Abstract
Background:
The cholinergic system plays a key role in cognitive impairment in Parkinson’s disease (PD). Previous acetylcholinesterase positron emission tomography imaging studies found memory, attention, and executive function correlates of global cortical cholinergic losses. Vesicular acetylcholine transporter positron emission tomography allows for more accurate topographic assessment of not only cortical but also subcortical cholinergic changes.
Objective:
The objectiveof this study was to investigate the topographic relationship between cognitive functioning and regional cholinergic innervation in patients with PD.
Methods:
A total of 86 nondemented patients with PD (mean ± SD age 67.8 ± 7.6 years, motor disease duration 5.8 ± 4.6 years), and 12 healthy control participants (age 67.8 ± 7.8 years) underwent cholinergic [18F] Fluoroethoxybenzovesamicol positron emission tomography imaging. Patients with PD underwent neuropsychological assessment. The z scores for each cognitive domain were determined using an age-matched, gender-matched, and educational level–matched control group. Correlations between domain-specific cognitive functioning and cholinergic innervation were examined, controlling for motor impairments and levodopa equivalent dose. Additional correlational analyses were performed using a mask limited to PD versus normal aging binding differences to assess for disease-specific versus normal aging effects.
Results:
Voxel-based whole-brain analysis demonstrated partial overlapping topography across cognitive domains, with most robust correlations in the domains of memory, attention, and executive functioning (P < 0.01, corrected for multiple comparisons). The shared pattern included the cingulate cortex, insula/operculum, and (visual) thalamus.
Conclusion:
Our results confirm and expand on previous observations of cholinergic system involvement in cognitive functioning in PD. The topographic overlap across domains may reflect a partially shared cholinergic functionality underlying cognitive functioning, representing a combination of disease-specific and aging effects.
Keywords: Parkinson’s disease, acetylcholine, cognition, PET
Cognitive impairment is a common nonmotor symptom with a debilitating effect on functional capacity and quality of life in people with Parkinson’s disease (PD).1,2 Mild cognitive impairment (MCI) in PD already manifests in 25% to 30% of newly diagnosed patients and approximately 80% of patients with PD eventually develop PD dementia during the course of the disease.3–5 The profile of cognitive impairment in PD is highly heterogeneous, with multiple domains variably affected in most patients.
The pathophysiology of cognitive impairment in PD is complex and includes cumulative and interactive effects of protein depositions, neuronal and synaptic changes, and alterations in various neurotransmitter systems, including the cholinergic system.6 Despite this multifaceted pathophysiology, the cholinergic system appears to play a particularly important role. For example, in vivo cholinergic imaging studies show more severe cholinergic losses in PD dementia compared with patients with PD without dementia.7–10 Even in the absence of dementia, cholinergic system degeneration is a major driver of cognitive impairment in PD.11
We previously showed that deficits in attention, executive functioning, and memory correlated with cholinergic losses in PD, at least at a global cortical level.10,12 Unlike traditional views of the cholinergic system as a diffuse cortical neuromodulator system,13 more recent studies emphasize the importance of regional deterministic activity of the cholinergic system.14–16 Therefore, there is a need for new studies that focus on cognitive effects of regional cholinergic alterations in PD. There are 3 major sources of cholinergic innervations in the brain. The basal forebrain cholinergic cell groups are the source of widespread cholinergic projections throughout the brain where specific subregions within the nucleus basalis of Meynert (Ch4) provide the majority of projections to the cortical mantle.17,18 The pedunculopontine nucleus– laterodorsal tegmental complex projects primarily to the thalamus, brainstem nuclei, and cerebellum. The third major source is represented by cholinergic interneurons mainly found in the striatum.14 Loss of structural integrity and connectivity of the basal forebrain subregions Ch1–2 has previously been associated with memory and visuospatial task performance in PD, whereas subregions Ch3–4 correlated with executive functions and more global cognitive performance.19
Our previous cholinergic imaging studies were performed using an acetylcholinesterase positron emission tomography (PET) ligand, which does not allow for reliable estimation of cholinergic activity in high binding areas, such as the striatum and cerebellum. [18F] Fluoroethoxybenzovesamicol ([18F]FEOBV) PET binds specifically to the vesicular acetylcholine transporter (VAChT) and allows for assessment of cholinergic nerve terminal integrity not only in the cortex but also in high binding subcortical regions, providing the opportunity of detailed assessment of regional cerebral cholinergic changes.20–22 The purpose of this study was to examine the topographic relationship between domain-specific cognitive functioning and regional cerebral VAChT binding in nondemented patients with PD.
Methods
Participants
A total of 86 patients with PD (67 men and 19 women) were included in this cross-sectional study. Patients had a mean ± SD age of 67.8 ± 7.6 years and motor disease duration of 5.8 ± 4.6 years. Inclusion criteria consisted of a clinical PD diagnosis in accordance with the UK PD Society Brain Bank clinical diagnostic criteria.23 Exclusion criteria included evidence of large-vessel stroke or mass lesions on anatomic imaging, the use of anticholinergic or cholinesterase inhibitor drugs, presence of severe depression as measured using the Geriatric Depression Scale,24 and presence of PD dementia. A healthy control (HC) group consisting of 5 men and 7 women with a mean age of 67.8 ± 7.8 years was included for normative PET imaging data. This study was approved by the institutional review boards of the University of Michigan School of Medicine and Veterans Affairs Ann Arbor Healthcare System. Written informed consent was obtained from all participants prior to any study procedures and conducted in accordance with the Declaration of Helsinki.
All patients with PD underwent motor examinations using the Movement Disorder Society Revised Unified PD Rating Scale Part III (MDS-UPDRS-III), with a mean score of 34.3 ± 12.1 and a Hoehn and Yahr score of 2.4 ± 0.6. Motor assessment was performed in the morning in the dopaminergic off state, that is, after overnight withdrawal of dopaminergic medication. Mean levodopa equivalent dose (LED)25 was 647 ± 410 mg. More details of the clinical and demographic characteristics are described in Table 1.
TABLE 1.
Demographic and clinical characteristics of included patients with PD
| PD | |
|---|---|
| Age | 67.8 (7.6) |
| Gender, male:female | 67:19 |
| Education, y | 15.6 (2.7) |
| Motor disease duration, y | 5.8 (4.6) |
| Hoehn and Yahr | 2.4 (0.6) |
| MDS-UPDRS Part III | 34.3 (12.1) |
| LED, mg | 647.2 (410) |
| PD-MCI, n (%) | 39 (45.3%) |
| MoCA | 26.2 (3.0) |
| z score memory | −0.44 (1.03) |
| z score attention | −0.41 (0.87) |
| z score executive function | −0.55 (1.27) |
| z score language | −0.48 (1.11) |
| z score visuospatial function | −0.12 (0.84) |
Means (standard deviations) are presented for numerical variables. Abbreviations: MDS-UPDRS, Movement Disorder Society–Unified Parkinson’s Disease Rating Scale; LED, levodopa equivalent dose; PD-MCI, Parkinson’s Disease Mild Cognitive Impairment; MoCA, Montreal Cognitive Assessment.
Neuropsychological Assessment
All patients with PD underwent a detailed neuropsychological assessment including at least 2 neuropsychological tests for each cognitive domain, in line with recommendations of the Movement Disorder Society (MDS) Task Force.26,27 The cognitive test battery consisted of the California Verbal Learning Test; Wechsler Memory Scale; Stroop Color Word Test; Delis-Kaplan Executive Function System Trail Making Test; the Wechsler Adult Intelligence Scale Digit Span, Matrix Reasoning Task, and Digit-Symbol Modalities Tests; the Letter and Semantic Verbal Fluency; Boston Naming Test; Benton Judgment of Line Orientation Test; and the Clock Copy Test of the PD–Cognitive Rating Scale. Conditions possibly influencing neuropsychological test performance, including visual problems, color blindness, dysarthria, and dyskinesia were taken into consideration when interpreting cognitive performance, excluding (sub)tasks if needed. Patients were considered PD-MCI based on MDS PD-MCI level II criteria.26 Neuropsychological tests and subtasks represented specific cognitive domains as shown in Table 2.
TABLE 2.
Neuropsychological tests per cognitive domain
| Domain | Neuropsychological (Sub)Test |
|---|---|
| Memory | California verbal learning test: immediate recall |
| California verbal learning test: delayed free recall | |
| Wechsler Memory Scale | |
| Attention | Stroop 2: color test |
| Delis–Kaplan Executive Function System, Trail Making Test 2: number sequencing | |
| Symbol Digit Modalities Test | |
| Wechsler Adult Intelligence Scale: digit span backward | |
| Executive function | Stroop 4: Adjusted Color Word Test |
| Delis–Kaplan Executive Function System, Trail Making Test 4: letter–number sequencing | |
| Wechsler Adult Intelligence Scale: matrix reasoning | |
| Letter fluency | |
| Language | Boston Naming Test |
| Verbal fluency: animals | |
| Visuospatial function | Judgment of line orientation |
| Parkinson’s Disease–Cognitive Rating Scale: Clock Copy Test |
A z score for every participant on specific tests was calculated based on a data set of a healthy control group of 77 older participants. Data from these controls were collected in our laboratory to provide normative data of a group with similar geographical and social–economic backgrounds and ensure the use of identical assessment techniques. The healthy control group was of similar age, gender, and educational-level distributions as the patient population. The z scores of the PD–Cognitive Rating Scale Clock Copy Test were based on the normative data described previously, correcting for age, gender, and educational level.28 By averaging all z scores on the tests or subtasks, an average z score for each cognitive domain was obtained (see Table 1). A global cognitive z score was computed as the average of all cognitive domains. A higher z score reflects better cognitive task performance. Neuropsychological testing of patients was performed while they were on their usual dopaminergic medications.
Imaging Acquisition and Analysis
All participants underwent brain magnetic resonance imaging (MRI) and VAChT [18F]FEOBV PET imaging. T1-weighted MRI was performed on a 3 Tesla Philips Achieva system (Philips, Best, The Netherlands). A 3-dimensional inversion recovery-prepared turbo field echo was performed in the sagittal plane using repetition time/echo time/inversion time = 9.8/4.6/1041 milliseconds, turbo factor = 200, single average, field of view = 240 × 200 × 160 mm, and acquired matrix = 240 × 200 × 160 slices and reconstructed to 1 mm isotropic resolution.
[18F]FEOBV was prepared as described previously.29 [18F]FEOBV delayed dynamic imaging was performed over 30 minutes (in six 5-minute frames) starting 3 hours after an intravenous bolus dose injection of 8 millicurie. [18F]FEOBV20 PET imaging was performed in 3-dimensional imaging mode using an ECAT Exact HR+ tomograph (Siemens Molecular Imaging, Inc., Knoxville, TN), which acquires 63 transaxial slices (slice thickness, 2.4 mm; intrinsic in-plane resolution, 4.1 mm full width at half maximum over a 15.2 cm axial field of view). [18F]FEOBV PET imaging was performed while patients were on their usual dopaminergic medication.
The PET imaging frames were spatially coregistered within subjects with a rigid-body transformation to reduce the effects of subject motion during the imaging session.30 Statistical parametric mapping software (SPM12; Wellcome Trust Centre for Neuroimaging, London, UK) was used for PET-MRI registration using the cropped T1-weighted magnetic resonance volumetric scan. Freesurfer software (Laboratory for Computational Neuroimaging, Athinoula A. Martinos Center for Biomedical Imaging, Boston, MA) was used to define cortical and subcortical magnetic resonance gray matter volume of interest (VOI). MRI-based partial volume correction of the PET data was performed.31
Cortical VOI labels from the Mindboggle-101 data set segmented in FreeSurfer were used to identify gray and white matter VOI.32 A white matter reference tissue approach was used to determine VAChT binding as previously reported.21,33 Distribution volume ratios were calculated from the ratio of averaged frames for gray matter targets and supratentorial white matter reference tissue.21 A single total neocortical VOI was created for the VOI-based statistical analysis.
Voxel-Based PET Analysis
Voxel-based PET analysis was performed as previously described.34 All brain images were spatially normalized to Montreal Neurological Institute template space using the diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL) normalization protocol35 and smoothed with a Gaussian kernel of 8 mm full width half maximum to adjust the anatomical variability between the individual brains and to enhance the signal-to-noise ratio. The relevant brain areas were displayed in Montreal Neurological Institute atlas coordinates (in millimeters) in the stereotactic space using the automated anatomical labeling toolbox.
Statistics
Analyses were performed using IBM SPSS Statistics for Windows, version 24.0 (IBM Corp., Armonk, NY). Correlations between VOI-based whole-brain neocortical VAChT binding and performance on specific cognitive domain z scores were analyzed using a partial Pearson correlation coefficient controlling for MDS-UPDRS-III scores and LED levels. The Holm-Bonferroni method was used to correct for effects of multiple testing.
Voxel-wise statistical analysis was performed using SPM12 software using the parametric [18F]FEOBV distribution volume ratio images of all patients to assess both positive and negative topographic correlations between specific cognitive domain z scores and whole-brain cholinergic binding. In addition, a voxel-wise group comparison between the PD and the HC group was performed. The result of this analysis, a topographic profile showing regions significantly different between PD and HC, was transformed into a mask. The domain-specific voxel-wise analyses were then repeated using this mask to identify PD-specific versus aging-related regions. Both domain-specific voxel-based analyses were controlled for MDS-UPDRS-III scores and LED levels. The false discovery rate (FDR) approach was used for correction for multiple testing effects in the voxel-based analysis.
Results
Cognitive Functioning
Based on MDS PD-MCI level II criteria, 39 (45.3%) participants in the PD group were classified as PD-MCI (Table 1). Only 3 of the 39 patients with PD-MCI presented with single-domain PD-MCI; all other patients with PD-MCI showed multidomain impairments. Attention was the most commonly affected domain (n = 30), followed by executive functions (n = 28) and memory (n = 27) in the 39 patients with PD-MCI. The language and visual domains were affected in 18 and 14 of the 39 patients, respectively, with PD-MCI.
Domain-Specific Cognitive Correlates of Global Neocortical [18F]FEOBV Distribution Volume Ratios
Significant correlations were present between VOI-based global cortical VAChT binding and the memory (r = 0.423, P < 0.001), executive function (r = 0.352, P < 0.001), and attention (r = 0.321, P = 0.003) domains (Table 3).
TABLE 3.
Correlations between global cortical vesicular acetylcholine transporter binding and performance on different cognitive domains, controlled for levodopa equivalent dose and MDS-UPDRS Part III
| Cognitive Domain | r | P |
|---|---|---|
| Memory | 0.423 | <0.001a |
| Executive functioning | 0.352 | <0.001a |
| Attention | 0.321 | 0.003a |
| Language | 0.128 | 0.247 |
| Visuospatial | 0.192 | 0.080 |
| Global cognition | 0.364 | 0.001a |
Global cognition is the average z score of all cognitive domains.
Significant after Holm-Bonferroni correction.
Abbreviation: MDS-UPDRS, Movement Disorders Society–Unified Parkinson’s Disease Rating Scale.
Voxel-Based Regional Cerebral [18F]FEOBV Binding Correlates of Different Cognitive Domains
Whole-brain voxel-based analyses were performed to explore the correlation between regional brain VAChT binding and the different cognitive domains, controlling for LED levels and parkinsonian motor impairment as measured by the MDS-UPDRS-III. Only positive correlations were found across domains with no negative correlations observed. Positive correlations indicate that a higher cholinergic binding is associated with better cognitive performance.
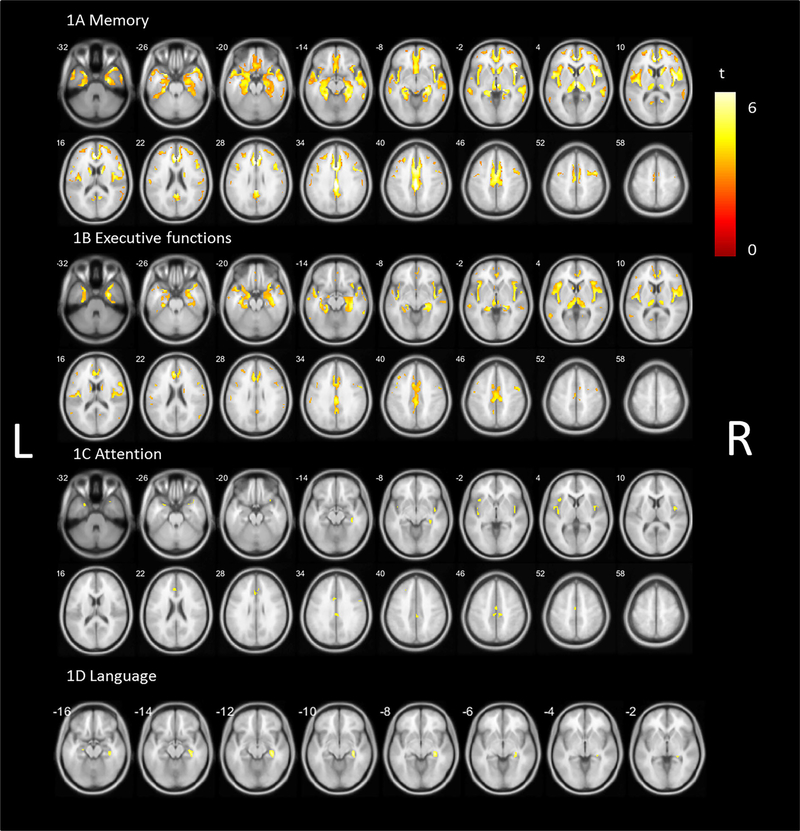
For the memory domain z score, regional cerebral cholinergic correlations (P < 0.01 FDR corrected) were seen in widespread cortical and subcortical brain regions (Fig. 1A). The topographic profile included the cingulate cortex (anterior, mid, posterior, and retrosplenial regions), prefrontal cortex (dorsolateral prefrontal cortex, orbitofrontal cortex, and gyrus rectus regions), insula and operculum, thalamus and visual thalamus with the pulvinar and lateral geniculate nucleus (LGN), caudate nucleus, hippocampus and parahippocampal regions, temporal lobe (superior, middle, and inferior temporal gyrus and temporal pole) bilaterally, and left lingual gyrus.
FIG. 1.
Statistical parametric voxel-based analysis (false discovery rate corrected P < 0.01) of the correlation between vesicular acetylcholine transporter binding and (A) memory domain z scores, (B) executive function domain z scores, (C) attention domain z scores, and (D) language domain z scores controlled for parkinsonian motor impairment and levodopa equivalent dose. L, left; R, right.
Results found for the executive function (Fig. 1B) and attention domains (Fig. 1C) showed partially overlapping topography with the memory domain. Overlapping regions across all 3 domains included the cingulate cortex, insula/operculum and visual thalamus (especially the LGN), and hippocampal region (P < 0.01 FDR corrected). Compared with the memory domain, involvement of the temporal lobe and prefrontal cortex was more limited for executive functions. The spatial extent of overlap was less for the attention domain but included the cingulate cortex, insula/operculum, temporal pole, right LGN, and LGN–fimbria transitional area (P < 0.01 FDR corrected).
The topographic profile for the language domain showed limited regional VAChT binding correlates, mainly including the right LGN, hippocampal fimbria, and LGN–fimbria transitional region (P < 0.01 FDR corrected, Fig. 1D).
Whole-brain voxel-based analysis for the visuospatial domain did not show significant voxels after correction for multiple comparisons.
Sensitivity Analysis of Voxel-Based Regional Cerebral [18F]FEOBV Binding Correlates of Different Cognitive Domains Superimposed on PD-Related Versus HC-Related Cholinergic Innervation Changes
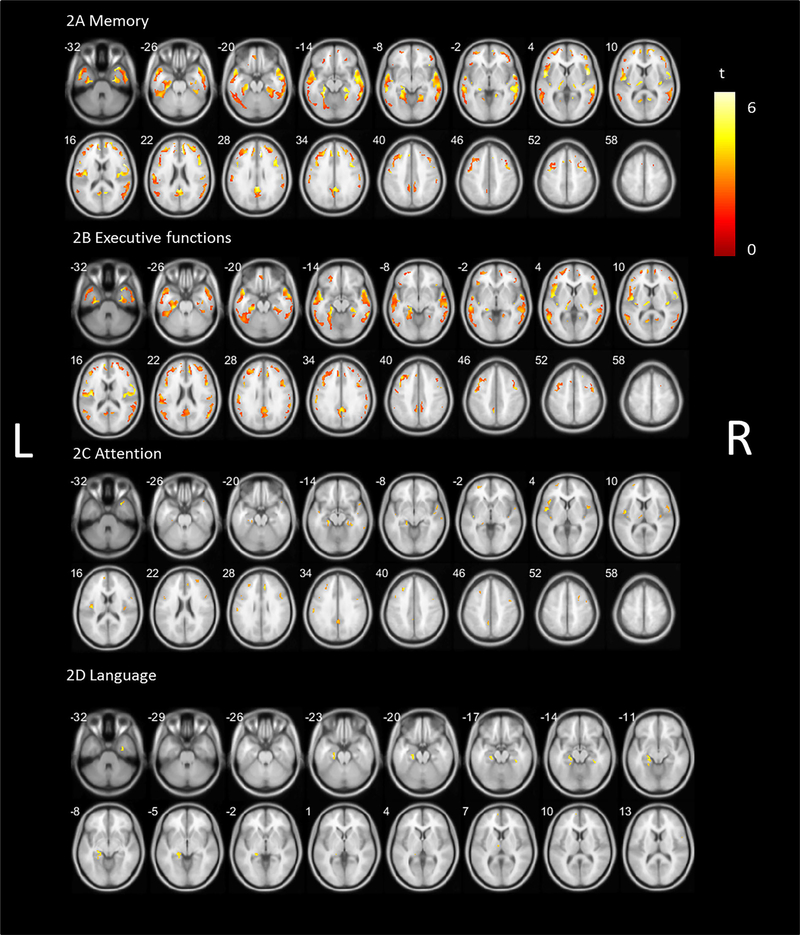
Whole-brain voxel-based group comparisons between the PD and the HC group were first performed to identify disease-specific VAChT binding differences (P < 0.05 FDR corrected; Fig. S1). Widespread predominant posterior cortical and subcortical differences were found. Subcortical regions included the thalamus and pallidum. Analyses in the opposite direction showed no significant higher cholinergic binding in the PD group.
The regional topography was then used as a mask to repeat the voxel-based cognitive domain-specific analyses. After correcting for multiple comparisons, the memory and executive function domains showed significant regional correlates with overlapping topography across both domains (Fig. 2; P < 0.05 FDR corrected). The significant regions for the memory domain (Fig. 2A) were less extensive but topographically comparable with the correlates in the non-PD-specific analysis (Fig. 1). The most prominent regions included the superior and medial temporal lobe, hippocampus and parahippocampal region, thalamus (including the left visual thalamus), insula, operculum, superior frontal region, postcentral gyrus, the anterior and posterior cingulum, and the precuneus. The cholinergic correlates of the executive function domain (Fig. 2B) show overlapping regions with the memory domain, including the temporal lobe, parahippocampal regions and left hippocampus, thalamus and left visual thalamus, insula and operculum, cingulum, and postcentral gyrus. The significant regions for the attention domain (Fig. 2C) included the insula, operculum, (para)hippocampal region, and the middle and anterior cingulate cortex. The language domain (Fig. 2D) showed significant regions in the (para)hippocampal region and LGN left more than right.
FIG. 2.
Statistical parametric voxel-based analysis (false discovery rate corrected P < 0.05) of the correlation between vesicular acetylcholine transporter binding and (A) memory domain z scores, (B) executive function domain z scores, (C) attention domain z scores, and (D) language domain z scores controlled for parkinsonian motor impairment and levodopa equivalent dose superimposed on a Parkinson’s disease impairment-related mask. L, left; R, right.
Discussion
Findings of this study confirm and expand on our previous studies. First, analyses of VOI-based global neocortical VAChT binding confirmed our previous observations of significant correlations between attention, memory, and executive function domains and global cortical acetylcholinesterase hydrolysis rates.10,12 Second, the voxel-based analysis demonstrated novel findings of a shared topographic pattern of vulnerability of brain anatomic cholinergic projections underlying multiple cognitive domains while controlling for the severity of PD-specific motor impairment and LED levels. Third, this cholinergic pattern represents a combination of disease-specific and aging effects. PD-specific regional brain changes included the cingulum, bilateral insula and operculum, hippocampal region, and visual thalamus.
These overlapping anatomic regions have previously been associated with cognitive functions. For example, the insula plays a key role in task set and control,36,37 and as part of the saliency network together with the anterior cingulate cortex, is of importance for stimuli detection, facilitating attention shifting and memory function.38,39 In addition, both the posterior cingulate cortex and hippocampus are involved in memory, showing coactivation during an episodic memory encoding tasks in patients with Alzheimer’s disease.40 Furthermore, reduced blood flow in the posterior cingulate cortex is predictive of more global cognitive decline rather than being limited to impaired memory functions in Alzheimer’s disease.41 Our findings suggest that cholinergic changes within these anatomic regions are of relevance for the cognitive impairment syndrome in PD. Furthermore, the overlapping cholinergic topography across different cognitive domains also suggests that these regions may serve a shared cognitive processing function underlying and serving multiple cognitive domains. Furthermore, cholinergic losses in these regions suggest vulnerability of not only the basal forebrain (both [para]limbic and neocortical projections) but also brainstem cholinergic projections and striatal cholinergic interneurons underlying the cognitive impairment syndrome in PD.
Our sensitivity analysis using a mask based on PD versus HC VAChT binding differences indicated both disease-specific as well as normal aging components contributing to the cholinergic topographic correlated of cognitive changes in the patients. Although less extensive, comparable cholinergic topography was observed for the domains of memory and executive functions, including the temporal lobe, (para)hippocampal region, thalamus, insula and operculum, and cingulate cortex. Topography was more limited when applying the PD mask, likely suggesting a component of normal aging contributing to cognitive impairment in PD. This is not unexpected as cholinergic changes, as measured with [18F]FEOBV, also occur with normal aging as previously reported.42 This is similar to nigrostrial dopaminergic losses in PD that effectively are a composite of normal aging and PD-specific dopaminergic losses.43 Given the reported symptomatic motor denervation threshold of about 50% loss of dopamine transporters in the putamen,44 findings explain the increasing incident of PD with older age. Similar to the dopaminergic system, we postulate that aging plays an important role in cholinergic denervation and its relationship with cognitive functioning, including the demonstrated topography. These regions could also be of particular interest in other age-related neurodegenerative disorders, including Alzheimer’s disease.
The overlapping topography across domains also suggests a role for attention and overall awareness. Attention is a prerequisite for cognitive functions, such as memory and executive functions. Therefore, attention may explain some element of the observed cholinergic communality underlying these cognitive domains.
However, the more limited spatial extent of the shared topographic pattern found for the attention domain suggests that other mechanisms may also play a role. Cholinergic losses in the visual thalamus, including the LGN, are consistent with vulnerability of the pedunculopontine nucleus–thalamic projections.18,45 Although typically viewed as a visual relay station, more recent literature describes the LGN as an active filtering center with an important role in modulating attention and cognitive control of visual information.46
Interestingly, when comparing cholinergic binding differences between PD and HC, the most prominent cholinergic denervation is found in the posterior cortical (parieto-occipital) regions, whereas cholinergic correlates of cognitive functioning are more prominent in more centrally located frontal and temporal regions. The predominant posterior cortical binding differences between PD and HC is in line with previous cholinergic imaging studies.7–9,22 Despite these prior observations, we did not find robust relationships between cognitive functioning and VAChT uptake in posterior cortical regions with the exception of the posterior cingulum and precuneus. However, these findings are mainly based on comparisons between patients and control groups rather than looking at regional cerebral correlates of cognition in PD or within control participants. It is possible that relatively isolated posterior cortical cholinergic losses in PD may not be sufficient to cause clinically manifest cognitive changes. This may be because of preservation of more anterior cholinergic projections. In other words, the symptomatic threshold for cognitive impairment attributed to cholinergic changes in PD may be more related to expanded networks rather than local cortical changes.
Another explanation may be that the assumed but not proven posterior-to-anterior cortical cholinergic denervation gradient may result in a statistical “floor” effect of cholinergic bindings measures related to cognitive performance in posterior brain regions when performing correlation analyses. This explanation is less likely as the thalamic complex and posterior cingulum are both part of the posterior (subcortical and limbocortical) brain that have strong functional and structural connectivity with the occipital cortex.47
A major strength of this study is the use of whole-brain voxel-based analyses to allow a more granular assessment of regional cerebral cholinergic correlates of cognitive functions in PD while controlling for PD-specific motor impairment and LED levels. This approach is particularly important to allow the identification of smaller regions that may be otherwise lost if only global cortical or large lobar cholinergic binding measures were used. For that reason, we did not apply a minimum large voxel cluster size to avoid missing small-sized regions that are of potential importance for cognitive functions for the same reason. For example, our novel observation of the cholinergic LGN correlating with cognitive domains of memory, language, and executive functions would have been easily missed otherwise. There are also several limitations of this study. This study included only a small control group for which no detailed neuropsychological assessment was available. However, we were able to perform a sensitivity analyses to determine PD-specific versus aging-related changes. Another limitation is that patients were studied on their usual dopaminergic medication during the [18F]FEOBV PET scan and cognitive assessment for reasons of patient comfort. However, our analyses were adjusted for LED levels.
Our findings may augur further research into a personalized medicine approach for use of cholinesterase inhibitors in patients with PD with cognitive complaints. In particular, PD-MCI patients with more prominent memory, executive function, or attentional deficits may be preferential candidates for such cholinergic augmentation studies.
To conclude, our findings confirm and expand on previous observations of robust cholinergic correlates of memory, attention, and executive functions in PD. Novel observations include evidence of a common cholinergic pattern with overlapping bilateral cholinergic topographic profiles associated with changes in these specific cognitive domains function in PD, including the insula, cingulate cortex, hippocampus, and thalamus, including the LGN.
Supplementary Material
Figure S1. Statistical parametric voxel-based analysis (false discovery rate corrected P < 0.05) showing the significant lower vesicular acetylcholine transporter binding in patients with Parkinson’s disease compared with healthy controls.
Acknowledgments:
Study funded by National Institutes of Health (P01 NS015655, RO1 NS070856, P50 NS091856), Department of Veterans Affairs grant (I01 RX001631), and the Michael J. Fox Foundation.
Footnotes
Relevant conflicts of interests/financial disclosures: Nothing to report.
Supporting Data
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
References
- 1.Post B, Muslimovic D, van Geloven N, et al. Progression and prognostic factors of motor impairment, disability and quality of life in newly diagnosed Parkinson’s disease. Mov Disord 2011;26(3):449–456. [DOI] [PubMed] [Google Scholar]
- 2.Lawson RA, Yarnall AJ, Duncan GW, et al. Severity of mild cognitive impairment in early Parkinson’s disease contributes to poorer quality of life. Parkinsonism Relat Disord 2014;20(10):1071–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aarsland D, Bronnick K, Williams-Gray C, et al. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology 2010;75(12):1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol 2003;60(3):387–392. [DOI] [PubMed] [Google Scholar]
- 5.Hely MA, Reid WGJ, Adena MA, Halliday GM, Morris JGL. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord 2008;23(6):837–844. [DOI] [PubMed] [Google Scholar]
- 6.Kalia LV, Lang AE. Parkinson’s disease. Lancet 2015;386(9996): 896–912. [DOI] [PubMed] [Google Scholar]
- 7.Hilker R, Thomas AV, Klein JC, et al. Dementia in Parkinson disease: functional imaging of cholinergic and dopaminergic pathways. Neurology 2005;65(11):1716–1722. [DOI] [PubMed] [Google Scholar]
- 8.Klein JC, Eggers C, Kalbe E, et al. Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology 2010;74(11):885–892. [DOI] [PubMed] [Google Scholar]
- 9.Shimada H, Hirano S, Shinotoh H, et al. Mapping of brain acetylcholinesterase alterations in Lewy body disease by PET. Neurology 2009;73(4):273–278. [DOI] [PubMed] [Google Scholar]
- 10.Bohnen NI, Müller MLTM, Kotagal V, et al. Heterogeneity of cholinergic denervation in Parkinson’s disease without dementia. J Cereb Blood Flow Metab 2012;32(8):1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bohnen NI, Albin RL, Müller MLTM, et al. Frequency of cholinergic and caudate nucleus dopaminergic deficits across the predemented cognitive spectrum of parkinson disease and evidence of interaction effects. JAMA Neurol 2015;72(2):194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bohnen NI, Kaufer DI, Hendrickson R, et al. Cognitive correlates of cortical cholinergic denervation in Parkinson’s disease and parkinsonian dementia. J Neurol 2006;253(2):242–247. [DOI] [PubMed] [Google Scholar]
- 13.Woolf N. Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol 1991;37:475–524. [DOI] [PubMed] [Google Scholar]
- 14.Ballinger EC, Ananth M, Talmage DA, Role LW. Basal forebrain cholinergic circuits and signaling in cognition and cognitive decline. Neuron 2016;91(6):1199–1218. 10.1016/j.neuron.2016.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarter M, Lustig C, Howe WM, Gritton H, Berry AS. Deterministic functions of cortical acetylcholine. Eur J Neurosci 2014;39(11): 1912–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaborszky L, Csordas A, Mosca K, et al. Neurons in the basal forebrain project to the cortex in a complex topographic organization that reflects corticocortical connectivity patterns: an experimental study based on retrograde tracing and 3D reconstruction. Cereb Cortex 2015;25(1):118–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu AKL, Chang RCC, Pearce RKB, Gentleman SM. Nucleus basalis of Meynert revisited: anatomy, history and differential involvement in Alzheimer’s and Parkinson’s disease. Acta Neuropathol 2015;129 (4):527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selden NR, Gitelman DR, Salamon-Murayama N, Parrish TB, Mesulam MM. Trajectories of cholinergic pathways within the cerebral hemispheres of the human brain. Brain 1998;121(Pt 1):2249–2257. [DOI] [PubMed] [Google Scholar]
- 19.Gargouri F, Gallea C, Mongin M, et al. Multimodal magnetic resonance imaging investigation of basal forebrain damage and cognitive deficits in Parkinson’s disease. Mov Disord 2019;34(4):516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrou M, KA F, Kilbourn MR, et al. In vivo imaging of human cholinergic nerve terminals with (-)-5–18F-Fluoroethoxybenzo-vesamicol: biodistribution, dosimetry, and tracer kinetic analyses. J Nucl Med 2014;55(3):396–404. [DOI] [PubMed] [Google Scholar]
- 21.Nejad-Davarani S, Koeppe RA, Albin RL, Frey KA, Müller MLTM, Bohnen NI. Quantification of brain cholinergic denervation in dementia with Lewy bodies using PET imaging with [18F]-FEOBV. Mol Psychiatry 2019;24(3):322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Zee S, Vallez Garcia D, Elsinga PH, et al. [18F] Fluoroethoxybenzovesamicol in Parkinson’s disease patients: Quantification of a novel cholinergic positron emission tomography tracer. Mov Disord 2019;34:924–926. [DOI] [PubMed] [Google Scholar]
- 23.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55(3):181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatry Res 1982;17(1):37–49. [DOI] [PubMed] [Google Scholar]
- 25.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 2010;25(15):2649–2653. [DOI] [PubMed] [Google Scholar]
- 26.Litvan I, Goldman JG, Tröster AI, et al. diagnostic criteria for mild cognitive impairment in Parkinson’s disease: movement Disorder Society task force guidelines. Mov Disord 2012;27(3):349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldman JG, Holden S, Ouyang B, Bernard B, Goetz CG, Stebbins GT. Diagnosing PD-MCI by MDS task force criteria: how many and which neuropsychological tests? Mov Disord 2015;30(3):402–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santangelo G, Lagravinese G, Battini V, et al. The Parkinson’s disease-cognitive rating scale (PD-CRS): normative values from 268 healthy Italian individuals. Neurol Sci 2017;38(5):845–853. [DOI] [PubMed] [Google Scholar]
- 29.Shao X, Hoareau R, Hockley BG, et al. Highlighting the versatility of the Tracerlab synthesis modules. Part 1: fully automated production of [18F]labelled radiopharmaceuticals using a Tracerlab FX(FN). J Labelled Comp Radiopharm 2011;54(6):292–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minoshima S, Koeppe RA, Fessler JA, et al. Integrated and Automated Data Analysis Method for Neuronal Activation Studying Using O15 Water PET. Tokyo, Japan: Excerpta Medica; 1993. [Google Scholar]
- 31.Muller-Gartner HW, Links JM, Prince JL, et al. Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. J Cereb Blood Flow Metab 1992;12(4):571–583. [DOI] [PubMed] [Google Scholar]
- 32.Klein A, Tourville J. 101 labeled brain images and a consistent human cortical labeling protocol. Front Neurosci 2012;6:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aghourian M, Legault-Denis C, Soucy JP, et al. Quantification of brain cholinergic denervation in Alzheimer’s disease using PET imaging with [18F]-FEOBV. Mol Psychiatry 2017;22(11):1531–1538. [DOI] [PubMed] [Google Scholar]
- 34.Kanel P, Müller MLTM, van der Zee S, et al. Topography of cholinergic changes in dementia with lewy bodies and key neural network hubs [published online ahead of print June 5, 2020]. J Neuropsychiatry Clin Neurosci. 10.1176/appi.neuropsych.19070165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashburner J A fast diffeomorphic image registration algorithm. Neuroimage 2007;38(1):95–113. [DOI] [PubMed] [Google Scholar]
- 36.Dosenbach NUF, Visscher KM, Palmer ED, et al. A core system for the implementation of task sets. Neuron 2006;50(5):799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dosenbach NUF, Fair DA, Miezin FM, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci 2007;104(26):11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christopher L, Koshimori Y, Lang AE, Criaud M, Strafella AP. Uncovering the role of the insula in non-motor symptoms of Parkinson’s disease. Brain 2014;137(8):2143–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 2010;214 (5–6):655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papma JM, Smits M, de Groot M, et al. The effect of hippocampal function, volume and connectivity on posterior cingulate cortex functioning during episodic memory fMRI in mild cognitive impairment. Eur Radiol 2017;27(9):3716–3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang C, Wahlund L-O, Svensson L, Winblad B, Julin P. Cingulate cortex hypoperfusion predicts Alzheimer’s disease in mild cognitive impairment. BMC Neurol 2002;2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albin RL, Bohnen NI, Muller MLTM, et al. Regional vesicular acetylcholine transporter distribution in human brain: a [(18) F] fluoroethoxybenzovesamicol positron emission tomography study. J Comp Neurol 2018;526(17):2884–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bohnen NI, Albin RL, Koeppe RA, et al. Positron emission tomography of monoaminergic vesicular binding in aging and Parkinson disease. J Cereb Blood Flow Metab 2006;26(9):1198–1212. [DOI] [PubMed] [Google Scholar]
- 44.Guttman M, Burkholder J, Kish SJ, et al. [11C]RTI-32 PET studies of the dopamine transporter in early dopa-naive Parkinson’s disease: implications for the symptomatic threshold. Neurology 1997;48(6): 1578–1583. [DOI] [PubMed] [Google Scholar]
- 45.Kotagal V, Muller MLTM, Kaufer, Koeppe RA, Bohnen NI. Thalamic cholinergic innervation is spared in Alzheimer disease compared to parkinsonian disorders. Neurosci Lett 2012;514(2): 169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Halassa MM, Kastner S. Thalamic functions in distributed cognitive control. Nat Neurosci 2017;20(12):1669–1679. [DOI] [PubMed] [Google Scholar]
- 47.Arcaro MJ, Pinsk MA, Kastner S. The anatomical and functional organization of the human visual pulvinar. J Neurosci 2015;35 (27):9848 LP–9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Statistical parametric voxel-based analysis (false discovery rate corrected P < 0.05) showing the significant lower vesicular acetylcholine transporter binding in patients with Parkinson’s disease compared with healthy controls.