified by ELISA. Data are presented as mean ± SD obtained with cells from three to four mice. ∗, p < 0.05; ∗∗, p < 0.01. B, A total of 200 μg of zymosan A (in 0.5 ml) was injected into the peritoneal cavity of mice. Peritoneal fluids were obtained 4 h after injection, and the concentration of MCP-1 and MCP-3 was measured by ELISA specific for MCP-1 and MCP-3, respectively. Data are presented as mean ± SD obtained with cells from three to seven mice. Although MCP-3 concentrations in peritoneal fluids of MCP-1Δ/Δ mice were slightly higher than those in peritoneal fluids of WT mice, there was no significant difference in MCP-3 concentration. C, Accumulation of macrophages 24 and 48 h after zymosan A injection. The number of macrophages in each peritoneal cavity was calculated by multiplying total cell number by the percentage of macrophages. ∗, p < 0.05; ∗∗, p < 0.01.
To examine in vivo production of MCP-1 and MCP-3, we injected 200 μg of zymosan A into the peritoneal cavity of WT, MCP-1Δ/Δ, and MCP-1 KO mice. A previous study indicated that MCP-1 concentration peaked at 2–4 h after i.p. injection with zymosan A (17). Therefore, we measured the concentration of MCP-1 and MCP-3 at 4 h after injection. As shown in Fig. 9B, ~1200 pg/ml MCP-1 was detected in the peritoneal fluids of WT mice injected with zymosan A. MCP-1 was not detectable in both MCP-1Δ/Δ and MCP-1 KO mice. MCP-3 concentration in the peritoneal fluids of WT mice was ~700 pg/ml. MCP-3 concentration in the peritoneal fluids of MCP-1Δ/Δ mice was slightly higher than that of WT mice, whereas it was lower in the peritoneal fluids of MCP-1 KO mice. Finally, the numbers of macrophages infiltrating the peritoneal cavities of zymosan A-injected mice were quantified (Fig. 9C). There was a significant decrease in the number of macrophages in MCP-1Δ/Δ and MCP-1 KO mice at both 24 and 48 h after zymosan A injection.
Discussion
In the present study, we generated a new model of MCP-1-deficient mice by deleting a 2.3-kb genomic DNA sequence from the MCP-1 gene, using the Cre-loxP system. Unexpectedly, this deletion resulted in increased production of the immediate downstream gene products, such as MCP-3 and MCP-5. The increased MCP-3 production appeared to be regulated at a transcriptional level because the expression of MCP-3 mRNA was markedly increased in LPS-activated MCP-1Δ/Δ macrophages, compared with MCP-1+/+ macrophages. In contrast to MCP-1Δ/Δ, the production of MCP-3 was markedly reduced in previously generated MCP-1 KO mice. Because MCP-1Δ/Δ and MCP-1 KO mice are both deficient in MCP-1, these two mouse models provided us with a unique opportunity to examine the role of MCP-3 in the local recruitment of monocytes. There was no significant difference in monocyte recruitment between MCP-1Δ/Δ and MCP-1 KO mice in response to i.p. TG or zymosan A injection, indicating that up-regulated MCP-3 production in MCP-1Δ/Δ mice did not compensate for the loss of MCP-1 and that MCP-3 does not appear to play a major role in monocyte recruitment in our acute peritonitis models.
The transcriptional mechanisms of the mouse MCP-1 gene, as well as the human gene, have been studied in detail. In both species, two well-conserved NF-κB binding sites are present upstream of the MCP-1 gene, and they, in cooperation with a GC box that is located proximal to the transcription start site, play a critical role in the transcription of this gene in response to proinflammatory stimuli, including LPS and TNF-α (18–20). In contrast, the mechanism of the MCP-3 gene transcription is not well understood. Murakami et al. (21) previously attempted to identify elements regulating the transcription of the human MCP-3 gene in response to IL-1 or PMA. They found a positive regulatory element located between −172 and −110 of the promoter region; however, there was no known PMA-responsive element in the region, including NF-κB binding sites. Notably, our results using the NF-κB inhibitor CAPE indicated that LPS-induced MCP-3 production in MCP-1+/+ macrophages was dependent on NF-κB. Up-regulated MCP-3 production in MCP-1Δ/Δ macrophages was also dependent on NF-κB. In our MCP-1Δ/Δ mice, two NF-κB binding sites in the MCP-1 gene promoter are still present. Thus, it is tempting to speculate that the two NF-κB binding sites of the MCP-1 gene promoter cooperated with the promoter of the MCP-3 gene and further increased the expression and production of MCP-3.
In contrast to the deletion in the MCP-1 gene locus, insertion of a 2-kb neo-gene cassette in the MCP-1 gene locus resulted in reduced production of MCP-1 and MCP-3. Reduced MCP-3 production was also detected in MCP-1 KO mice in which the MCP-1 gene was disrupted by inserting a neo-gene cassette in exon 2, very close to the position where a neo cassette was inserted in our MCP-1neo/neo mice (5). We demonstrated that peritoneal resident macrophages from MCP-1 KO mice expressed a lower level of MCP-3 mRNA and produced a lower level of MCP-3 in response to LPS than those from WT mice. The production of MCP-3 by LPS-activated MCP-1 KO macrophages was previously demonstrated by immunoprecipitating metabolically labeled MCP-3 in the culture supernatants of LPS-activated peritoneal macrophages from MCP-1 KO mice (5). However, because ELISA was not available at the time, quantitative analysis of MCP-3 in MCP-1 KO mice was difficult. The reduced production of MCP-3 in MCP-1neo/neo and MCP-1 KO mice could be due to the activity of neo gene as previously implicated in lymphotoxin α KO mice (22) and recently in MCP-2 and −5 double-KO mice (7). The reduced MCP-3 production in MCP-1 KO mice may have contributed to the phenotypes observed in various disease models.
To examine in vivo production of MCP-1 and MCP-3 and their functional role, we used a TG-induced acute peritonitis model. The kinetics of MCP-1 production was previously characterized (14), and this model was previously used to evaluate the role for MCP-1 and its receptor CCR2 in monocyte recruitment (5, 10, 23, 24). Consistent with our in vitro results, the levels of MCP-3 and MCP-5 4 h after TG injection were increased in the peritoneal fluids of MCP-1Δ/Δ mice, whereas the levels of MCP-3 and MCP-5 were reduced in the peritoneal fluids of MCP-1 KO mice. Previous studies using MCP-1 KO or CCR2 KO mice demonstrated a critical, nonredundant role for MCP-1 in the recruitment of monocytes in TG-induced peritonitis (5). However, the role for other MCPs, such as MCP-3 and MCP-5, remains unclear. Despite the fact that the concentration of MCP-3 in the peritoneal fluids of MCP-1Δ/Δ mice at 4 h was 3- to 4-fold higher than that of MCP-1+/+ mice, and more than 10-fold higher than that of MCP-1 KO mice after TG injection, the number of infiltrating macrophages in the peritoneal fluids of MCP-1Δ/Δ mice at 24 and 96 h was comparable to that of MCP-1 KO mice and much lower than that of MCP-1+/+ mice. Similar observations were made in response to zymosan A injection. Monocyte chemotactic activity of human MCP-3 was previously examined. Although human MCP-3 attracted human monocytes in vitro, the potency of MCP-3 was much lower than that of MCP-1 and bound to CCR2 with ~35-fold lower affinity than MCP-1 (25). These data suggest that the increased MCP-3 production in MCP-1Δ/Δ mice was not sufficient to compensate for the loss of MCP-1 and that MCP-1 is the primary chemokine required for monocyte recruitment in this model.
Although we did not find a primary role for MCP-3 in monocyte recruitment in TG- and zymosan A-induced peritonitis, a recent study of MCP-3 KO mice provided evidence that MCP-3 plays a distinct role in monocyte mobilization from bone marrow and recruitment to inflammatory sites (7). MCP-1 KO mice and MCP-3 KO mice had a significant reduction in a monocyte population in peripheral blood characterized by bright staining with an Ab specific to the surface marker 7/4. In response to i.p. TG injection, a normal level of 7/4 bright monocytes appeared in peripheral blood, but the recruitment of these cells into the peritoneal cavity was dramatically reduced. More recently, it was found that both MCP-1 and MCP-3 provided parallel contributions to CCR2-mediated inflammatory, Ly6-high monocyte recruitment, and that both chemokines were required for optimal innate immune response against Listeria monocytogenes infection (26). Although we did not evaluate the number of specific monocyte population in blood by flow cytometry, there was no reduction in the number of circulating monocytes in our MCP-1Δ/Δ mice determined by hematological examination (data not shown). More detailed studies using our MCP-1Δ/Δ mice will help us define the role for MCP-3 in monocyte recruitment and immune responses.
In addition to CCR2 expressed on monocytes, MCP-3 has been shown to bind other chemokine receptors, including CCR1, CCR3, and CCR5, and to play a role in the recruitment of other cell types (27). For example, MCP-3 is reported to play a significant role in the allergen-induced eosinophilic inflammation of the airways (28) and in type 2 hypersensitivity pulmonary granulomas (29). In the present study, we did not observe increased eosinophil accumulation in MCP-1Δ/Δ mice in an acute inflammation model. It will be interesting to examine using our MCP-1Δ/Δ mice whether the infiltration of eosinophils could be increased in response to allergen or Ag challenge.
Because a number of studies have indicated the critical role for MCP-1 in the development of many inflammatory diseases and cancer, MCP-1 has become a target for the treatment of patients suffering from chronic inflammatory diseases and cancer. However, it is not clear whether inhibiting only MCP-1 is effective to prevent monocyte infiltration due to the presence of other MCPs. Our results presented in this study indicated that MCP-1 is the primary chemokine regulating the recruitment of monocytes in TG- and zymosan A-induced peritonitis. MCP-3 was produced at a level similar to MCP-1 in MCP-1+/+ mice, but the role for MCP-3 appeared to be minimal. The production of MCP-5 was much lower than that of MCP-1 in MCP-1+/+ mice, suggesting its minimal role. MCP-2 does not appear to be a functional ligand for CCR2 (7). Thus, blocking MCP-1 may be as effective as blocking CCR2. However, the infiltration of monocytes was still detected in all MCP-1Δ/Δ, MCP-1 KO, and CCR2 KO mice. This indicated that the interaction of MCPs with CCR2 is not the only mechanism regulating the recruitment of monocytes during TG- and zymosan A-induced peritonitis and there must be additional mechanisms for the process. Defining the exact role for each chemoattractant in monocyte recruitment is critical to design a strategy for a successful intervention of disease development.
Acknowledgments
We are grateful to Dr. Joost J. Oppenheim for reviewing this manuscript.
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research
Footnotes
Disclosures
The authors have no financial conflict of interest.
Abbreviations used in this paper: KO, knockout; CAPE, caffeic acid phenethyl ester; ES, embryonic stem; PEC, peritoneal exudate cell; TG, thioglycolate; WT, wild type.
References
- 1.Van Coillie E, Van Damme J, and Opdenakker G. 1999. The MCP/eotaxin subfamily of CC chemokines. Cytokine Growth Factor Rev. 10: 61–86. [DOI] [PubMed] [Google Scholar]
- 2.Wagner K, Dendorfer U, Chilla S, Schlöndorff D, and Luckow B. 2001. Identification of new regulatory sequences far upstream of the mouse monocyte chemoattractant protein-1 gene. Genomics 78: 113–123. [DOI] [PubMed] [Google Scholar]
- 3.Yoshimura T, Yuhki N, Moore SK, Appella E, Lerman MI, and Leonard EJ. 1989. Human monocyte chemoattractant protein-1 (MCP-1): full length cDNA cloning, expression in mitogen-stimulated blood mononuclear leukocytes, and sequence similarity to mouse competence gene JE. FEBS Lett. 244: 487–493. [DOI] [PubMed] [Google Scholar]
- 4.Matsushima K, Larsen CG, DuBois GC, and Oppenheim JJ. 1989. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J. Exp. Med 169: 1485–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu B, Rutledge BJ, Gu L, Fiorillo J, Lukacs NW, Kunkel SL, North R, Gerard C, and Rollins BJ. 1998. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J. Exp. Med 187: 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerard C, and Rollins BJ. 2001. Chemokines and disease. Nat. Immunol 2: 108–115. [DOI] [PubMed] [Google Scholar]
- 7.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, and Charo IF. 2007. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J. Clin. Invest 117: 902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshimura T, and Ueda A. 1996. Monocyte chemoattractant protein-1 In Human Cytokines: Handbook for Basic and Clinical Research, II. Aggarwal BB and Gutterman JU, eds. Blackwell Science, Cambridge, pp. 198–221. [Google Scholar]
- 9.Natarajan K, Singh S, Burke TR Jr., Grunberger D, and Aggarwal BB. 1996. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-κB. Proc. Natl. Acad. Sci. USA 93: 9090–9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuziel WA, Morgan SJ, Dawson TC, Griffin S, Smithies O, Ley K, and Maeda N. 1997. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc. Natl. Acad. Sci. USA 94: 12053–12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holzenberger M, Lenzner C, Leneuve P, Zaoui R, Hamard G, Vaulont S, and Le Bouc Y. 2000. Cre-mediated germline mosaicism: a method allowing rapid generation of several alleles of a targeted gene. Nucleic Acids Res. 28: e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshimura T 1993. cDNA cloning of guinea pig monocyte chemoattractant protein-1 and expression of the recombinant protein. J. Immunol 150: 5025–5032. [PubMed] [Google Scholar]
- 13.Wang M-H, Cox GW, Yoshimura T, Sheffler LA, Skeel A, and Leonard EJ. 1994. Macrophage-stimulating protein inhibits induction of nitric oxide production by endotoxin- or cytokine-stimulated mouse macrophages. J. Biol. Chem 269: 14027–14031. [PubMed] [Google Scholar]
- 14.Matsukawa A, Kudo S, Maeda T, Numata K, Watanabe H, Takeda K, Akira S, and Ito T. 2005. Stat3 in resident macrophages as a repressor protein of inflammatory response. J. Immunol 175: 3354–3359. [DOI] [PubMed] [Google Scholar]
- 15.Tylaska LA, Boring L, Weng W, Aiello R, Charo IF, Rollins BJ, and Gladue RP. 2002. Ccr2 regulates the level of MCP-1/CCL2 in vitro and at inflammatory sites and controls T cell activation in response to alloantigen. Cytokine 18: 184–190. [DOI] [PubMed] [Google Scholar]
- 16.Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, and Aderem A. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 410: 811–815. [DOI] [PubMed] [Google Scholar]
- 17.Ajuebor MN, Flower RJ, Hannon R, Christie M, Bowers K, Verity A, and Perretti M. 1998. Endogenous monocyte chemoattractant protein-1 recruits monocytes in the zymosan peritonitis model. J. Leukocyte Biol 63: 108–116. [DOI] [PubMed] [Google Scholar]
- 18.Ueda A, Okuda K, Ohno S, Shirai A, Igarashi T, Matsunaga K, Fukushima J, Kawamoto S, Ishigatsubo Y, and Okubo T. 1994. NF-κB and Sp1 regulate transcription of human monocyte chemoattractant protein-1 gene. J. Immunol 153: 2052–2063. [PubMed] [Google Scholar]
- 19.Ueda A, and Yoshimura T. 1996. Characterization of cis-acting elements of the human macrophage stimulating protein gene: the involvement of positive and negative regulatory elements. J. Biol. Chem 271: 20265–20272. [DOI] [PubMed] [Google Scholar]
- 20.Ping D, Boekhoudt GH, Rogers EM, and Boss JM. 1999. Nuclear factor-κB p65 mediates the assembly and activation of the TNF-responsive element of the murine monocyte chemoattractant-1 gene. J. Immunol 162: 727–734. [PubMed] [Google Scholar]
- 21.Murakami K, Nomiyama H, Miura R, Follens A, Fiten P, Van Coillie E, Van Damme J, and Opdenakker G. 1997. Structural and functional analysis of the promoter region of the human MCP-3 gene: transactivation of expression by novel recognition sequences adjacent to the transcription initiation site. DNA Cell Biol. 16: 173–183. [DOI] [PubMed] [Google Scholar]
- 22.Liepinsh DJ, Grivennikov SI, Klarmann KD, Lagarkova MA, Drutskaya MS, Lockett SJ, Tessarollo L, McAuliffe M, Keller JR, Kuprash DV, and Nedospasov SA. 2006. Novel lymphotoxin (LTα) knockout mice with unperturbed tumor necrosis factor expression: reassessing LTα biological functions. Mol. Cell. Biol 26: 4214–4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boring L, Gosling J, Cleary M, and Charo IF. 1998. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 394: 894–897. [DOI] [PubMed] [Google Scholar]
- 24.Kurihara T, Warr G, Loy J, and Bravo R. 1997. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J. Exp. Med 186: 1757–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franci C, Wong LM, Van Damme J, Proost P, and Charo IF. 1995. Monocyte chemoattractant protein-3, but not monocyte chemoattractant protein-2, is a functional ligand of the human monocyte chemoattractant receptor. J. Immunol 154: 6511–6517. [PubMed] [Google Scholar]
- 26.Jia T, Serbina NV, Brandl K, Zhong MX, Leiner IM, Charo IF, and Pamer EG. 2008. Additive roles for MCP-1 and MCP-3 in CCR2-mediated recruitment of inflammatory monocytes during Listeria monocytogenes infection. J. Immunol 180: 6846–6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menten P, Wuyts A, and Van Damme J. 2001. Monocyte chemotactic protein-3. Eur. Cytokine Network 12: 554–560. [PubMed] [Google Scholar]
- 28.Stafford S, Li H, Forsythe PA, Ryan M, Bravo R, and Alam R. 1997. Monocyte chemotactic protein-3 (MCP-3)/fibroblast-induced cytokine (FIC) in eosinophilic inflammation of the airways and the inhibitory effects of an anti-MCP-3/FIC antibody. J. Immunol 158: 4953–4960. [PubMed] [Google Scholar]
- 29.Shang XZ, Chiu BC, Stolberg V, Lukacs NW, Kunkel SL, Murphy HS, and Chensue SW. 2002. Eosinophil recruitment in type-2 hypersensitivity pulmonary granulomas: source and contribution of monocyte chemotactic protein-3 (CCL7). Am. J. Pathol 161: 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]