Abstract
Social mammals often live in groups in which a dominance hierarchy is an important determinant of access to mates. In addition to competing individually, males may form coalitions of two or more to attack or intimidate rivals. Coalition formation could be particularly advantageous for adolescent males by helping them compensate for their physical and social immaturity. However, adolescents may struggle to attract effective coalition partners because of these inadequacies. Here, we examine the behavior of maturing male chimpanzees to test whether coalitions are more frequent among more or less powerful individuals. Our longitudinal study followed 18 males (ages 5 through 25 years) and utilized 1517 coalitions across 12 years of observation of the Kanyawara chimpanzee community in Kibale National Park, Uganda. We found that rates of coalition formation increased across maturation and that this increase was independent of a rise in the overall use of aggression. Juveniles formed coalitions almost exclusively with their mothers, while adolescents partnered primarily with peers and adult males. When adolescents and adult males formed coalitions with each other, the adolescents were more likely to join the adults than vice versa. Finally, adolescents engaged in joint behavior with adult males more often in non-aggressive vocal displays than in aggressive coalitions. Taken together, our results suggest that adolescent males are largely unable to attract the most powerful coalition partners and that they “make the best of a bad job” by joining adult males in less competitive situations, when the risk of receiving aggression from opponents is lower.
Keywords: Cooperation, Adolescence, Aggression, Testosterone, Dominance rank, Pant-hoot chorusing
Introduction
Coalitions are acts of joint aggression directed by two or more individuals against one or more conspecific targets (Harcourt and de Waal 1992; Nishida and Hosaka 1996). Coalitionary aggression during intragroup conflicts has been observed in multiple orders, including passerines, anseriformes, artiodactyls, perissodactyls, proboscideans, carnivorans, cetaceans, and primates (reviewed by Smith et al. 2010). Participation in coalitions yields a range of direct benefits, including improved access to mates (e.g., bottlenose dolphins (Tursiops spp.): Connor 1992; Connor et al. 1992; lions (Panthera leo): Bertram 1975; Bygott et al. 1979; Packer and Pusey 1982; Packer et al. 1988; cheetahs (Acinonyx jubatus): Caro 1994; Barbary macaques (Macaca sylvanus): Kuester and Paul 1992; Bissonnette et al. 2011; savanna baboons (Papio cynocephalus): Noë 1992; chimpanzees (Pan troglodytes): Watts 1998; Duffy et al. 2007; Gilby et al. 2013; Bray et al. 2016) or food resources (e.g., squirrel monkeys (Saimiri sciureus): Mitchell et al. 1991; capuchin monkeys (Cebus capucinus): Perry 1997; Vogel et al. 2007), and maintenance or improvement of dominance rank (e.g., fallow deer (Dama dama): Jennings et al. 2011; African wild dogs (Lycaon pictus): de Villiers et al. 2003; spotted hyenas (Crocuta crocuta): Zabel et al. 1992; Smale et al. 1993; Engh et al. 2000; Assamese macaques (Macaca assamensis): Schülke et al. 2010; chimpanzees: de Waal 1982; Watts 2002; Gilby et al. 2013). When coalitions are formed with kin, indirect benefits are also possible (e.g., spotted hyenas: Wahaj et al. 2004; Smith et al. 2010; savanna baboons: Silk et al. 2004).
Among animals that engage in coalitions, primates are notable for extended juvenile periods (i.e., from the end of weaning to sexual maturity), during which they must learn the skills necessary to forage, maintain relationships with conspecifics, and reproduce within their complex societies (Pereira and Fairbanks 1993; Joffe 1997). Chimpanzees are additionally noteworthy for experiencing adolescence, a life history stage between the juvenile period and adulthood (Goodall 1986). Adolescents are sexually mature (i.e., biologically capable of siring offspring), but have not completed their social or physical development, making them less effective at competing for mating opportunities (Pusey 1990). Coalitions potentially allow individuals that are small, inexperienced, or low ranking to leverage the strength of a conspecific, compensating for their own shortcomings in dyadic competition (e.g., Foster et al. 2009). Thus, adolescent males could presumably benefit from cooperating in coalitions, provided they can obtain willing partners, but little is known about their involvement or the process through which males become invested in coalitions as they mature.
In this paper, our goal is to test whether male coalitions in chimpanzees are more frequent among more or less powerful individuals during the period of maturation from adolescence to prime adulthood, and to test how and to what extent males exercise partner choice when forming coalitions. These are important questions for understanding the evolution of coalitions because they can reveal how competitive ability influences coalitionary involvement in a species of primate known for relatively high levels of competition and cooperation (e.g., Muller and Mitani 2005). Previous studies of other chimpanzee communities (e.g., Gombe (Kasekela): Bygott 1979; Mahale (M group): Hayaki et al. 1989; Ngogo: Sandel et al. 2017) have reported that adolescent males rarely engage in competition with other males and cannot be assigned ranks relative to one another. In our study community, however, adolescent males often formed unidirectional dominance relationships with each other and were clearly involved in male-male competition (see Electronic Supplementary Material). Therefore, the males in our study had obvious reasons for participating in coalitions prior to adulthood, particularly by the second half of adolescence (i.e., ages 12 through 14 years).
Male chimpanzees first express interest in dominating others at a young age. They begin with mostly harmless branchwaving and stick-throwing as juveniles, followed by more serious advances and actual attacks against adult females throughout adolescence (Pusey 1990; Nishida 2003). By the time males reach late adolescence and early adulthood, they start to challenge fully grown adult males, initiating their ascent up the male dominance hierarchy (Goodall 1986; Takahata 1990). During this period of status striving, it could prove especially useful for a male to form coalitions with other individuals to climb the hierarchy. Watts (2018) recently analyzed longitudinal data on coalitions and dominance rank at Ngogo and found that coalitionary participation by young adult male chimpanzees aged 22–24 years was associated with higher peak ranks at later ages. The direction of causality between rank and participation in coalitions remains unclear (Watts 2018), but if coalitions improve rank attainment, then their use prior to adulthood could be particularly advantageous.
Close-aged peers are expected to be relatively easy for adolescent males to secure as coalition partners, due to their familiarity and likelihood of shared social goals (Noë 1994; Mitani et al. 2002). In a captive pack of African wild dogs, coalition formation was strongest between males in the same age cohort, and a cohort of sub-adult males cooperatively challenged an older cohort and successfully took over their dominant rank positions (de Villiers et al. 2003). In bottlenose dolphins (Tursiops aduncus), close adolescent male associates were more likely to persist as alliance (i.e., recurring coalition) partners in adulthood, the more similar they were in age (Gerber et al. 2020). Females are also known to form coalitions with their peers. For example, in Hanuman langurs (Presbytis entellus), immature females cooperatively harassed adult females using revolutionary coalitions, which helped the young females achieve high rank positions by the time they reached menarche (Borries et al. 1991). Adolescent male chimpanzees might likewise be inclined to cooperate with close-aged peers, but such partnerships would lack the overall strength that an adolescent could achieve through a coalition with a fully grown adult male (unless coalitions between peers are highly synergistic; see Bissonnette et al. 2015).
The mature physical and social power of adult male chimpanzees would seem to make them the most effective coalition partners. However, it may be difficult for adolescent males to secure an adult male as a partner (Watts 2015), given adolescents’ inability to contribute substantially to the strength of a coalition (Noë 1994; Noë and Sluijter 1995). Nevertheless, some long-term studies of chimpanzees report instances of adolescent males successfully forming coalitions with adult males that helped them challenge others and rise in rank (Pusey 1983; Goodall 1986; Pusey 1990; Watts and Pusey 1993). Male chimpanzees are known to exchange grooming for agonistic support (Watts 2002), so adolescent males could potentially compensate for their lack of competitive ability, and gain the support of adult males, by grooming them (see Foster et al. (2009) for evidence of smaller alpha male chimpanzees compensating for their shortcomings in body size by allocating more time to grooming for coalitionary support). Because male-male chimpanzee dyads can form strong social bonds that last for many years (e.g., Gilby and Wrangham 2008; Mitani 2009), tolerance of or support given to an adolescent coalition partner could also lead to future benefits for an adult male, if the adolescent will soon mature into a formidable adult.
Given these factors, a critical question for understanding the ontogeny of coalition formation is how changes in competitive ability from adolescence to prime adulthood influence coalition participation and partner selection. Drawing from 12 years of data collected on the Kanyawara community of chimpanzees in Kibale National Park, Uganda, we analyze instances of males forming coalitions against other members of the community. To assess coalition participation, we test the hypothesis (H1) that fully mature males are more likely to form coalitions, building on their competitive strengths. Our alternative hypothesis (H2) is that younger, weaker males may be more likely to form coalitions, compensating for their competitive inadequacies. In addition to considering age and dominance rank as proxies for competitive ability, we consider whether coalition formation is predicted by muscle mass (as assayed by urinary creatinine levels) or testosterone, a steroid hormone that promotes mating effort, including the motivation to dominate others and to avoid being dominated by them (reviewed by Muller 2017).
To assess partner selection, we test between the hypotheses that adolescent males form coalitions that maximize either (H3) coalition strength or (H4) partner similarity (in terms of age and rank). Specifically, we predict that adolescents will primarily form coalitions with adult males according to H3, but with close-aged peers according to H4. We additionally investigate any coalitions that occurred between adolescent and adult males to elucidate whether their formation was driven by the adolescents joining the adults, or the adults joining the adolescents.
Finally, we consider joint vocal displays as an alternative means by which adolescent males could engage in joint behavior with higher-ranking males, but in a non-competitive context. Joint vocal displays, which have been shown to reflect social preferences among male chimpanzees (Mitani and Brandt 1994; Fedurek et al. 2013), are primarily a non-aggressive form of long-distance communication through the production of simultaneous pant-hoot vocalizations by two or more males (Bygott 1979; Ghiglieri 1984; Clark Arcadi 1996; Mitani and Gros-Louis 1998). Chimpanzees are capable of extracting social information from vocal signals when exposed to playbacks of aggressor and victim screams (Slocombe et al. 2010a), and they can modify their vocalizations according to the identities (Slocombe et al. 2010b) and knowledge states (Crockford et al. 2012) of audience members (reviewed by Clay and Zuberbühler 2014). Given these findings, we expect that chimpanzees can infer which individuals are engaging in joint behavior with each other (or at least in close proximity to each other) by attuning to pant-hoot choruses. Joint vocal displays could therefore provide adolescent males with a low-risk opportunity to participate in joint behavior with powerful males and be perceived as associating with those males. Below, we compare our findings for coalitions and joint vocal displays to determine whether males differentially engaged in these alternative types of joint behavior during adolescence.
Methods
Study population
We studied the Kanyawara community of wild chimpanzees (Pan troglodytes schweinfurthii) in Kibale National Park, southwestern Uganda. All members of the community were fully habituated to human presence, due to continuous observation of the population since 1987. We included data from January 2005 through December 2016, incorporating a total of 45,017 observation hours in which at least one male aged 5 through 25 years was present. During the study period, the size of the community ranged from 44 to 54 chimpanzees. Chimpanzees form flexible sub-groups in which varying compositions of individuals temporarily come together for minutes, hours, or days at a time, so the same community members are not always present in the same sub-group, or “party” (Nishida 1968; Sugiyama 1968; Goodall 1986).
In order to examine longitudinal changes in coalition formation, most of our analyses focused on males from adolescence (9 through 14 years, N = 10 males) and young to prime adulthood (15 through 25 years, N = 10). For comparative purposes, some analyses contrast adolescents with all adult males (15+ years; N = 17) or with juvenile males (ages 5 through 8 years, N = 12). Most males transitioned between at least two age categories during the study period (mean years per male: 7.4; range: 2–12). The majority of the juvenile to prime adult males in the study (N = 15) were known from birth, and their ages are estimated to within 1 month. The remaining subjects (N = 3) were infants or juveniles when first identified (ca. 1983–1989) and were fully adult by the time the study period began.
Data collection
Teams of 2–4 long-term Ugandan field assistants and university-based researchers attempted to observe parties of chimpanzees from night-nest to night-nest (i.e., throughout the active period) on a daily basis (it was not possible to record data blind because our study involved focal animals in the field). At 15-min intervals, a field assistant recorded all chimpanzees present within 50 m of one another, which was possible since the observers collected data collaboratively from different viewpoints. Aggression, copulations, and other notable behaviors were recorded using all-occurrence sampling (Altmann 1974). Because chimpanzee agonism is typically loud and conspicuous, it is reasonable to assume that assistants were able to record most occurrences of aggression within the focal party. However, as the identities and actions of aggressors and victims were sometimes obscured by vegetation, individual rates reported here may underestimate true rates. Prior work at Kanyawara established that dyadic rates of aggression recorded during long-term data sampling were highly correlated with those derived from focal data collected by a single observer, suggesting that they provide an unbiased estimate of aggression across the larger party (Muller et al. 2007).
Definition of aggression and coalitionary behavior
We counted any directed charge, chase, or attack as an act of aggression, following definitions provided by Muller (2002). We considered an instance of targeted aggression or a non-vocal display without a clear target to be coalitionary if two or more individuals jointly acted to direct the aggression against at least one victim or to perform the display. Non-vocal displays function to intimidate conspecifics through bouts of exaggerated locomotion and piloerection lasting several seconds to several minutes, accompanied by actions such as slapping the ground, dragging vegetation, and throwing sticks or stones (Muller and Mitani 2005). We combined coalitionary cases of targeted aggression and non-vocal displays into a single sample of coalitions for all analyses except those related to coalition context, which necessarily are limited to coalitions with a clear target. Because our hypotheses are specific to how coalitions function in aggression, we compared these results to parallel analyses on joint vocal displays to distinguish how individuals engaged in joint behavior in an aggressive versus non-aggressive context.
Our unit of analysis was the individual chimpanzee rather than the overall composition of the coalition. Thus, a single coalition could be represented multiple times, once for each member in the target age groups. For example, a coalition between an adolescent and adult male was counted once for the adolescent and once for the adult. It was not always possible to determine the direction of coalition formation (i.e., who joined whom). Consequently, analyses involving directionality relied on a subset of the data in which it was clear who joined whom.
Coalition formation rate analyses (H1 vs. H2)
We calculated frequencies of coalitions formed, and of non-coalitionary aggression given, per male and calendar year. All male-years contained at least 100 observation hours of the male under social contexts in which a coalition was possible (i.e., when at least one potential partner and target were present). We excluded one male (“MX”) from our analyses because he was missing both feet due to snare injuries and was, therefore, often solitary and unusually low ranking for his age, never formally entering the adult male hierarchy. During the 10 years of the study period during which MX was an adolescent or adult, he participated in only 11 total coalitions, 6 of which were with his mother.
To test H1 and H2, we used generalized linear mixed models (GLMMs). In order to account for different opportunities to observe males forming coalitions, we included coalition count as the outcome variable and the log of hours observed as an offset term, in addition to male ID as a random effect. Age, proportional dominance rank (i.e., rank corrected for the number of males in the hierarchy), lean body mass (i.e., creatinine residual), and testosterone (corrected for time of collection) were incorporated as fixed predictors. See Electronic Supplementary Material for detailed methods of how we calculated dominance rank and obtained creatinine and testosterone values from opportunistically collected urine samples. In order to distinguish between developmental changes in overall aggressiveness and, specifically, the propensity to form coalitions, our analyses included annual rates of non-coalitionary aggression for each male as a control. Observation hours specific to this control variable considered the presence of the focal male and at least one potential target. Prior to analysis, we standardized the predictors and control variable using z-scores. For the models, we entered data as 3-month averages (our findings were qualitatively the same whether we analyzed the data in quarterly, biannual, or annual periods).
We used multi-model inference (e.g., Burnham and Anderson 2002; Johnson and Omland 2004; Stephens et al. 2007) to determine which combination of predictors was best able to explain variance in the outcome variable. All candidate models included an offset term of log(hours observed), non-coalitionary aggression rate as a control, and a random effect for male ID. We considered models in order of highest to lowest Akaike weight and rejected any models that occurred outside of a cumulative model weight of 0.95 (Burnham and Anderson 2002; Symonds and Moussalli 2011). We validated models with likelihood ratio tests and checked model assumptions with Q-Q and residual plots. All variance inflation factors (VIFs) were below 4, suggesting that our models were minimally impacted by multicollinearity. Due to data overdispersion, we ran the models with a negative binomial distribution and a log link function.
Coalition partner selection analyses (H3 vs. H4)
To test H3 and H4, we examined the correlation between male age and the mean rank difference between each male and his dyadic coalition partners. We determined rank differences according to relative ordinal ranks on the date that a coalition was formed between two males and calculated the average for each male per calendar year. We additionally compared the distribution of coalition partner types (i.e., male or female; juvenile, adolescent, or adult) across male age classes. For coalitions formed between adolescent and adult males, we determined whether they resulted from the adolescents joining the adults, or the adults joining the adolescents.
Statistical analyses
We performed all statistical analyses in R 3.6.3 (R Core Team 2020). We used the lme4 package to run the models (Bates et al. 2015), the lmtest package to conduct likelihood ratio tests for model comparison (Zeileis and Hothorn 2002), and the MuMIn package to carry out multi-model inference (Barton 2018). All figures were generated with the ggplot2 package (Wickham 2016). Some of our analyses contained sample sizes which were not large enough to run GLMMs, so we utilized correlations instead. Because correlational analyses can encounter issues of pseudoreplication, we visually inspected our data and verified that none of our results were driven by individual males.
Results
Coalition participation
During the 12-year study period, our team recorded 1517 coalitions and 2407 joint vocal displays involving at least one male. Of these, 178 coalitions (11.7%) and 395 joint vocal displays (16.4%) contained an adolescent male. A majority (N = 11/13; 84.6%) of the targeted coalitions formed between early adolescents (9 through 11 years) and other males were directed against females, compared with less than half (N = 12/26; 46.2%) for late adolescents (12 through 14 years). Additionally, 41.8% (N = 23/55) of all coalitions in which early adolescents participated contained a female partner, versus only 10.4% (N = 13/125) for late adolescents. Therefore, in order to keep the focus on coalitions with a direct effect on male ranks (rather than male-female competition), we considered only adolescents at least 12 years old in our analyses of coalition rate.
Coalition formation rate (H1 vs. H2)
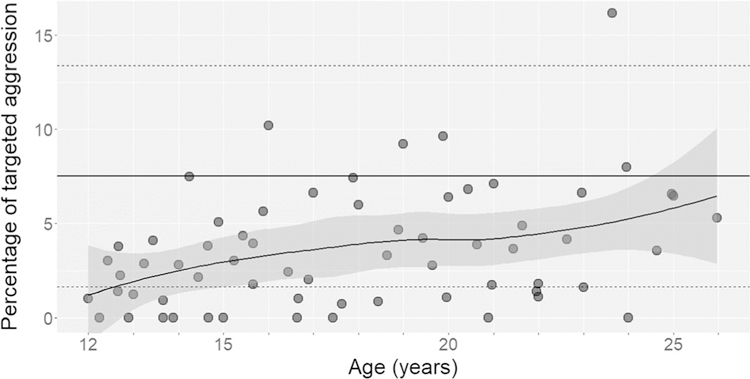
Rates of coalition formation (Fig. 1a) and joint vocal displays (Fig. 1b) increased steadily through late adolescence and young adulthood. Males reached mean adult rates of coalitions at 18–19 years of age and joint vocal displays at 16–17 years. These increases in coalition formation with age were not merely products of increases in overall aggressiveness. The percentage of all targeted aggression that was coalitionary also increased with age, though males did not reach the mean adult percentage until approximately 27 years of age (Fig. 2).
Fig. 1.
Rates of formation of a coalitions and b joint vocal displays. Each point represents an individual male plotted at the midpoint of his age during a calendar year. Each plot contains 64 male-years across 10 males and a LOESS curve fitted to the points (the shaded area indicates the 95% confidence interval). The horizontal, solid lines mark the mean rate of coalition or display formation for all adult males (ages 15 years and older, up to 58 years), and the dashed lines represent one standard deviation above and below the mean
Fig. 2.
The percentage of targeted aggression that was coalitionary rather than non-coalitionary. Each point represents an individual male plotted at the midpoint of his age during a calendar year. The plot contains 64 male-years across 10 males and a LOESS curve fitted to the points (the shaded area indicates the 95% confidence interval). We included male-years during which the male directed at least 10 total instances of coalitionary or non-coalitionary aggression against others. The horizontal lines represent the adult male mean and standard deviations as in Fig. 1
Before running a full multivariate statistical model, we ran four exploratory models with the rate of non-coalitionary aggression as a control, and we found that age, dominance rank, lean body mass, and testosterone each showed a positive correlation with the rate of coalition formation. These results are not surprising, as all of the predictor variables increase during maturation. To disentangle these correlated effects, we then considered all of the predictors together and conducted a multi-model inference procedure. This procedure allowed us to determine which variable, or combination of variables, exerted the strongest influence. We found that the top model contained dominance rank as the sole predictor (Table 1). This model was 2.4 times more likely to be the best approximating model than was the model with the second-greatest weight. Dominance rank appeared in all of the models within the 95% confidence set of cumulative model weight. When we conducted the multi-model inference procedure with joint vocal display rate, rather than coalition rate, as the outcome variable, the results were qualitatively the same.
Table 1.
Results of multi-model inference procedure performed on predictors of targeted coalition formation (N = 136 three-month periods across males). Out of 16 candidate models, the top 6 fell within the 95% confidence set of cumulative model weight and are shown here. The table contains model diagnostics, parameter estimates, and weighted averages for estimates and error based on these models
| Model | 1 | 2 | 3 | 4 | 5 | 6 | ||
|---|---|---|---|---|---|---|---|---|
| Model diagnostics | ||||||||
| df | 5 | 6 | 6 | 6 | 7 | 7 | ||
| ΔAICc | 0 | 1.76 | 2.03 | 2.13 | 3.90 | 3.98 | ||
| Weight | 0.388 | 0.161 | 0.140 | 0.134 | 0.055 | 0.053 | ||
| Cumulative weight | 0.388 | 0.549 | 0.689 | 0.823 | 0.878 | 0.931 | ||
| Predictors | ||||||||
| Age | 0.088 | 0.066 | 0.082 | 0.224 | ||||
| Dominance rank | 0.755 | 0.726 | 0.687 | 0.754 | 0.676 | 0.725 | 0.733 | 0.142 |
| Lean body mass | 0.014 | − 0.006 | 0.008 | 0.058 | ||||
| Testosterone | 0.046 | 0.043 | 0.050 | 0.046 | 0.073 | |||
| Control predictors | ||||||||
| Intercept | − 4.202 | − 4.200 | − 4.203 | − 4.203 | − 4.200 | − 4.200 | − 4.202 | 0.154 |
| Non-coalitionary aggression rate | 0.203 | 0.199 | 0.195 | 0.202 | 0.193 | 0.199 | 0.200 | 0.067 |
Coalition partner selection (H3 vs. H4)
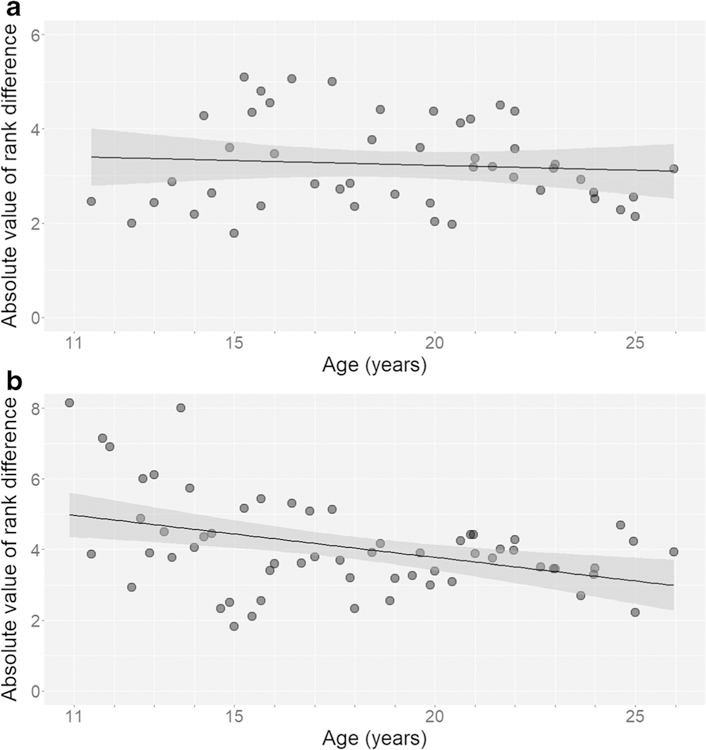
The rank difference between a male and his coalition partners did not change with age across maturation (Pearson’s r = − 0.084, p = 0.571, N = 48 male-years; Fig. 3a). By contrast, as males matured, they participated in joint vocal displays with males that were more similarly ranked to themselves (r = − 0.406, p < 0.01, N = 60; Fig. 3b). We found the same pattern when we used age differences, instead of rank differences, between partners (coalitions: r = 0.209, p = 0.155, N = 48; joint vocal displays: r = − 0.291, p < 0.05, N = 60). Compared with adult males, adolescents engaged in joint behavior with males that were closer in rank to themselves for agonistic coalitions but males that were further ranked from themselves for non-competitive vocal displays.
Fig. 3.
The difference in dominance rank between a male subject and other males with whom he formed dyadic a coalitions and b joint vocal displays. Each point represents an individual male plotted at the midpoint of his age during a calendar year. The coalition plot contains 48 male-years across 9 males, the display plot contains 60 male-years across 10 males, and both plots contain a linear regression line fitted to the points (the shaded area indicates the 95% confidence interval). We included male-years during which the male participated in at least 5 coalitions or displays
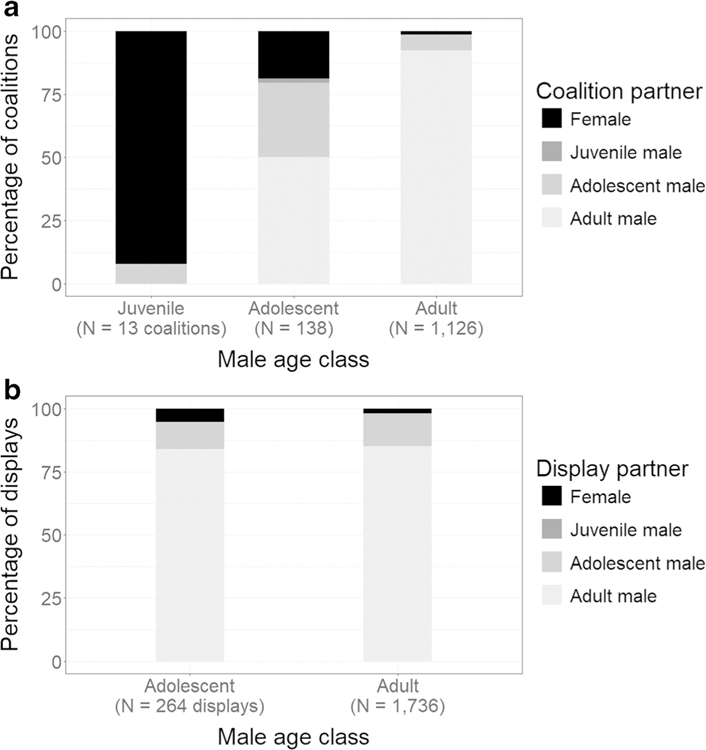
Adolescents and adults formed coalitions and joint vocal displays with adult males at the greatest percentages (Figs. 4a, b, respectively). Adolescents cooperated with each other in coalitions 29.7% of the time (Fig. 4a), as compared with only 10.6% in the context of joint vocal displays (Fig. 4b). In contrast to adolescents and adults, juveniles formed most of their coalitions with females (Fig. 4a). Of the 12 coalitions between a juvenile male and a female, 11 contained a mother-son dyad.
Fig. 4.
Partners of dyadic a coalitions and b joint vocal displays by male age class. We did not include a stacked bar for juvenile male displays due to a comparatively small sample size (N < 10 displays). Additionally, juvenile males participated in coalitions and joint vocal displays infrequently enough that they are not visible as a partner category in most of the stacked bars
Our hypotheses assume that there may be a disparity between the motivation of adolescent males to form coalitions with adult males, and the willingness of those adults to join them. Thus, we examined which party was responsible for forming these coalitions, using the subset of coalitions in which this was clearly observed. As a male’s age increased, so did his likelihood of being joined by an adult male (Pearson’s r = 0.524, p < 0.001, N = 41 male-years; Fig. 5a). Specifically, subjects were more likely to join an adult male partner in a coalition before reaching their early 20s and were subsequently more likely to be joined by an adult male partner after their early 20s, around prime adulthood. The joint vocal display data exhibited the same pattern (r = 0.704, p < 0.0001, N = 56; Fig. 5b). See Electronic Supplementary Material for additional analyses of rank sorting in coalitions, partner availability, potential limitations on successful coalition participation, and coalition context.
Fig. 5.
The percentage of a coalitions and b joint vocal displays in which a male subject participated with an adult male and in which the subject was joined by the adult partner. Each point represents an individual male plotted at the midpoint of his age during a calendar year. The coalition plot contains 41 male- years across 8 males, the display plot contains 56 male-years across 10 males, and both plots contain a linear regression line fitted to the points (the shaded area indicates the 95% confidence interval). We included male-years during which the male engaged in at least 5 coalitions or displays with a clear direction of formation. The horizontal, dashed line at 50% indicates when the individual that joined is equally likely to be the subject or adult male partner
Discussion
While adolescent male chimpanzees potentially have much to gain by forming coalitions with adult males, our data show that adults largely chose each other as coalition partners. This likely explains why adolescents formed coalitions primarily with closely ranked males and utilized non-competitive vocal displays to engage in joint behavior with higher-ranking adults. During the juvenile years, males participated in coalitions at low rates, and most of these coalitions were with their mothers (11/13 coalitions) and in response to aggression received by either the son or mother (10/11 coalitions). As males passed through adolescence and early adulthood, they became increasingly involved in coalitions. This pattern was independent of increases in the overall rate of aggression: it reflected a growing likelihood that any particular aggressive event would involve a coalition. Thus, we found support for the hypothesis (H1) that fully mature males (i.e., prime-aged adults) were more likely to form coalitions than were younger, weaker males (i.e., adolescents). Our findings suggest that adolescent males were generally unable to use coalitions to compensate for their competitive disadvantages, and were constrained by the desire of other males to select the most competitive partners.
While adolescent males were more likely to select adult males than immature males as coalition partners, a more detailed analysis of the actual rank differences between partners revealed that adolescents tended to choose partners of similar rank to themselves rather than the highest-ranking males. This finding is broadly consistent with Pandit and van Schaik’s (2003) model of coalition formation (which focused on leveling coalitions), which predicts that most partners are adjacent in rank. The salience of partner similarity in adolescent male coalition formation may result from the low level of interest shown by adult males in forming coalitions with adolescents. Specifically, most of the coalitions that we observed between adolescent and adult males were formed because of an effort by the adolescent to join the adult rather than the reverse.
We distinguished between coalitions and joint vocal displays in our analyses. This distinction was uninformative for adult males, which tended to engage in both types of joint behavior with similar partners, but produced interesting contrasts for adolescents. Namely, adolescent males participated in more than double the number of joint vocal displays than coalitions throughout the study period, and they joined adult males, and males more distant in rank from themselves, in joint vocal displays more often than in coalitions. These findings suggest that adolescent males used vocal displays in an aspirational way, by engaging in joint behavior with adult males when the potential costs were low. It is also possible that adult males are simply more tolerant when adolescents attempt to join them for non-aggressive joint vocal displays than for aggressive coalitions. Regardless, joint vocal displays appear to be a viable alternative to coalitions for adolescents attempting to integrate themselves into the adult male social world. In this way, adolescents begin to invest in social bonds with powerful males (or at least take advantage of the opportunity to have others associate them with a powerful figure) in a safer setting, thereby “making the best of a bad job” ( Dawkins 1980) by minimizing their competitive disadvantages.
Juvenile males in our study rarely engaged in coalitions, but when they did, it was almost exclusively with their mothers, usually in a retaliatory context (see Electronic Supplementary Material). In bonobos (Pan paniscus), mothers aid their sons in competition against other males, but this support persists into adulthood (Surbeck et al. 2011). There are also extensive data from matrilineal species, such as spotted hyenas (Smale et al. 1993; Engh et al. 2000; East et al. 2009; Smith et al. 2010) and various cercopithecines (Pereira 1989; Chapais et al. 1997, 2001; Silk et al. 2004; Lea et al. 2014; reviewed by Chapais 1992; Langergraber 2012; Bissonnette et al. 2015), showing that juvenile females receive coalitionary support from their mothers and other maternal kin, which helps them with rank acquisition in the female hierarchy. Coalitions between mothers and sons at Kanyawara may help males become experienced with the process of coalition formation early in life. Chapais (1995) proposed that young primates initially gain coalitionary experience through opportunistic bridging interventions. By siding with their mothers in low-cost bridging coalitions (i.e., against targets whom their mothers outrank), juvenile male chimpanzees could become increasingly familiar with the social situations in which coalitions are appropriate and better prepared for status striving in adolescence and early adulthood.
The developmental period is accompanied by rapid changes in lean body mass and testosterone, which affect the ability and motivation to compete individually (reviewed by Muller 2017). While it is difficult to disentangle these effects, we did not find any evidence that males that were relatively large or had relatively high testosterone for their age or rank were more or less likely to form coalitions. The best model for explaining variance in rates of formation of both coalitions and joint vocal displays contained dominance rank as the sole predictor. Likewise, research on male savanna baboons by Bercovitch (1988) and Noë (1989) indicated that rank was the key determinant of coalition formation (reviewed by Noë 1992). Recent work on adult chimpanzees also supports a clear link between dominance rank and participation in coalitions (e.g., Gilby et al. 2013; Hasegawa and Kutsukake 2015; Watts 2018). However, the authors of these studies emphasized that the direction of causality between these variables is not clear. In the same way, the results of our present study cannot address whether an increase in rank led to greater coalition participation in maturing males, or whether increased engagement in coalitions led to the acquisition of higher rank.
Our analysis of alternative coalition partner availability (see Electronic Supplementary Material) revealed that, more often than not, adolescent and adult males had the potential to form a coalition with a male from either age class (i.e., such partners were present in the party). Given this opportunity to exercise choice between potential coalition partners of varying strength, we might expect males to “shop for partners” (Noë 1992) in accordance with biological market mechanisms, in which individuals compete for commodities that cannot be taken by force and are only attainable through the consent of the partner (Noë et al. 1991; Noë and Hammerstein 1994). What could determine the likelihood of a coalition forming between two individuals in such situations? Noë’s (1994) model and Noë and Sluijter’s (1995) observational data of three savanna baboon groups concluded that the central factor in coalition formation is the relative fighting ability of coalition dyads compared with the fighting ability of their target(s). Noë and Sluijter’s (1995) corresponding finding that low-ranking males seldom exhibited coalitionary behavior because of their inability to form effective coalitions is consistent with our results for adolescent males, which participated in coalitions at lower rates than did higher-ranking adult males.
Adolescent males are at a disadvantage in competing for powerful allies, because they have less ability to contribute social power to a cooperative dyad. Even when a variety of possible coalition partners are present in a social party, the partner choices of higher-ranking males and their pre-existing alliances likely limit willingness to form coalitions with adolescents. Seyfarth (1977) presented a model of social grooming among adult female monkeys, which illustrates how competition for grooming partners could result in the highest-ranking females consistently grooming with each other, leaving the rest of the females to sort with similarly ranked partners down the hierarchy. Our analyses demonstrate that, consistent with Seyfarth’s (1977) model, males at Kanyawara sorted with coalition partners on the basis of rank similarity (see Electronic Supplementary Material). Closer inspection of our data further revealed that adolescent males toward the bottom of the hierarchy drove these correlations, suggesting that low-ranking adolescents were especially affected by this process of coalition formation according to rank.
Ultimately, adolescent male chimpanzees are limited in the extent to which they can freely exercise preferences for powerful coalition partners. They appear to compromise by utilizing less competitive situations (e.g., non-aggressive vocal displays) as a means of engaging in joint behavior with adult males, at least until they mature into young adults with sufficient fighting ability to form highly competitive coalitions.
Supplementary Material
Significance statement.
Adolescent males are at a disadvantage in competing for powerful allies, because they have less strength to contribute to a cooperative dyad. Even when a variety of possible coalition partners are present, the partner choices of higher-ranking males likely limit the number of remaining individuals that are available and willing to form coalitions with adolescents. Accordingly, we found that males sorted with closely ranked coalition partners down the hierarchy, leaving adolescents to form coalitions with partners of similar rank to themselves rather than high-ranking adult males. There was no evidence for rank sorting in joint vocal displays, however. Additionally, adolescents participated in more than twice as many joint vocal displays as coalitions. These data suggest that joint vocal displays represent a viable alternative to coalitions for adolescents attempting to integrate themselves into the adult male social world.
Acknowledgments
For collecting field data, we thank Daniel Akaruhanga, Seezi Atwijuze, Fred Baguma, the late John Barwogeza, Richard Karamagi, Christopher Katongole, James Kyomuhendo, Francis Mugurusi, the late Donor Muhangyi, the late Christopher Muruuli, Solomon Musana, Japan Musunguzi, Denis Sebugwawo, John Sunday, Peter Tuhairwe, and Wilberforce Tweheyo. We thank Edgar Mugenyi, Christine Abbe, and Jovia Mahoro for field data entry, Emily Otali for research oversight, and Kamden Cornell for providing laboratory assistance in the assaying of testosterone. For sponsoring long-term research in Kibale National Park, we thank the Uganda Wildlife Authority and Makerere University Biological Field Station. For helpful comments on the manuscript, we thank David Watts and three anonymous reviewers.
Funding information Research was supported by the National Science Foundation Graduate Research Fellowship Grant No. DGE-0237002; National Science Foundation Grants No. 1355014, 9807448, 0416125, and NCS-FO-1926352; the National Institutes of Health (National Institute on Aging/Office of Research on Women’s Health R01AG049395); the Wenner-Gren Foundation; the Leakey Foundation; Harvard University; and the University of New Mexico.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Ethical approval All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures performed in this study were in accordance with the ethical standards of the University of New Mexico Institutional Animal Care and Use Committee (no. 19-200862-MC), the Uganda National Council for Science and Technology, and the Uganda Wildlife Authority.
Data availability The datasets generated and analyzed during the current study are available from the corresponding authors on reasonable request.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00265-020-02872-7) contains supplementary material, which is available to authorized users.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Altmann J (1974) Observational study of behavior: sampling methods. Behaviour 49:227–267 [DOI] [PubMed] [Google Scholar]
- Barton K (2018) MuMIn: multi-model inference. R package version 1.42.1, https://CRAN.R-project.org/package=MuMIn
- Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48 [Google Scholar]
- Bercovitch FB (1988) Coalitions, cooperation and reproductive tactics among adult male baboons. Anim Behav 36:1198–1209 [Google Scholar]
- Bertram BCR (1975) Social factors influencing reproduction in wild lions. J Zool 177:463–482 [Google Scholar]
- Bissonnette A, Bischofberger N, van Schaik CP (2011) Mating skew in Barbary macaque males: the role of female mating synchrony, female behavior, and male-male coalitions. Behav Ecol Sociobiol 65: 167–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonnette A, Perry S, Barrett L, Mitani JC, Flinn M, Gavrilets S, de Waal FBM (2015) Coalitions in theory and reality: a review of pertinent variables and processes. Behaviour 152:1–56 [Google Scholar]
- Borries C, Sommer V, Srivastava A (1991) Dominance, age and reproductive success in free-ranging female Hanuman langurs (Presbytis entellus). Int J Primatol 12:231–257 [Google Scholar]
- Bray J, Pusey AE, Gilby IC (2016) Incomplete control and concessions explain mating skew in male chimpanzees. Proc R Soc B 283: 20162071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York [Google Scholar]
- Bygott JD (1979) Agonistic behavior, dominance, and social structure in wild chimpanzees of the Gombe National Park. In: Hamburg DA, McCown ER (eds) The great apes. Benjamin/Cummings, Menlo Park, pp 405–427 [Google Scholar]
- Bygott JD, Bertram BCR, Hanby JP (1979) Male lions in large coalitions gain reproductive advantages. Nature 282:839–841 [Google Scholar]
- Caro TM (1994) Cheetahs of the Serengeti plains. University of Chicago Press, Chicago [Google Scholar]
- Chapais B (1992) The role of alliances in social inheritance of rank among female primates. In: Harcourt AH, de Waal FBM (eds) Coalitions and alliances in humans and other animals. Oxford University Press, Oxford, pp 29–59 [Google Scholar]
- Chapais B (1995) Alliances as a means of competition in primates: evolutionary, developmental, and cognitive aspects. Yearb Phys Anthropol 38:115–136 [Google Scholar]
- Chapais B, Gauthier C, Prud’Homme J, Vasey P (1997) Relatedness threshold for nepotism in Japanese macaques. Anim Behav 53: 1089–1101 [Google Scholar]
- Chapais B, Savard L, Gauthier C (2001) Kin selection and the distribution of altruism in relation to degree of kinship in Japanese macaques (Macaca fuscata). Behav Ecol Sociobiol 49:493–502 [Google Scholar]
- Clark Arcadi A (1996) Phrase structure of wild chimpanzee pant hoots: patterns of production and interpopulation variability. Am J Primatol 39:159–178 [DOI] [PubMed] [Google Scholar]
- Clay Z, Zuberbühler K (2014) Vocal communication and social awareness in chimpanzees and bonobos: insights from studies of vocal communication. In: Dor D, Knight C, Lewis J (eds) The social origins of language. Oxford University Press, Oxford, pp 141–156 [Google Scholar]
- Connor RC (1992) Dolphin alliances and coalitions. In: Harcourt AH, de Waal FBM (eds) Coalitions and alliances in humans and other animals. Oxford University Press, Oxford, pp 415–443 [Google Scholar]
- Connor RC, Smolker RA, Richards AF (1992) Two levels of alliance formation among male bottlenose dolphins (Tursiops sp.). Proc Natl Acad Sci U S A 89:987–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockford C, Wittig RM, Mundry R, Zuberbühler K (2012) Wild chimpanzees inform ignorant group members of danger. Curr Biol 22: 142–146 [DOI] [PubMed] [Google Scholar]
- Dawkins R (1980) Good strategy or evolutionarily stable strategy. In: Barlow GW, Silverberg J (eds) Sociobiology: beyond nature/nurture. Westview Press, Boulder, pp 331–370 [Google Scholar]
- de Villiers MS, Richardson PRK, van Jaarsveld AS (2003) Patterns of coalition formation and spatial association in a social carnivore, the African wild dog (Lycaon pictus). J Zool 260:377–389 [Google Scholar]
- de Waal FBM (1982) Chimpanzee politics. Harper and Row, New York [Google Scholar]
- Duffy KG, Wrangham RW, Silk JB (2007) Male chimpanzees exchange political support for mating opportunities. Curr Biol 17:R586–R587 [DOI] [PubMed] [Google Scholar]
- East ML, Honer OP, Wachter B, Wilhelm K, Burke T, Hofer H (2009) Maternal effects on offspring social status in spotted hyenas. Behav Ecol 20:478–483 [Google Scholar]
- Engh AL, Esch K, Smale L, Holekamp KE (2000) Mechanisms of maternal rank ‘inheritance’ in the spotted hyaena, Crocuta crocuta. Anim Behav 60:323–332 [DOI] [PubMed] [Google Scholar]
- Fedurek P, Machanda ZP, Schel AM, Slocombe KE (2013) Pant hoot chorusing and social bonds in male chimpanzees. Anim Behav 86: 189–196 [Google Scholar]
- Foster MW, Gilby IC, Murray CM, Johnson A, Wroblewski EE, Pusey AE (2009) Alpha male chimpanzee grooming patterns: implications for dominance “style”. Am J Primatol 71:136–144 [DOI] [PubMed] [Google Scholar]
- Gerber L, Connor RC, King SL, Allen SJ, Wittwer S, Bizzozzero MR, Friedman WR, Kalberer S, Sherwin WB, Wild S, Willems EP, Krützen M (2020) Affiliation history and age similarity predict alliance formation in adult male bottlenose dolphins. Behav Ecol 31: 361–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiglieri MP (1984) The chimpanzees of Kibale Forest: a field study of ecology and social structure. Columbia University Press, New York [Google Scholar]
- Gilby IC, Wrangham RW (2008) Association patterns among wild chimpanzees (Pan troglodytes schweinfurthii) reflect sex differences in cooperation. Behav Ecol Sociobiol 62:1831–1842 [Google Scholar]
- Gilby IC, Brent LJN, Wroblewski EE, Rudicell RS, Hahn BH, Goodall J, Pusey AE (2013) Fitness benefits of coalitionary aggression in male chimpanzees. Behav Ecol Sociobiol 67:373–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall J (1986) The chimpanzees of Gombe: patterns of behavior. Harvard University Press, Cambridge [Google Scholar]
- Harcourt AH, de Waal FBM (1992) Coalitions and alliances in humans and other animals. Oxford University Press, Oxford [Google Scholar]
- Hasegawa M, Kutsukake N (2015) Bayesian competitiveness estimation predicts dominance turnover among wild male chimpanzees. Behav Ecol Sociobiol 69:89–99 [Google Scholar]
- Hayaki H, Huffman MA, Nishida T (1989) Dominance among male chimpanzees in the Mahale Mountains National Park, Tanzania: a preliminary study. Primates 30:187–197 [Google Scholar]
- Jennings DJ, Carlin CM, Hayden TJ, Gammell MP (2011) Third-party intervention behaviour during fallow deer fights: the role of dominance, age, fighting and body size. Anim Behav 81:1217–1222 [Google Scholar]
- Joffe TH (1997) Social pressures have selected for an extended juvenile period in primates. J Hum Evol 32:593–605 [DOI] [PubMed] [Google Scholar]
- Johnson JB, Omland KS (2004) Model selection in ecology and evolution. Trends Ecol Evol 19:101–108 [DOI] [PubMed] [Google Scholar]
- Kuester J, Paul A (1992) Influence of male competition and female mate choice on male mating success in Barbary macaques (Macaca sylvanus). Behaviour 120:192–217 [Google Scholar]
- Langergraber KA (2012) Cooperation among kin. In: Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB (eds) The evolution of primate societies. University of Chicago Press, Chicago, pp 491–513 [Google Scholar]
- Lea AJ, Learn NH, Theus MJ, Altmann J, Alberts SC (2014) Complex sources of variance in female dominance rank in a nepotistic society. Anim Behav 94:87–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani JC (2009) Male chimpanzees form enduring and equitable social bonds. Anim Behav 77:633–640 [Google Scholar]
- Mitani JC, Brandt KL (1994) Social factors influence the acoustic variability in the long-distance calls of male chimpanzees. Ethology 96: 233–252 [Google Scholar]
- Mitani JC, Gros-Louis J (1998) Chorusing and call convergence in chimpanzees: tests of three hypotheses. Behaviour 135:1041–1064 [Google Scholar]
- Mitani JC, Watts DP, Muller MN (2002) Recent developments in the study of wild chimpanzee behavior. Evol Anthropol 11:9–25 [Google Scholar]
- Mitchell CL, Boinski S, van Schaik CP (1991) Competitive regimes and female bonding in two species of squirrel monkeys (Saimiri oerstedi and S. sciureus). Behav Ecol Sociobiol 28:55–60 [Google Scholar]
- Muller MN (2002) Agonistic relations among Kanyawara chimpanzees. In: Boesch C, Hohmann G, Marchant LF (eds) Behavioural diversity in chimpanzees and bonobos. Cambridge University Press, New York, pp 112–124 [Google Scholar]
- Muller MN (2017) Testosterone and reproductive effort in male primates. Horm Behav 91:36–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MN, Mitani JC (2005) Conflict and cooperation in wild chimpanzees. Adv Stud Behav 35:275–331 [Google Scholar]
- Muller MN, Kahlenberg SM, Emery Thompson M, Wrangham RW (2007) Male coercion and the costs of promiscuous mating for female chimpanzees. Proc R Soc B 274:1009–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida T (1968) The social group of wild chimpanzees in the Mahali Mountains. Primates 9:167–224 [Google Scholar]
- Nishida T (2003) Harassment of mature female chimpanzees by young males in the Mahale Mountains. Int J Primatol 24:503–514 [Google Scholar]
- Nishida T, Hosaka K (1996) Coalition strategies among adult male chimpanzees of the Mahale Mountains, Tanzania. In: McGrew WC, Marchant LF, Nishida T (eds) Great ape societies. Cambridge University Press, New York, pp 114–134 [Google Scholar]
- Noë R (1989) Coalition formation among male baboons. PhD thesis, University of Utrecht [Google Scholar]
- Noë R (1992) Alliance formation among male baboons: shopping for profitable partners. In: Harcourt AH, de Waal FBM (eds) Coalitions and alliances in humans and other animals. Oxford University Press, Oxford, pp 233–257 [Google Scholar]
- Noë R (1994) A model of coalition formation among male baboons with fighting ability as the crucial parameter. Anim Behav 47:211–213 [Google Scholar]
- Noë R, Hammerstein P (1994) Biological markets: supply and demand determine the effect of partner choice in cooperation, mutualism and mating. Behav Ecol Sociobiol 35:1–11 [Google Scholar]
- Noë R, Sluijter AA (1995) Which adult male savanna baboons form coalitions? Int J Primatol 16:77–105 [Google Scholar]
- Noë R, van Schaik CP, van Hooff JARAM (1991) The market effect: an explanation for pay-off asymmetries among collaborating animals. Ethology 87:97–118 [Google Scholar]
- Packer C, Pusey AE (1982) Cooperation and competition within coalitions of male lions: kin selection or game theory? Nature 296:740–742 [Google Scholar]
- Packer C, Herbst L, Pusey AE, Bygott JD, Hanby JP, Cairns SJ, Borgerhoff-Mulder M (1988) Reproductive success in lions. In: Clutton-Brock TH (ed) Reproductive success. University of Chicago Press, Chicago, pp 363–383 [Google Scholar]
- Pandit SA, van Schaik CP (2003) A model for leveling coalitions among primate males: toward a theory of egalitarianism. Behav Ecol Sociobiol 55:161–168 [Google Scholar]
- Pereira ME (1989) Agonistic interactions of juvenile savanna baboons. II Agonistic support and rank acquisition. Ethology 80:152–171 [Google Scholar]
- Pereira ME, Fairbanks LA (1993) Juvenile primates: life history, development and behavior. Oxford University Press, Oxford [Google Scholar]
- Perry S (1997) Male-female social relationships in wild white-faced capuchins (Cebus capucinus). Behaviour 134:477–510 [Google Scholar]
- Pusey AE (1983) Mother-offspring relationships in chimpanzees after weaning. Anim Behav 31:363–377 [Google Scholar]
- Pusey AE (1990) Behavioural changes at adolescence in chimpanzees. Behaviour 115:203–246 [Google Scholar]
- R Core Team (2020) R: a language and environment for statistical com. R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/ [Google Scholar]
- Sandel AA, Reddy RB, Mitani JC (2017) Adolescent male chimpanzees do not form a dominance hierarchy with their peers. Primates 58:39–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schülke O, Bhagavatula J, Vigilant L, Ostner J (2010) Social bonds enhance reproductive success in male macaques. Curr Biol 20: 2207–2210 [DOI] [PubMed] [Google Scholar]
- Seyfarth RM (1977) A model of social grooming among adult female monkeys. J Theor Biol 65:671–698 [DOI] [PubMed] [Google Scholar]
- Silk JB, Alberts SC, Altmann J (2004) Patterns of coalition formation by adult female baboons in Amboseli, Kenya. Anim Behav 67:573–582 [Google Scholar]
- Slocombe KE, Kaller T, Call J, Zuberbühler K (2010a) Chimpanzees extract social information from agonistic screams. PLoS One 5:e11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slocombe KE, Kaller T, Turman L, Townsend SW, Papworth S, Squibbs P, Zuberbühler K (2010b) Production of food-associated calls in wild male chimpanzees is dependent on the composition of the audience. Behav Ecol Sociobiol 64:1959–1966 [Google Scholar]
- Smale L, Frank LG, Holekamp KE (1993) Ontogeny of dominance in free-living spotted hyaenas: juvenile rank relations with adult females and immigrant males. Anim Behav 46:467–477 [Google Scholar]
- Smith JE, van Horn RC, Powning KS, Cole AR, Graham KE, Memenis SK, Holekamp KE (2010) Evolutionary forces favoring intragroup coalitions among spotted hyenas and other animals. Behav Ecol 21: 284–303 [Google Scholar]
- Stephens PA, Buskirk SW, Martínez del Rio C (2007) Inference in ecology and evolution. Trends Ecol Evol 22:192–197 [DOI] [PubMed] [Google Scholar]
- Sugiyama Y (1968) Social organization of chimpanzees in the Budongo Forest, Uganda. Primates 9:225–258 [Google Scholar]
- Surbeck M, Mundry R, Hohmann G (2011) Mothers matter! Maternal support, dominance status and mating success in male bonobos (Pan paniscus). Proc R Soc B 278:590–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds MRE, Moussalli A (2011) A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav Ecol Sociobiol 65:13–21 [Google Scholar]
- Takahata Y (1990) Social relationships among adult males. In: Nishida T (ed) The chimpanzees of the Mahale Mountains. University of Tokyo Press, Tokyo, pp 149–170 [Google Scholar]
- Vogel ER, Munch SB, Janson CH (2007) Understanding escalated aggression over food resources in white-faced capuchin monkeys. Anim Behav 74:71–80 [Google Scholar]
- Wahaj SA, van Horn RC, van Horn TL, Dreyer R, Hilgris R, Schwarz J, Holekamp KE (2004) Kin discrimination in the spotted hyena (Crocuta crocuta): nepotism among siblings. Behav Ecol Sociobiol 56:237–247 [Google Scholar]
- Watts DP (1998) Coalitionary mate guarding by male chimpanzees at Ngogo, Kibale National Park, Uganda. Behav Ecol Sociobiol 44: 43–55 [Google Scholar]
- Watts DP (2002) Reciprocity and interchange in the social relationships of wild male chimpanzees. Behaviour 139:343–370 [Google Scholar]
- Watts DP (2015) Mating behavior of adolescent male chimpanzees (Pan troglodytes) at Ngogo, Kibale National Park, Uganda. Primates 56: 163–172 [DOI] [PubMed] [Google Scholar]
- Watts DP (2018) Male dominance relationships in an extremely large chimpanzee community at Ngogo, Kibale National Park, Uganda. Behaviour 165:969–1009 [Google Scholar]
- Watts DP, Pusey AE (1993) Behavior of juvenile and adolescent great apes. In: Pereira ME, Fairbanks LF (eds) Juvenile primates: life history, development and behavior. Oxford University Press, New York, pp 148–167 [Google Scholar]
- Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, New York [Google Scholar]
- Zabel CJ, Glickman SE, Frank LG, Woodmansee KB, Keppel G (1992) Coalition formation in a colony of prepubertal spotted hyenas. In: Harcourt AH, de Waal FBM (eds) Coalitions and alliances in humans and other animals. Oxford University Press, Oxford, pp 112–135 [Google Scholar]
- Zeileis A, Hothorn T (2002) Diagnostic checking in regression relationships. R News 2:7–10. https://CRAN.R-project.org/doc/Rnews/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.