Abstract
Background:
The majority of current pharmacological treatments for Parkinson’s disease (PD) were approved for clinical use in the second half of the last century and they only provide symptomatic relief. Derivatives of these therapies continue to be explored in clinical trials, together with potentially disease modifying therapies that can slow, stop or reverse the condition.
Objective:
To provide an overview of the pharmacological therapies— both symptomatic and disease modifying— currently being clinically evaluated for PD, with the goal of creating greater awareness and opportunities for collaboration amongst commercial and academic researchers as well as between the research and patient communities.
Methods:
We conducted a review of clinical trials of drug therapies for PD using trial data obtained from the ClinicalTrials.gov database and performed a breakdown analysis of studies that were active as of January 21, 2020.
Results:
We identified 145 registered and ongoing clinical trials for therapeutics targeting PD, of which 51 were Phase 1 (35% of the total number of trials), 66 were Phase 2 (46% ), and 28 were Phase 3 (19% ). There were 57 trials (39% ) focused on long-term disease modifying therapies, with the remaining 88 trials (61% ) focused on therapies for symptomatic relief. A total of 50 (34% ) trials were testing repurposed therapies.
Conclusion:
There is a broad pipeline of both symptomatic and disease modifying therapies currently being tested in clinical trials for PD.
Keywords: Clinical trials, studies, Parkinson’s, disease modification, neuroprotection, immunotherapy, inflammation, gene therapy
INTRODUCTION
Parkinson’s disease (PD) is a neurodegenerative condition for which there are currently no curative therapies, and the available symptomatic treatments have increasingly limited impact as the condition progresses [1, 2]. In addition, the incidence of PD is increasing, with the number of cases expected to double worldwide by 2040 [3– 5]. Given these trends, novel disease modifying therapies (DMTs) are urgently needed to enhance future quality of life for the current PD community and reduce the potential burden both on society and on financially challenged healthcare systems worldwide.
The development of new treatments has been slow since the U.S. FDA approval in 1970 of levodopa, the primary symptomatic therapy (ST) [6– 8]. Amantadine, monoamine oxidase type B (MAO-B) inhibitors, apomorphine and dopamine agonists were all discovered and tested before the 1970s [9– 11]. Derivatives and improved reformulations have subsequently been developed in the intervening years, and non-pharmacological treatments, such as deep brain stimulation, pallidotomy/subthalamotomy and high-intensity focused ultrasound, have also been approved [12, 13]. None of these ST approaches, however, target the underlying pathological biology of the condition and they do little to halt the progression of the disease.
With the discovery of the first genetic risk factors for PD at the turn of this century [14], researchers have begun to develop a better understanding of the possible biological pathways that may be governing/influencing the progressive neurodegeneration associated with PD [15]. These discoveries have led to a growing number of clinical trials targeting an increasing number of potentially disease-relevant mechanisms of action (MOA) [16].
In addition to improved understanding of the possible biology of the condition, unprecedented government/industry/foundation research consortia have been organized with the goals of identifying and validating novel treatments, new biomarkers, more reliable measuring tools, and improvements to the clinical trial process. These consortia include ‘Accelerating Medicines Partnership: Parkinson’s disease’ [17], ‘Aligning Science Across Parkinson’s’ [18], ‘Parkinson’s Progression Markers Initiative’ [19], and Critical Path for Parkinson’s Consortium [20].
Recent efforts have provided better guidance on clinical trials focused on specific PD targets [21], improved outcome measures [22], accelerated repurposing of clinically available drugs [23], and more refined protocols for cell replacement therapy [24]. As a result, the PD research community is better positioned for clinical trials evaluating new STs and the complexities of potential DMTs. A list of various clinical trial related tools, projects and organizations is provided on the Journal of Parkinson’s Disease website resource page [25].
As the number of clinical trials for PD grows, it is useful and important for the research and Parkinson’s communities to stay abreast of the broad, ever-changing landscape in order to highlight trends and better manage expectations. Annual reviews of clinical drug development have been provided for Alzheimer’s disease [26], and with this current report we are seeking to provide opportunities for collaboration by raising awareness of the clinical trial landscape in PD, both with researchers and the wider PD community. We are also offering data that may prompt discussion about trial design, and we are providing people with Parkinson’s (PwP) with information that may increase their likelihood to participate in clinical trials and enhance their ability to act as advocates for PwP.
Here, we will provide an overview and analysis of the current PD drug development pipeline, based on ongoing clinical trials registered on the ClinicalTrials.gov database as of January 21, 2020, in the hope of gaining some insights into how the management of this condition is evolving and what novel treatment options may lie on the horizon. We would also like to note that this review incorporates the perspective of PwP, as two of the authors are PwP and one is a PwP care partner.
METHODS
Data collection
Data on active clinical trials of PD drug therapies were obtained from ClinicalTrials.gov for analysis in this review. ClinicalTrials.gov provides information on publicly and privately supported clinical studies conducted worldwide and is the world’s most comprehensive clinical trial registry. To date, it includes over 2500 PD trials that have been registered since its launch in 2000. Data on the registry is maintained directly by trial sponsors and includes information about a trial’s design, outcome measures, enrollment targets, recruiting status, locations, expected start and end dates, and more.
To generate our dataset for analysis of active PD drug trials, data was downloaded from ClinicalTrials.gov, as of January 21, 2020, based on the following search criteria:
•
-
•
Condition: Parkinson disease
-
•
Study type: Interventional
-
•
Phase: Early Phase 1, Phase 1, Phase 2, Phase 3
-
•
Status parameter: “Recruiting”, “Not yet recruiting”, “Active, not recruiting”, or “Enrolling by invitation”.
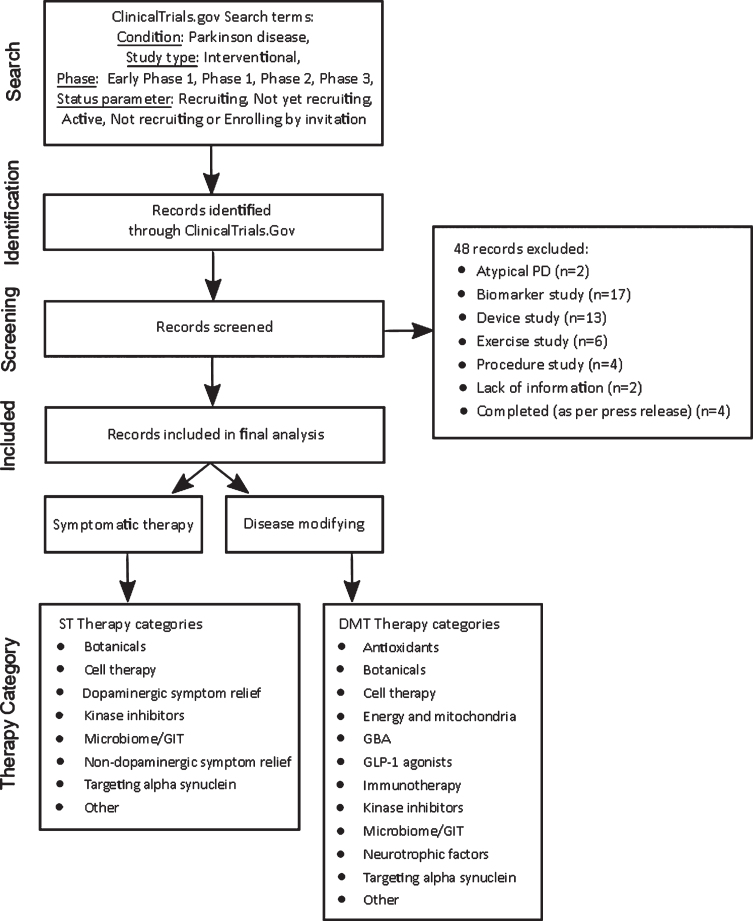
The downloaded dataset included 193 trials. The therapy for each interventional trial registered on ClinicalTrials.gov is classified by the trial sponsor as “Drug”, “Behavioral”, “Biological”, “Combination Product”, “Device”, “Diagnostic Test”, “Dietary Supplement”, “Genetic”, “Other”, “Procedure”, or “Radiation” study. Since our pipeline review is focused on PD drug trials, we filtered out trials that were evaluating devices, biomarkers, or behavioral interventions (for example, exercise), as well as drug trials i) for which no supporting information could be found online, ii) that were already completed, though still listed as active, or iii) applied to atypical Parkinson’s only. The filter excluded 48 trials. The remaining 145 trials were included in our dataset for analysis of active Phase 1, 2, and 3 PD drug trials. Trials whose phase was classified “as early Phase 1” on ClinicalTrials.gov were grouped with Phase 1 trials in this report. If a trial was classified as Phase 1/Phase 2 or Phase 2/3 on ClinicalTrials.gov, we included the study in the lower of the two phases in our analyses (for example, a Phase 1/2 was considered Phase 1).
Trial categorization
Each trial in our dataset was first determined to be ST or DMT, and then assigned to one of 14 categories according to the mechanism being tested.
ii)
-
i)
Each trial was classified as ST or DMT based on a review of the trial investigator’s stated hypothesis on ClinicalTrials.gov, as well as an online literature search to help determine the MOA when it was not otherwise clear. Our working definition of a DMT required a MOA, whether proven or proposed, that would interfere with the pathology of PD and/or outcome measures investigating disease-modifying properties. Trial coordinators were contacted when further information was needed to make an assessment. For each trial classified as ST, the symptom(s) for which the trial was evaluating treatment were identified based on information on ClinicalTrials.gov, including “Study Title”, “Study Description”, and “Outcome Measures”, as well as information on the sponsor’s website.
-
ii)
The categories to which each trial was allocated were developed using an iterative process in which the MOA, target, and source of the drugs in our trial dataset were all considered, and we accepted an element of overlap where a trial could have been allocated to more than one category. For example, immunotherapy trials could have been allocated to “targeting alpha-synuclein”, but we felt that immunotherapy deserved its own category given the focus and investment it has received, not just in PD. This process yielded 14 categories, ten of which were primarily based on MOA, three on drug target, and one on drug source (botanicals). “Botanicals” were a separate category as they are mixtures with a number of potential active agents and have attracted a lot of attention in the Parkinson’s community as a source of current and future medicines. Each of the 145 trials was then assigned to one of the 14 categories based on the characteristics of the trial’s agent.
The categories, together with the type of category, were:
•
-
•
‘Dopaminergic symptom relief therapies’ (MOA) applied to ST agents that either restore, replace or mimic the neurotransmitter dopamine.
-
•
‘Non-dopaminergic symptom relief therapies’ (MOA) applied to ST agents that impacted any neurotransmitters, other than dopamine, which include noradrenergic, serotonergic, glutamatergic, and cholinergic systems.
-
•
‘Antioxidants’ (MOA) were agents that are primarily focused on reducing oxidative stress.
-
•
‘Botanicals’ (source) was applied to agents derived from herbal extracts where the mechanism of action was unknown or unclear.
-
•
‘Cell therapy’ (MOA) were trials including either intracerebral cell transplantation or peripheral delivery of cells.
-
•
‘Energy and mitochondria’ (target) was a category for agents seeking to stimulate improvements in mitochondrial function.
-
•
‘GBA’ (MOA) agents were focused on enhancing the activity of glucocerebrosidase (GCase).
-
•
‘GLP-1 agonists’ (MOA) are a specific class of drugs activating the glucagon-like peptide-1 receptor.
-
•
‘Immunotherapy’ (MOA) was designed to cover antibody-based agents.
-
•
‘Kinase inhibitors’ (MOA) represented a broad category encompassing agents blocking specific kinase activity.
-
•
‘Microbiome/GIT’ (target) were agents specifically targeting the activity of the gastrointestinal system.
-
•
‘Neurotrophic factors’ (MOA) was assigned to therapies involving the delivery of GDNF or CDNF.
-
•
‘Targeting alpha synuclein’ (target) covered small molecules specifically focused on alpha synuclein inhibition or disaggregation.
-
•
‘Other’ (MOA) was assigned to trials whose therapy had a MOA that did not match another therapy. In situations where the MOA was unclear, a designation of ‘Other’ was also given.
A flow chart depicting the data collection and trial categorization methodology is provided in Fig. 1.
Fig.1.
Data collection and trial categorization methodology.
RESULTS
Overview
Our dataset for analysis included 145 active Phase 1– 3 clinical trials of PD drug therapeutics. Of these trials, 37 (26% ) were Phase 1, 14 (10% ) were Phase 1/Phase 2, 61 (42% ) were Phase 2, 5 (3% ) were Phase 2/Phase 3, and 28 (19% ) were Phase 3 (Table 1; see Supplemental File 1 for the complete list of trials and extracted data and Supplemental File 2 for a summary view). As mentioned previously, for our analyses, the Phase 1/Phase 2 and Phase 2/Phase 3 studies were incorporated into the Phase 1 and Phase 2 grouping, respectively.
Table 1.
Active PD drug trials by phase, as of January 21, 2020, ClinicalTrials.gov
| Phase | # Trials | % Trials |
| Phase 1 | 37 | 26% |
| Phase 1/2 | 14 | 10% |
| Phase 2 | 61 | 42% |
| Phase 2/3 | 5 | 3% |
| Phase 3 | 28 | 19% |
| Total | 145 | 100% |
Of the 145 trials, 57 (39% ) were considered to be DMT trials and 88 (61% ) were considered to be ST trials, as shown in Fig. 2. Of the DMT trials, 30 (53% ) were in Phase 2, while only 3 (5% ) of the DMT trials were in Phase 3. 36 (41% ) of the ST trials were in Phase 2, with the remainder split closely between Phase 1 and Phase 3.
Fig.2.
Number of trials of disease-modifying and symptomatic therapies by phase
About 2/3 of the ST studies (58/88) were focused on motor symptoms, including overall movement (45 trials), levodopa-induced dyskinesia (LID; 6 trials), gait and balance (4 trials) and tremor (3 trials). One of the ST studies was focused on both motor and non-motor symptoms. The remaining approximately 1/3 of the ST trials targeted a range of non-motor symptoms, including neurogenic orthostatic hypertension (nOH; 5 trials), general non-motor symptoms (4 trials), impulse control disorders (ICD; 3 trials), pain (3 trials), PD dementia (2 trials), PD psychosis (2 trials) and 1 trial each for anxiety, constipation, depression, fatigue, hallucinations, neuropsychiatric symptoms, PD MCI, sialorrhea, sleepiness, and urinary symptoms.
The therapeutic for each of the 145 trials in the dataset was mapped to one of 14 categories, resulting in the breakout shown in Table 2. Of the agents under evaluation in our dataset of 145 trials, we found that 49 agents (21 DMT/ 28 ST) were being evaluated in Phase 1, 60 agents (28 DMT/32 ST) in Phase 2, and 22 agents (3 DMT/19 ST) in Phase 3 (Fig. 3).
Table 2.
Number of active PD drug trials by therapy category, as of January 21, 2020, ClinicalTrials.gov
| Number of trials | ||||
| Therapy Category | Phase 1 | Phase 2 | Phase 3 | Total |
| Antioxidants | 0 | 2 | 0 | 2 |
| Botanicals | 0 | 3 | 1 | 4 |
| Cell therapy | 8 | 1 | 0 | 9 |
| Dopaminergic symptom relief | 12 | 6 | 16 | 34 |
| Energy and mitochondria | 1 | 3 | 0 | 4 |
| GBA | 1 | 3 | 0 | 4 |
| GLP-1 agonists | 1 | 4 | 1 | 6 |
| Immunotherapy | 5 | 2 | 0 | 7 |
| Kinase inhibitors | 3 | 1 | 0 | 4 |
| Microbiome/GIT | 3 | 3 | 0 | 6 |
| Neurotrophic factors | 3 | 0 | 0 | 3 |
| Non-dopaminergic symptom relief | 6 | 26 | 9 | 41 |
| Targeting alpha synuclein | 2 | 2 | 1 | 5 |
| Other | 6 | 10 | 0 | 16 |
| Total | 51 | 66 | 28 | 145 |
Fig.3.
Agents in active PD drug trials, as of January 21, 2020 on ClinicalTrials.gov (by phase, DMT/ST and therapy category).
Therapy category analysis
Phase 1
Of the registered and active trials we covered in this analysis, 35% (n = 51) were in Phase 1 clinical testing (Fig. 4). A large portion of the trials (42% ) in our analysis that were considered DMT were in Phase 1 testing (n = 24), while only 31% (n = 27) of the ST trials were in Phase 1. Gene therapy trials feature strongly in Phase 1. These include ST trials of VY-AADC01 (n = 2), OXB-102 (AXO-Lenti-PD), & ProSavin, as well as DMT studies of AAV2-GDNF (n = 2) & PR001A. The Phase 1 category contained most of the cell therapy (n = 8) and immunotherapy clinical trials (n = 5). The immunotherapy studies include ABBV-0805, BIIB054, Lu AF82422, MEDI1341, and UB-312. ‘Kinase inhibitors’ involved clinical trials exploring Leucine-rich repeat kinase 2 (LRRK2) inhibition (DNL151), c-Abl inhibition (FB-101), and Src/Bcr-Abl dual inhibition (saracatinib). The ‘Other’ category in Phase 1 included BIIB094 (LRRK2 anti-sense oligonucleotide), Cu(II)ATSM (metal chelation), plasma infusions (healthy young plasma administration), sargramostim (immunomodulation), and terazosin (PGK1 activation).
Fig.4.
Therapy Categories for Trials in Phase 1 (as of January 21, 2020, ClinicalTrials.gov).
Most of the dopaminergic ST trials involved reformulation and/or different delivery mechanisms. All but one of the non-dopaminergic projects were repurposed from other indications.
Phase 2
Phase 2 had the largest array of therapies in trials. In total, 60 different agents were being studied in 66 trials (Fig. 5). Of these, 30 (45% ) were considered DMT trials. These DMT trials included 4 studies targeting α-synuclein reduction, involving both immunotherapy (BIIB054 & prasinezumab) and small molecule inhibitors of α-synuclein aggregation (mannitol & ENT-01). There were four DMT trials testing glucagon-like peptide 1 (GLP-1) agonists, three of which are already approved for use in diabetes and being repurposed for PD (liraglutide, lixisenatide, and semaglutide), and one novel GLP-1 agonist, NLY01, a pegylated form of exenatide, which has been specifically designed for central nervous system (CNS) penetration. There were three trials evaluating drugs that focused on improving cellular energy and mitochondrial defects (EPI-589, UDCA & CNM_Au8 (gold nanocrystals)). Glucocerebrosidase, the product of the GBA gene, is the target of two potentially DMT drugs in Phase 2 testing, ambroxol (two separate trials) and venglustat. One drug, K0706, is currently in trial for c-Abl kinase inhibition. Of the 10 DMT trials classified as ‘Other’, the therapies and mechanisms include a statin (simvastatin), a sigma-1 receptor agonist (ANAVEX2-73), an antibiotic for PD dementia (ceftriaxone), a cholinesterase blocker (donepezil), a nootropic for PD with mild cognitive impairment (MCI); adrenergic blocker (2 trials involving carvedilol), fractions of human plasma (GRF6021), MAO-B inhibition (rasagiline), and metabolic cofactor supplementation. Finally, there is a DMT trial investigating the potential of autologous mesenchymal stem cell therapy as a therapeutic treatment.
Fig.5.
Therapy Categories for Trials in Phase 2 (as of January 21, 2020, ClinicalTrials.gov).
36 (59.0% ) of the Phase 2 trials were for drugs that were considered ST. Of these, six drugs were targeting dopamine levels (either directly or as a dopamine agonist), two of which were new formulations of levodopa/carbidopa (accordion pill and ND0612) and one was a gene therapy approach (VY-AADC02). Two new agents, KDT-3594 a dopamine agonist, and LY3151207, a D1 receptor modulator, are in trials for symptomatic treatment.
27 trials were for drugs which are targeting symptom relief though non-dopaminergic mechanisms. This classification involves new agents (including CVN424, CX8998, JM-010, KW-6356, NYX-458, pridopidine, foliglurax, SEP-363856 and THN102) which were being tested as potential therapies for a variety of PD-related symptoms. However, following our data download the results of the foliglurax study were announced, showing the study did not meet its target outcome measures.
The ST classification also included drugs already approved for other PD symptoms or other conditions, such as BoNT-A (botulinum toxin), bumetanide, clonidine, droxidopa, glycopyrrolate, ondansetron, and pimavanserin. There were also three trials testing the therapeutic benefits of cannabis or cannabis-based agents. In addition to dopaminergic and non-dopaminergic therapies, four ST trials were designated as ‘Targeting α-synuclein’ (ENT01), ‘Microbiome/GIT’ (gastrointestinal tract; resistant maltodextrin and Ecologic BARRIER849), and ‘Botanical’ (SQJZ herbal mixtures).
Phase 3
In total, 22 different agents were being studied in 28 Phase 3 trials (Fig. 6). Of these, 3 trials (11% ) were considered DMT trials. These DMT trials included evaluations of exenatide (a GLP-1R agonist), lingzhi (a fungal extract), and memantine (an NMDA receptor antagonist, which is being evaluated for its ability to inhibit α-synuclein cell-cell transmission).
Fig.6.
Therapy Categories for Trials in Phase 3 (as of January 21, 2020, ClinicalTrials.gov).
25 (89% ) of the Phase 3 trials were for drugs that were considered ST. Of these, 16 trials were dopaminergic symptom relief trials evaluating 11 different dopaminergic symptom relief agents, including 3 trials involving apomorphine and 2 trials involving tavapadon; plus safinamide, IPX203 (an extended-release capsule of carbidopa-levodopa), Accordion pill (levodopa/carbidopa), and APL-130277 (a sublingual film of apomorphine). The Accordion Pill has been included in our analysis despite news that the study did not reach its target outcome measures, announced in 2020. The trial is still listed as active and the company is undertaking further analysis of the results [27]. The remaining nine trials were non-dopaminergic symptom relief trials evaluating six non-dopaminergic symptom relief agents, including three trials of the oral norepinephrine reuptake inhibitor, ampreloxetine.
Sponsor/collaborator information
ClinicalTrials.gov defines a trial sponsor as “the single person or entity who initiates the study, by preparing and/or planning the study, and who has authority and control over the study” and defines collaborators as “Other organizations (if any) providing support. Support may include funding, design, implementation, data analysis or reporting.” The sponsor is responsible for registering a trial on ClinicalTrials.gov and for maintaining the accuracy and timeliness of the data provided. The sponsor is not necessarily the funder of the trial. Table 3 shows the breakdown of sponsors/collaborators across the 145 active trials in our analysis (Supplemental File 1 provides the full list of these). In the analysis, a sponsor/collaborator that was a pharmaceutical or biotechnology company was classified as “Industry”, a university or hospital as “Academic Medical Center”, a U.S. or non-U.S. Government entity as “Government, and a non-profit such as a charity, foundation or consortium as “Foundation/Consortium”. Of note, industry are the primary sponsors/collaborators in PD clinical development, with involvement in over 60% of the trials in our dataset.
Table 3.
Sponsors/Collaborators for Active Phase 1–3 PD Drug Trials (n
| Sponsor Type | # Trials as sponsor or collaborator | % Trials as sponsor or collaborator |
| Industry | 92 | 63% |
| Academic Medical Center | 66 | 46% |
| Foundation/Consortium | 12 | 8% |
| Government | 11 | 8% |
Enrollment analysis
The total target enrollment for the Phase 1– 3 trials was 16,023 (excluding the 100,000 target enrollment for a Phase 2 cannabis trial) (Fig. 7). There were 51 trials in Phase 1, which had a total target enrollment of 1,613 (10% of the total for all phases). The average number of participants being recruited in Phase 1 was 32 per trial. At the Phase 2 level, there were 65 trials that had total enrollment targets of 7,447 (46.5% of the total for all phases). In Phase 2, an average of 113 participants were being recruited per trial. Finally, there were 28 Phase 3 trials that had enrollment targets of 6,963, which averaged 249 participants per trial. Of interest, only 14 out of 145 trials involved in our analysis were recruiting healthy volunteers, of which 11 were Phase 1.
Fig.7.
Target enrollment by Phase for Active Phase 1– 3 PD Drug Trials (as of January 21, 2020, ClinicalTrials.gov).
Trial site analysis
The trials included in this review were being conducted at research institutes around the world, as shown on the map in Fig. 8. The majority of these studies were in the USA and Europe, and many were conducted at sites across different geographic regions. The total number of trial sites for each region is presented on the map. Fourteen of the 145 trials in our dataset did not have trial sites listed, so our analysis and the map in Fig. 8 reflects data available for the remaining 131 studies.
Fig.8.
Geographical location of active PD drug trials sites (as of January 21, 2020, ClinicalTrials.gov). Map source: ClinicalTrials.gov (enlarged font size).
60% of Phase 1 and Phase 1/2 trials (28/47) had one trial site and 30% (14/47) had 2– 5 sites (Fig. 9). No trials in Phase 1 had more than 10 sites. In contrast, 53% of the Phase 2 and Phase 2/3 trials (31/59) had one trial site, and 37% had >10 sites. 48% of Phase 3 trials had between 1– 5 sites (12/25), while 52% (13/25) had >10 sites. Interestingly, none of the trials in Phase 2, Phase 2/3 or Phase 3 trials had 6– 10 sites, but 35 trials had >10 sites.
Fig.9.
Distribution of number of sites per trial across Phases 1– 3.
Novel and existing compounds
In our review, we explored whether trials were evaluating novel or existing compounds. Existing compounds were classified as either “repurposed”, “reformulation” or “new claim”. Repurposing was defined as the testing of a therapy already approved by regulatory authorities for a different indication (i.e., disease or condition) besides PD. “New claim” was applied to therapies already approved for treatment of a PD symptom(s) now being explored for a different PD symptom. A reformulation was defined as an agent already in use in one delivery mode, such as an immediate release tablet, that was being delivered in a different way, for example by sub-cutaneous infusion.
Of the 145 Phase 1– 3 trials in our analysis, 66 (46% ) were evaluating novel agents, 50 (34% ) were testing repurposed agents, 20 (14% ) were evaluating reformulations, and 6% (9/145) were testing “New Claims” (Table 4; see Supplemental Files 1 and 2).
Table 4.
Number of active Phase 1–3 PD drug trials with novel vs. existing agents (as of January 21, 2020, ClinicalTrials.gov)
| Number of trials | ||||
| Trial agent | Phase 1 | Phase 2 | Phase 3 | Total |
| Novel | 32 | 28 | 6 | 66 |
| Repurposed | 13 | 29 | 8 | 50 |
| Reformulation | 6 | 3 | 11 | 20 |
| New Claim | 0 | 6 | 3 | 9 |
| Total | 51 | 66 | 28 | 145 |
Notably, about one third of the trials were evaluating repurposed agents. An example of repurposing is the use of GLP-1 agonists, originally developed for use in type 2 diabetes, of which there were four molecules in five trials classified as repurposed in our analysis. Other indications from which therapies were being repurposed for PD were Alzheimer’s disease (five trials), antibiotics (four trials), hypertension (four trials), pain relief (four trials), and two trials each for nausea and vomiting (antiemetics), anxiety disorders, diuretics, gallstones/primary biliary cirrhosis, and respiratory diseases. PD trials were also evaluating compounds approved for the following 18 conditions: ADHD, adjunct to cancer treatment, anesthesia, bone transplantation stimulation, constipation, depression, epilepsy, hypercholesterolemia, excess iron reduction, MS spasticity, hepatic oxidation enhancement, neurogenic orthostatic hypotension, paracetamol/acetaminophen overdose, peptic ulcers, PET imaging, gut health, sleep and sleepiness.
New claims for existing PD drugs included three trials evaluating droxidopa (for fatigue, gait and balance, and nOH), two trials testing pimavanserin (ICD and neuropsychiatric symptoms), a Duodopa (ABT-SLV187) trial for non-motor symptoms, two trials evaluating safinamide (one for movement and one for LID), and a trial evaluating rasagiline as a DMT. Reformulated compounds in trial included apomorphine, Accordion Pill, CTC-413, Infudopa, IPX203, LD-CD intestinal gel, LY03003, ND0612, opicapone, P2B001, ropinirole, SER-214 (rotigotine polymer conjugate), and solifenacin.
DISCUSSION
Recently completed studies
This report of PD trials active as of January 21, 2020 has provided an overview of a broad spectrum of therapeutics being evaluated across all phases of clinical development. The breadth of this pipeline is both encouraging and inspiring. In addition to these ongoing efforts, other trials investigating therapies for PD have recently completed. These include high profile Phase 3 DMT trials, such as the STEADY-PD (evaluating isradipine) and SURE-PD (testing inosine) studies, which both announced that they had not reached their primary endpoints [28, 29], as well as Phase 1 and 2 studies that provided more encouraging results. The latter include the Herantis CDNF trial and the Denali-sponsored evaluation of their LRRK2 inhibitor, DNL-201, both of which focused on novel targets and achieved their primary endpoints. [30, 31]. We are also still awaiting the results of some studies that recently completed, such as the Phase 2 trial assessing the DMT potential of the iron chelator, deferiprone.
Recently approved therapeutics
Four therapeutics to treat PD “off” periods, when used as an adjunct to levodopa/carbidopa, recently received regulatory approval by the U.S. FDA. In December 2018, a new inhaled version of levodopa from Acorda Therapeutics, Inbrija, was approved for use in alleviating “off” “episodes [32]. In October 2019, Kyowa Kirin’s novel therapeutic, adenosine A2A receptor antagonist Nourianz/istradefylline, was approved in the U.S. for treating “off” episodes, as well. It was originally launched in Japan in 2013 [33]. In April 2020, the FDA approved the COMT inhibitor opicapone (Ogentys) from Neurocrine Biosciences, also for treatment for PD “off” episodes [34]. In May 2020, Kynmobi (APL-130277) was also approved for the treatment of PD “off” episodes[35].
Symptomatic therapies
Our analysis has highlighted some trends that will be interesting to revisit in future annual reviews. For example, the majority of ST trials aiming to deliver symptomatic relief via the dopaminergic pathway use well-established molecules (for example, levodopa/carbidopa or dopamine agonists), but there were some trials exploring novel approaches, including gene therapies aiming to deliver genes for biosynthetic enzymes to enable the striatum to synthesise dopamine, (such as Voyager Therapeutics’s VY-AADC01 and Axovant’s OXB-102 (AXO-Lenti-PD)). Cell therapy trials, which are also dopamine replacement ST efforts, were all at the early stages of the clinical development process, with eight studies in Phase 1 and one study in Phase 2. These are long term projects primarily assessing the safety of the transplanted tissue over time, but we did note additional developments suggesting interest from major pharmaceutical companies in this area (for example, the acquisition of stem cell-based biotech firm Bluerock Therapeutics by Bayer).
Our analysis also showed that only about a third of the ST trials were aimed at specific non-motor symptoms, prompting us to stress the importance of increasing research and development for the treatment of non-motor symptoms. Research has shown that non-motor symptoms are at least as prevalent and severe as motor symptoms, and also suggest that, in the opinion of PwP, these symptoms are being poorly treated and have a significant impact on their quality of life [36, 37]. This, of course, does not include the treatment of non-motor symptoms using medicines that address the symptom regardless of the presence of PD.
Disease-modifying therapies
As indicated in the introduction of this report, there have been significant developments in terms of our understanding of the biology potentially underlying PD, and this is now reflected in the range of DMT clinical trials. For example, recent research on the mechanisms of PD have identified mitochondria and lysosomes as organelles prone to malfunction [38, 39]. In our dataset, there were four studies focused on mitochondrial deficiencies, two of which involve ursodeoxycholic acid (UDCA). The others were BioElectron’s EPI-589 (also known as BioE-589) and CNM_Au8 (gold nanocrystals) from Clene Nanomedicine. Four studies were targeting the activity of the product of the GBA gene, glucocerebrosidase, closely linked to lysosomal function. Two studies were on repurposing the respiratory medication ambroxol; Sanofi has venglustat in Phase 2 which aims to reduce sphingolipid production; and finally, Prevail Therapeutics were investigating a gene therapy approach for PD patients who have mutations in their GBA gene.
Following the encouraging results of a Phase 2 clinical trial of exenatide in PD [40], GLP-1 agonists were well represented in our analysis. These molecules have been shown to have neuroprotective effects in models of PD, via multiple pathways [41]. The five GLP-1 agonist trials in our analysis consist of one in Phase 1, three in Phase 2 and one in Phase 3. In addition, as mentioned above, there is another Phase 2 study on exenatide in Sweden that was not registered on ClinicalTrials.gov but is listed on EudraCT (ID no 2019-000732-26). Peptron of South Korea registered a Phase 2 study on ClinicalTrials.gov for their version of sustained release exenatide after the cut-off date of our analysis. In all, we have identified eight trials of GLP-1 agonists – exenatide (n = 5), liraglutide, lixisenatide, semaglutide – currently in clinical evaluation for PD.
There is significant genetic, histological and pathological evidence pointing towards a role for α-synuclein in the biology of PD [42, 43], thus it is no surprise that there is intense clinical activity aimed at unravelling (or preventing) the formation of α-synuclein oligomers/fibrils and their intercellular spread. Five studies were using small molecules to target α-synuclein: anle138b, ENT-01 (2 trials), mannitol, and memantine. There were also seven trials of immunotherapy drugs against α-synuclein in our dataset, two of which were in Phase 2 (Biogen (BIIB054) and Roche (RO7046015/prasinezumab). This area is being watched closely by scientists and patients alike, as the potential for modifying the progression of PD through interrupting the spread of α-synuclein is strong. This is tempered somewhat by the recent experience of immunotherapy in Alzheimer’s disease, where this approach has had limited successto date.
Of the four kinase inhibitors in our analysis, three were targeting the c-Abl protein kinase [44]. This enzyme remains a target despite the recent disappointing outcome of two Phase 2 trials of nilotinib, although this may have been an issue of access through the blood-brain barrier rather than a failure of target engagement and MOA. FB-101, K0706 and saracatinib (dual Src & c-Abl inhibitor) were all targeting c-Abl in clinical trials of PD. The remaining kinase inhibitor study was a LRRK2 inhibitor (DNL151), the first in what is likely to be an active field in the future.
The influence of the gut microbiota in the context of PD has recently become an intense area of preclinical research [45] and this has given rise to a number of clinical trials targeting dysbiosis in the gastrointestinal system. There were five studies seeking to positively influence the content and activity of gut microbiota, all of which were under way in academic institutions. They included active intervention with two trials on rifaximin, one prebiotic (resistant maltodextrin), a lyophilised fecal extract (PRIM-DJ2727), and fecal microbialtransplantation.
In 2019, the results of the Bristol glial cell-derived neurotrophic factor (GDNF) clinical trial were published [46, 47] and while that study did not meet its primary endpoints, there is still significant interest in the potential use of neurotrophic factors for the treatment of PD. In our dataset, there were three studies evaluating neurotrophic factors. Two were gene therapies to introduce the gene for GDNF, and the other was assessing infusion of CDNF. The results of one of the GDNF gene therapy trials has been published [48], and the topline results of the CDNF study have been made available [49], both providing support for further evaluation of this experimental treatment class.
Biomarkers
In addition to a broad range of pharmacological agents being tested, it is also encouraging to see numerous biomarkers being used in the assessment aspects of many of these trials, although a detailed analysis of biomarker research was beyond the scope of this PD drug pipeline review. However, we believe that the importance of this research warrants a brief discussion. The lack of accurate and reliable biomarkers for PD causes great difficulty in early diagnosis, as well as in measuring disease progression. Better biomarkers may also allow for shorter and more efficient clinical trials.
In addition to the 145 trials in our analysis, there were more than 40 trials currently listed on ClinicalTrials.gov that involve research into specific biomarkers for PD. They were listed as either interventional studies or observational studies. Many of the 145 DMT and ST studies that we reviewed were measuring biomarkers for some of the outcome results, which were typically secondary. Most often, these involve testing for dopamine metabolites, amino acids and various forms of α-synuclein in blood and cerebrospinal fluid. There is also hope that microRNA analysis may bring progress to this area of research.
Definitions
An important consideration in our analysis was the absence of a universally accepted definition of whether a particular therapy for PD is “disease-modifying” or “symptomatic” [50, 51], which made it challenging to determine whether the therapies in some trials were DMT or ST. As discussed in the methods section, our working definition of a DMT required a mode of action, whether proven or proposed, that would interfere with the pathology of PD and/or outcome measures investigating disease-modifying properties. An example of the latter would be the post-treatment washout period prior to outcome measurement to assess residual disease-modifying effects in the Phase 2 lixisenatide study or the delayed start design of the prasinezumab Phase 2 trial. Despite this working definition, the allocation to DMT or ST was not always obvious. In those cases, we used a combination of judgment and consultation with experts in the relevant scientific field. There have been efforts to provide research ontology for neurodegenerative conditions (such as ‘Common Alzheimer’s Disease Research Ontology’ (or CADRO) [52] and ‘Parkinson’s disease ontology’ (PDON) [53]. The potential of similar standards may be relevant to future reviews of the PD drug development field.
Additional trials
Based on information in the literature and press releases, we identified additional active PD drug trials not listed on ClinicalTrials.gov that we would like to note, as they are part of the trial pipeline. The information provided on other registries was not a complete match to that on ClinicalTrials.gov, complicating like-for-like analyses. Thus, these trials were left out of our analyses. These trials include resTORbio’s Phase 1b study of their mTORC1 inhibitor RTB101, registered on the Australian New Zealand Clinical Trials Registry (ANZCTR) [54], Yumanity Therapeutics’ Phase 1 trial of Stearoyl-CoA-Desaturase inhibitor YTX-7739 [55], the Lysosomal Therapies trials of their GCase activator LTI-29, listed on the Netherlands Trial Registry [56, 57], the Neuropore and UCB Phase 1b trial of UCB0599, an alpha-synuclein misfolding inhibitor [58], and a Phase 2 trial of Exenatide in Sweden listed on the EU Clinical Trials Register [59].
Conclusions
Our analysis has provided a broad overview of the pharmacological therapies currently being clinically tested for PD. While it is encouraging to see such a wide range of therapeutic approaches being applied to PD across multiple phases of clinical development, the process of bringing DMTs into clinical use in PD remains challenging. Improvements in the clinical trial process may accelerate the assessment of ST and DMTs, and we look forward with hope to further therapies becoming available for people with PD.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Helen Matthews of The Cure Parkinson’s Trust and Prof Tanya Simuni of Northwestern University for reading the manuscript and providing constructive feedback. They would also like to thank all of the trial participants, their families, and researchers involved in the ongoing clinical research for Parkinson’s disease.
Supplemental File 2
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-202128.
REFERENCES
- [1]. DeMaagd G, Philip A (2015) Parkinson’s disease and its management: Part 1: Disease entity, risk factors, pathophysiology, clinical presentation, and diagnosis. P T 40, 504–532. [PMC free article] [PubMed] [Google Scholar]
- [2]. Simon DK, Tanner CM, Brundin P (2020) Parkinson disease epidemiology, pathology, genetics, and pathophysiology. Clin Geriatr Med 36, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. GBD 2016 Neurology Collaborators (2019) Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18, 459–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Dorsey ER, Sherer T, Okun MS, Bloemd BR (2018) The emerging evidence of the Parkinson pandemic. J Parkinsons Dis 8, S3–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Dorsey ER, Bloem BR (2018) The Parkinson pandemic—a call to action. JAMA Neurol 75, 9–10. [DOI] [PubMed] [Google Scholar]
- [6]. Cotzias GC, Papavasiliou PS, Gellene R (1969) Modification of Parkinsonism–chronic treatment with L-dopa. N Engl J Med 280, 337–345. [DOI] [PubMed] [Google Scholar]
- [7]. Yahr MD, Duvoisin RC, Schear MJ, Barrett RE, Hoehn MM (1969) Treatment of parkinsonism with levodopa. Arch Neurol 21, 343–354. [DOI] [PubMed] [Google Scholar]
- [8]. Abbott A (2010) Levodopa: The story so far. Nature 466, S6–S7. [DOI] [PubMed] [Google Scholar]
- [9]. Schwab RS, England AC, Poskanzer DC, Young RR (1969) Amantadine in the treatment of Parkinson’s disease. JAMA 208, 1168–1170. [PubMed] [Google Scholar]
- [10]. Birkmayer W, Riederer P, Youdim MB, Linauer W (1975) The potentiation of the anti akinetic effect after L-dopa treatment by an inhibitor of MAO-B, Deprenil. J Neural Transm 36, 303–326. [DOI] [PubMed] [Google Scholar]
- [11]. Tolosa E, Martí MJ, Valldeoriola F, Molinuevo JL (1998) History of levodopa and dopamine agonists in Parkinson’s disease treatment. Neurology 50(6 Suppl 6), S2–10. [DOI] [PubMed] [Google Scholar]
- [12]. Sironi VA (2011) Origin and evolution of deep brain stimulation. Front Integr Neurosci 5, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Lee D, Dallapiazza R, De Vloo P, Lozano A (2018) Current surgical treatments for Parkinson’s disease and potential therapeutic targets. Neural Regen Res 13, 1342–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL (1997) Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science 276, 2045–2047. [DOI] [PubMed] [Google Scholar]
- [15]. Blauwendraat C, Nalls MA, Singleton AB (2020) The genetic architecture of Parkinson’s disease. Lancet Neurol 19, 170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Bandres-Ciga S, Diez-Fairen M, Kim JJ, Singleton AB (2020) Genetics of Parkinson’s disease: An introspection of its journey towards precision medicine. Neurobiol Dis 137, 104782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].AMP-PD (2020) https://amp-pd.org/, Accessed April 02 2020.
- [18].ASAP: Aligning Science Across Parkinson’s (2020) https://parkinsonsroadmap.org/, Accessed April 02 2020.
- [19].Parkinson’s Progression Markers Initiative (PPMI) (2020) https://www.ppmi-info.org/, Accessed April 02, 2020.
- [20].CPP | Critical Path Institute (2020) https://c-path.org/programs/cpp/, Accessed April 02 2020.
- [21]. Espay AJ, Hausdorff JM, Sánchez-Ferro Á, Klucken J, Merola A, Bonato P, Paul SS, Horak FB, Vizcarra JA, Mestre TA, Reilmann R, Nieuwboer A, Dorsey ER, Rochester L, Bloem BR, Maetzler W (2019) A roadmap for implementation of patient-centered digital outcome measures in Parkinson’s disease obtained using mobile health technologies. Mov Disord 34, 657–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Merchant KM, Cedarbaum JM, Brundin P, Dave KD, Eberling J, Espay AJ, Hutten SJ, Javidnia M, Luthman J, Maetzler W, Menalled L, Reimer AN, Stoessl AJ, Weiner DM (2019) A proposed roadmap for Parkinson’s disease proof of concept clinical trials investigating compounds targeting alpha-synuclein. J Parkinsons Dis 9, 31–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Brundin P, Wyse RK (2019) The Linked Clinical Trials initiative (LCT) for Parkinson’s disease. Eur J Neurosci 49, 307–315. [DOI] [PubMed] [Google Scholar]
- [24]. Barker RA, Studer L, Cattaneo E, Takahashi J (2015) G-Force PD: A global initiative in coordinating stem cell-based dopamine treatments for Parkinson’s disease. NPJ Parkinsons Dis 1, 15017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Clinical Trials Highlights: Resource List | Journal of Parkinson’s Disease (2020) https://www.journalofparkinsonsdisease.com/clinical-trials-highlights-resource-list, Accessed April 02 2020.
- [26]. Cummings J, Lee G, Ritter A, Sabbagh M, Zhong K (2019) Alzheimer’s disease drug development pipeline: 2019. Alzheimers Dement (N Y) 5, 272–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Intec Pharma Issues Letter to Shareholders | Intec Pharma (2020) https://ir.intecpharma.com/news-releases/news-release-details/intec-pharma-issues-letter-shareholders, Accessed April 02 2020.
- [28]. The Parkinson Study Group STEADY-PD III Investigators (2020) Isradipine versus placebo in early Parkinson disease. Ann Intern Med 172, 591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Study of Urate Elevation in Parkinson’s Disease, Phase 3 (SURE-PD3) | National Institute of Neurological Disorders and Stroke (2019) https://www.ninds.nih.gov/Disorders/Clinical-Trials/Study-Urate-Elevation-Parkinsons-Disease-Phase-3-SURE-PD3, Accessed April 02, 2020.
- [30].Herantis Pharma Plc Announces Topline Results of Phase 1-2 CDNF Trial (2020) https://www.prnewswire.co.uk/news-releases/herantis-pharma-plc-announces-topline-results-of-phase-1-2-cdnf-trial-882045188.html, Accessed April 02, 2020.
- [31].Denali Therapeutics Announces Broad Pipeline Progress Including Positive Results From Its LRRK2 Program for Parkinson’s Disease (2020) https://www.globenewswire.com/news-release/2020/01/14/1970308/0/en/Denali-Therapeutics-Announces-Broad-Pipeline-Progress-Including-Positive-Results-From-Its-LRRK2-Program-for-Parkinson-s-Disease.html, Accessed May 10, 2020.
- [32].ADDING MULTIMEDIA Acorda Therapeutics Announces FDA Approval of INBRIJATM (levodopa inhalation powder) (2018) https://www.businesswire.com/news/home/20181221005620/en/ADDING-MULTIMEDIA-Acorda-Therapeutics-Announces-FDA-Approval, Accessed May 10, 2020.
- [33].Kyowa Kirin Announces NOURIANZ™ (Istradefylline) Now Available in the U.S. for Treatment of Parkinson’s Disease “Off” Episodes (2019) https://www.businesswire.com/news/home/20191014005706/en/Kyowa-Kirin-Announces-NOURIANZ%E2%84%A2-Istradefylline-U.S.-Treatment
- [34].Neurocrine Biosciences Announces FDA Approval of Once-Daily ONGENTYS® (opicapone) as an Add-On Treatment for Patients with Parkinson’s Disease Experiencing “Off” Episodes (2020), https://www.prnewswire.com/news-releases/neurocrine-biosciences-announces-fda-approval-of-once-daily-ongentys-opicapone-as-an-add-on-treatment-for-patients-with-parkinsons-disease-experiencing-off-episodes-301047469.html
- [35].Sunovion Announces U.S. FDA Approval of KYNMOBI™ (apomorphine hydrochloride) Sublingual Film for the Treatment of Parkinson’s Disease OFF Episodes (2020) https://www.businesswire.com/news/home/20200521005786/en/Sunovion-Announces-U.S.-FDA-Approval-KYNMOBI%E2%84%A2-apomorphine
- [36]. Mischley LK, Lau RC, Weiss NS (2017) Use of a self-rating scale of the nature and severity of symptoms in Parkinson’s Disease (PRO-PD): Correlation with quality of life and existing scales of disease severity. NPJ Parkinsons Dis 3, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Chaudhuri KR, Sauerbier A, Rojo JM, Sethi K, Schapira AHV, Brown RG, Antonini A, Stocchi F, Odin P, Bhattacharya K, Tsuboi Y, Abe K, Rizos A, Rodriguez-Blazquez C, Martinez-Martin P (2015) The burden of non-motor symptoms in Parkinson’s disease using a self-completed non-motor questionnaire: A simple grading system. Parkisonism Relat Disord 21, 287–291. [DOI] [PubMed] [Google Scholar]
- [38]. Nguyen M, Wong YC, Ysselstein D, Severino A, Krainc D (2019) Synaptic, mitochondrial, and lysosomal dysfunction in Parkinson’s disease. Trends Neurosci 42, 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Plotegher N, Duchen MR (2017) Crosstalk between lysosomes and mitochondria in Parkinson’s disease. Front Cell Dev Biol 5, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Athauda D, Maclagan K, Skene SS, Bajwa-Joseph M, Letchford D, Chowdhury K, Hibbert S, Budnik N, Zampedri L, Dickson J, Li Y, Aviles-Olmos I, Warner TT, Limousin P, Lees AJ, Greig NH, Tebbs S, Foltynie T (2017) Exenatide once weekly versus placebo in Parkinson’s disease: A randomised, double-blind, placebo-controlled trial. Lancet 390, 1664–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Athauda D, Foltynie T (2016) The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson’s disease: Mechanisms of action. Drug Discov Today 21, 802–818. [DOI] [PubMed] [Google Scholar]
- [42]. Brás IC, Dominguez-Meijide A, Gerhardt E, Koss D, Lázaro DF, Santos PI, Vasili E, Xylaki M, Outeiro TF (2020) Synucleinopathies: Where we are and where we need to go. J Neurochem 153, 433–454. [DOI] [PubMed] [Google Scholar]
- [43]. Fields CR, Bengoa-Vergniory N, Wade-Martins R (2019) Targeting alpha-synuclein as a therapy for Parkinson’s disease. Front Mol Neurosci 12, 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Brahmachari S, Karuppagounder SS, Ge P, Lee S, Dawson VL, Dawson TM, Ko HS (2017) C-Abl and Parkinson’s disease: Mechanisms and therapeutic potential. J Parkinsons Dis 7, 589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. Chen QQ, Haikal C, Li W, Li JY (2019) Gut inflammation in association with pathogenesis of Parkinson’s disease. Front Mol Neurosci 12, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46]. Whone A, Luz M, Boca M, Woolley M, Mooney L, Dharia S, Broadfoot J, Cronin D, Schroers C, Barua NU, Longpre L, Barclay CL, Boiko C, Johnson GA, Fibiger HC, Harrison R, Lewis O, Pritchard G, Howell M, Irving C, Johnson D, Kinch S, Marshall C, Lawrence AD, Blinder S, Sossi V, Stoessl AJ, Skinner P, Mohr E, Gill SS (2019) Randomized trial of intermittent intraputamenal glial cell line-derived neurotrophic factor in Parkinson’s disease. Brain 142, 512–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]. Whone AL, Boca M, Luz M, Woolley M, Mooney L, Dharia S, Broadfoot J, Cronin D, Schroers C, Barua NU, Longpre L, Lynn Barclay C, Boiko C, Johnson GA, Christian Fibiger H, Harrison R, Lewis O, Pritchard G, Howell M, Irving C, Johnson D, Kinch S, Marshall C, Lawrence AD, Blinder S, Sossi V, Stoessl AJ, Skinner P, Mohr E, Gill SS (2019) Extended treatment with glial cell line-derived neurotrophic factor in Parkinson’s disease. J Parkinsons Dis 9, 301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Heiss JD, Lungu C, Hammoud DA, Herscovitch P, Ehrlich DJ, Argersinger DP, Sinharay S, Scott G, Wu T, Federoff HJ, Zaghloul KA, Hallett M, Lonser RR, Bankiewicz KS (2019) Trial of magnetic resonance–guided putaminal gene therapy for advanced Parkinson’s disease. Mov Disord 34, 1073–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Herantis Pharma Plc Announces Topline Results of Phase 1-2 CDNF Trial (2020) https://www.prnewswire.co.uk/news-releases/herantis-pharma-plc-announces-topline-results-of-phase-1-2-cdnf-trial-882045188.html, Accessed April 02, 2020.
- [50]. Lang AE, Espay AJ (2018) Disease modification in Parkinson’s disease: Current approaches, challenges, and future considerations. Mov Disord 33, 660–677. [DOI] [PubMed] [Google Scholar]
- [51]. Espay AJ, Kalia LV, Gan-Or Z, Williams-Gray CH, Bedard PL, Rowe SM, Morgante F, Fasano A, Stecher B, Kauffman MA, Farrer MJ, Coffey CS, Schwarzschild MA, Sherer T, Postuma RB, Strafella AP, Singleton AB, Barker RA, Kieburtz K, Olanow CW, Lozano A, Kordower JH, Cedarbaum JM, Brundin P, Standaert DG, Lang AE (2020) Disease modification and biomarker development in Parkinson disease: Revision or reconstruction? Neurology 94, 481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52]. Refolo LM, Snyder H, Liggins C, Ryan L, Silverberg N, Petanceska S, Carrillo MC (2012) Common Alzheimer’s disease research ontology: National institute on aging and Alzheimer’s association collaborative project. Alzheimers Dement 8, 372–375. [DOI] [PubMed] [Google Scholar]
- [53]. Younesi E, Malhotra A, Gündel M, Scordis P, Kodamullil AT, Page M, Müller B, Springstubbe S, Wüllner U, Scheller D, Hofmann-Apitius M (2015) PDON: Parkinson’s disease ontology for representation and modeling of the Parkinson’s disease knowledge domain. Theor Biol Med Model 12, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Phase 1b study to determine the safety, tolerability and pharmacokinetics of RTB101 and sirolimus alone or in combination in patients with Mild Parkinson’s Disease, ANZCTR - Registration (2019) https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=376782, Accessed April 02 2020.
- [55].SAD and food-effect study of YTX-7739, Netherlands Trial Registry (2019) https://www.trialregister.nl/trial/8258, Accessed April 02 2020.
- [56].Multiple Ascending Dose study of LTI-291, Netherlands Trial Registry (2017) https://www.trialregister.nl/trial/6516, Accessed April 02 2020.
- [57].GBA-PD Patient Imaging study of LTI-291 versus placebo, Netherlands Trial Registry (2018) https://www.trialregister.nl/trial/7061, Accessed April 02 2020.
- [58].UCB Initiates Phase 1b US-Based Multicenter Clinical Trial in Parkinson’s Disease Patients with UCB0599, a Compound Arising from the Neuropore-UCB Collaboration (2019) https://www.businesswire.com/news/home/20190528005062/en, Accessed May 10, 2020.
- [59].Effect of Exenatide on disease progression in early Parkinson’s disease, EU Clinical Trials Register (2019) https://www.clinicaltrialsregister.eu/ctr-search/trial/2019-000732-26/SE, Accessed May 10, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.