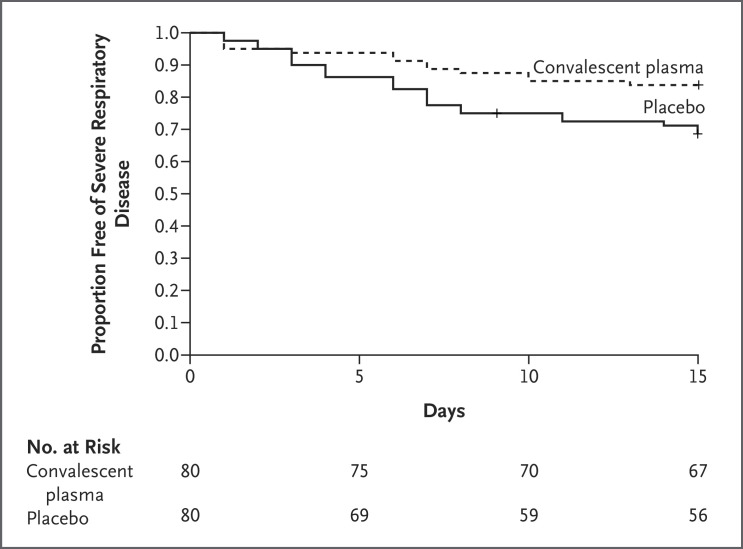
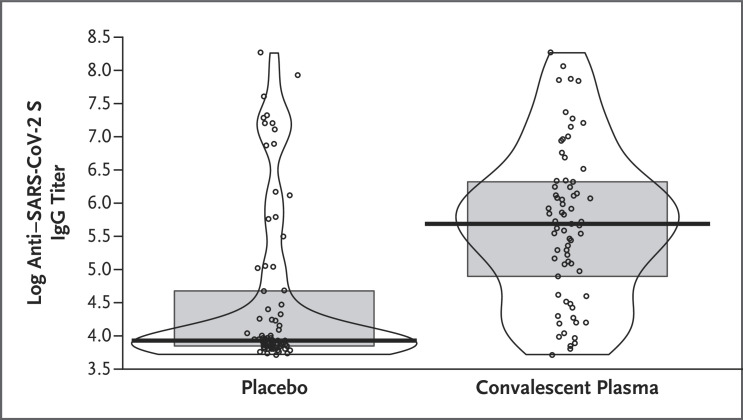
Romina Libster
Romina Libster, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Gonzalo Pérez Marc
Gonzalo Pérez Marc, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Diego Wappner
Diego Wappner, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Silvina Coviello
Silvina Coviello, M.S.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Alejandra Bianchi
Alejandra Bianchi
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Virginia Braem
Virginia Braem
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Ignacio Esteban
Ignacio Esteban, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Mauricio T Caballero
Mauricio T Caballero, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Cristian Wood
Cristian Wood, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Mabel Berrueta
Mabel Berrueta, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Aníbal Rondan
Aníbal Rondan, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Gabriela Lescano
Gabriela Lescano, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Pablo Cruz
Pablo Cruz, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Yvonne Ritou
Yvonne Ritou, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Valeria Fernández Viña
Valeria Fernández Viña, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Damián Álvarez Paggi
Damián Álvarez Paggi, Ph.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Sebastián Esperante
Sebastián Esperante, Ph.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Adrián Ferreti
Adrián Ferreti
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Gastón Ofman
Gastón Ofman, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Álvaro Ciganda
Álvaro Ciganda, B.I.T.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Rocío Rodriguez
Rocío Rodriguez
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Jorge Lantos
Jorge Lantos, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Ricardo Valentini
Ricardo Valentini, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Nicolás Itcovici
Nicolás Itcovici, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Alejandra Hintze
Alejandra Hintze, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
M Laura Oyarvide
M Laura Oyarvide, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Candela Etchegaray
Candela Etchegaray, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Alejandra Neira
Alejandra Neira, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Ivonne Name
Ivonne Name, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Julieta Alfonso
Julieta Alfonso, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Rocío López Castelo
Rocío López Castelo, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Gisela Caruso
Gisela Caruso, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Sofía Rapelius
Sofía Rapelius, M.S.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Fernando Alvez
Fernando Alvez, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Federico Etchenique
Federico Etchenique, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Federico Dimase
Federico Dimase, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Darío Alvarez
Darío Alvarez, M.S.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Sofía S Aranda
Sofía S Aranda, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Clara Sánchez Yanotti
Clara Sánchez Yanotti
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Julián De Luca
Julián De Luca
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Sofía Jares Baglivo
Sofía Jares Baglivo
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Sofía Laudanno
Sofía Laudanno
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Florencia Nowogrodzki
Florencia Nowogrodzki
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Ramiro Larrea
Ramiro Larrea, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
María Silveyra
María Silveyra, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Gabriel Leberzstein
Gabriel Leberzstein, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Alejandra Debonis
Alejandra Debonis, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Juan Molinos
Juan Molinos, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Miguel González
Miguel González, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Eduardo Perez
Eduardo Perez, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Nicolás Kreplak
Nicolás Kreplak, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Susana Pastor Argüello
Susana Pastor Argüello, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Luz Gibbons
Luz Gibbons, Ph.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Fernando Althabe
Fernando Althabe, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Eduardo Bergel
Eduardo Bergel, Ph.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,
Fernando P Polack
Fernando P Polack, M.D.
1From Fundación INFANT (R. Libster, S.C., A.B., I.E., M.T.C., C.W., D.A.P., S.E., A.F., G.O., S.S.A., C.S.Y., J.D.L., S.J.B., S.L., F.N., F.P.P.), iTrials (R. Libster, G.P.M., D.W., S.C., A.B., V.B., S.S.A., F.P.P.), Swiss Medical Group (D.W., J.L., F.E., J.M.), National Scientific and Technical Research Council (M.T.C., D.A.P., S.E.), Hospital Dr. Carlos Bocalandro (A.R., G. Lescano), Centro Gallego (P.C.), Instituto de Efectividad Clínica y Sanitaria (M.B., A.C., R.R., L.G., E.B.), Hospital Simplemente Evita (V.F.V.), CEMIC (R.V.), Fundación Hematológica Sarmiento (F. Alvez), Hospital Municipal San Isidro, (R. Larrea), Sanatorio Anchorena Recoleta (M.S.), Sanatorio Sagrado Corazón, Obra Social de los Empleados de Comercio y Actividades Civiles (G. Leberzstein), Ministerio de Salud de la Provincia de Buenos Aires (A.D., N.K.), Sanatorio Finochietto (M.G.), Programa de Atención Médica Integral (E.P.), Hospital Militar Central (G.P.M., V.B., C.W., N.I., A.H., M.L.O., C.E., A.N., I.N., J.A., R.L.C., G.C., S.R., F.D., D.A., S.P.A.), and Hospital San Juan de Dios (Y.R.) — all in Buenos Aires; the University of Oklahoma, Norman (G.O.); and the United Nations Development Program–United Nations Population Fund–United Nations Children’s Fund–World Health Organization–World Bank Special Program of Research, Development, and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva (F. Althabe).
1,✉,
the Fundación INFANT–COVID-19 Group