Abstract
Axonal damage has been highlighted recently as a cause of neurological disability in various demyelinating diseases, including multiple sclerosis, either as a primary pathological change or secondary due to myelin loss. To characterize and quantify axonal damage and loss in canine distemper demyelinating leukoencephalomyelitis (DL), formalin‐fixed paraffin‐embedded cerebella were investigated histochemically and immunohistochemically using the modified Bielschowsky's silver stain as well as antibodies against nonphosphorylated (n‐NF), phosphorylated neurofilament (p‐NF) and β‐amyloid precursor protein (β‐APP). Injured axons characterized by immunoreactivity against n‐NF and β‐APP were detected in early distemper lesions without demyelination. In subacute and chronic demyelinating lesions the number of injured axons increased. Moreover, a significant decrease in axonal density was observed within lesions and in the normal appearing white matter in DL as determined by morphometric analyses using Bielschowsky's silver stain and p‐NF immunohistochemistry. Summarized, the observed findings indicate that axonal damage (i) occurs early in DL; (ii) can be detected before myelin loss; and (iii) represents a pivotal feature in advanced lesions. It must be postulated that axonal damage plays an important role in the initial phase as a primary event and during progression of nervous distemper as a result of demyelination.
Keywords: axonal pathology, β‐APP, canine distemper virus
INTRODUCTION
Infection with canine distemper virus (CDV), a single‐stranded, negative‐sense morbillivirus of the family Paramyxoviridae, often results in lesions of the central nervous system (CNS; 3). Though both gray and white matter changes can be observed in distemper, demyelinating leukoencephalomyelitis (DL) represents the main sequel in dogs 5, 42. Interestingly, the early phase of CNS manifestation is characterized by an infection of the gray matter followed by virus spread to the white matter in most cases (5). Due to the morphological similarities between the neuropathological changes in DL and Multiple Sclerosis (MS), nervous distemper advanced to an important spontaneously occurring animal model of human demyelinating diseases 3, 11, 49. Types of plaques in canine distemper are distinguished according to their morphological appearance and are presumed to be related to the age of the lesion 5, 18, 41, 51. Demyelination in DL is supposed to be a biphasic process. Initiation of demyelination has been ascribed to a direct action of the virus, infiltrating CD8‐positive cytotoxic T cells and an upregulation of proinflammatory cytokines 4, 17, 20. In contrast, plaque progression seems to be an immunopathologic process associated with a decrease or an elimination of virus antigen within lesions, strong upregulation of major histocompatibility complex (MHC) class II and an infiltration of CD4‐ and CD8‐positive lymphocytes, plasma cells and macrophages 3, 42. Although the hallmark of this process is demyelination, destruction of axons has also been observed ultrastructurally (22).
The key contributing role of axonal damage in both the gray and white matters in demyelination disorders other than DL has been emphasized in previous studies 6, 28, 32, 47. Axonal damage has recently attracted attention, because it may be the major pathological correlate of permanent functional deficits 13, 26, 43. However, pathogenesis and mechanisms for axonal damage remain unclear and are still discussed controversially and include neurotoxic mediators, MHC‐I‐dependent cytotoxicitiy, antibody‐mediated mechanisms, cytokines and reactive oxygen species 9, 36, 37, 43.
Common immunohistochemical markers for axonal pathology consist of phosphorylated and nonphosphorylated neurofilaments (p‐NF and n‐NF) and β‐amyloid precursor protein (β‐APP). Following pathological injuries, the expression of p‐NF decreases and n‐NF represents the dominating isoform 24, 34, 47. β‐APP is a highly sensitive marker for early axonal damage 7, 16, 26, 27 that accumulates due to turbulences of the fast axonal transport, thus indicating functional changes of damaged axons.
Both β‐APP and n‐NF expression were observed in dystrophic axons in acute and chronic MS lesions as well as in traumatic brain injury, Alzheimer's disease and amyotrophic lateral sclerosis 6, 14, 16, 26, 27, 38, 39, 43. Common animal models of MS‐like experimental allergic encephalomyelitis (EAE) in mice or non‐human primates or infection of mice with Theiler's murine encephalomyelitis virus (TMEV) also displayed loss of axonal density and morphological alterations of axonal structures 34, 45, 47, 48, 50.
However, it is still unknown whether the same pathogenetic mechanisms apply for myelin loss and axonal damage. Moreover, it is still a matter of debate whether demyelination is a prerequisite for axonal injury or axonal damage precedes myelin loss or whether both represent independent processes in certain disease entities. To further investigate the pathogenesis of axonal injury and its association to myelin loss and to dissect the temporal‐spatial relationship of both processes in DL, a detailed histochemical and immunohistochemial study was performed.
MATERIALS AND METHODS
Animals, histology, and neuropathological classification
The cerebella of four CDV‐negative, healthy control dogs (group 1) and 17 dogs with spontaneously occurring and immunohistochemically confirmed DL (groups 2–8) were examined. The dogs with distemper represented submissions to the routine diagnostic service of the Department of Pathology, University of Veterinary Medicine, Hannover, Germany. Animals died spontaneously or were humanely killed. During necropsy, tissues including CNS were collected and fixed in 10% non‐buffered formalin, embedded in paraffin wax and serial sections, 3 µm thick, were prepared. Sections for histochemistry and immunohistochemistry were mounted on SuperFrost‐Plus slides (Menzel Gläser, Braunschweig, Germany). Neuropathological diagnoses were done on hematoxylin and eosin (HE)‐ and luxol fast blue–cresyl echt violet (LFB–CV)‐stained sections. In addition, an impregnation with a modified Bielschowsky's silver stain was performed (10). Moreover, detection of CDV‐nucleoprotein (NP) by immunohistochemistry was carried out as described below (18). DL white matter lesions were classified into different types as described 18, 20, 21, 41. Group 2 areas represent normal appearing white matter (NAWM) regions of the cerebellum of infected dogs. These areas did not show pathological alterations in the HE stain and lacked CDV antigen. Group 3 consisted of areas lacking lesions in HE‐stained slides and contained CDV antigen‐positive cells. Lesions, which showed mild white matter vacuolation without gliosis but detectable CDV antigen, were placed in group 4. Group 5 acute lesions consisted of focal vacuolation, mild gliosis with activated astrocytes and microglia and CDV‐NP‐positive cells. No loss of myelin was found in the white matter areas and lesions of groups 1–5. Group 6 plaques included subacute lesions without inflammation, characterized by demyelination, moderate gliosis with gemistocytes and macrophages/microglia and CDV‐NP‐positive cells. Group 7 lesions were composed of subacute inflammatory changes, similar to group 6; however, additionally perivascular mononuclear infiltrations up to two or three layers of thickness were present. Group 8 plaques consisted of chronic lesions, similar to group 7 lesions; however, a more prominent perivascular infiltration with more than three layers of monocytic inflammatory cells was seen. Cytoplasmic and intranuclear inclusion bodies could be frequently found in acute and subacute lesions. Several plaque types may occur simultaneously in the brain of one individual.
Immunohistochemistry and determination of axon density
In order to detect cellular and viral antigens, a standard avidin‐biotin‐peroxidase complex (ABC; Vector Laboratories, Burlingame, CA, USA) method was used as described 1, 41. Briefly, sections were dewaxed and hydrated through graded alcohols. Endogenous peroxidase activity was quenched by methanol with 0.5% H2O2. Sections were incubated with a CDV‐specific monoclonal antibody (10H3, kindly provided by L. Haas, Department of Virology, University of Veterinary Medicine, Hannover, Germany). Additionally, p‐NF‐ and n‐NF‐ (SMI 312 and SMI 311; Sternberger Monoclonals, Lutherville, MD, USA) as well as β‐APP‐ (MAB348; Chemicon International Inc., Temecula, CA, USA) specific antibodies were used. Control sections were incubated with isotype‐matched control antibodies or with non‐immune sera. Brain tissue of an adult dog as well as the cerebellum of a distemper dog served as positive controls for the various antigens. Positive antigen‐antibody reactions were visualized by incubation with 3,3′‐diaminobenzidine tetrahydrochloride (DAB) with 0.03% H2O2, pH 7.2 for 5 minutes followed by slight counterstaining with Mayer's hemalaun.
The obtained brown signal following incubation with n‐NF and β‐APP‐specific antibodies was evaluated quantitatively by counting the number of positive axons using a morphometric grid (number of immunoreactive axons/mm2). According to the individual size of the lesioned areas, the total area of white matter lesions or, in cases of large lesions, a maximum of 15 randomly distributed high power fields of 0.16 mm2 in size within the lesion were counted. For each animal, five NAWM areas were analyzed as well. The percentage of p‐NF‐ or Bielschowsky's silver stain‐positive axons in white matter areas (lesions and NAWM) was evaluated by digitalizing the cerebellar section with a color video camera (Color View II, 3.3 Megapixel CCD; Soft Imaging System, Münster, Germany) mounted on an Axiophot microscope (Zeiss, Oberkochen, Germany) with a 5× objective. The positive structures were measured interactively after manually outlining the total white matter area using the analysis 3.1 software package (Soft Imaging System). Data are presented as percentage of labeled axonal profiles in relation to the total lesioned area. Because of the generally lower density of axons in the deeper and periventricular white matter compared to the cerebellar lamellae, the same number of these different types of white matter was chosen in infected and control animals.
Combination of immunohistochemistry and histochemistry to identify axonal lesions
Selected sections were investigated for the detection of LFB‐positive white matter areas and β‐APP‐positive axons or Bielschowsky's silver stain‐positive structures, respectively. After visualization of β‐APP immunoreactivity by using DAB or 3‐amino‐9‐ethylcarbazole, sections remained in phosphate‐buffered saline followed by LFB–CV or Bielschowsky's silver stain, respectively, as previously described (10). Colocalization of β‐APP (brown/black) and LFB‐positive white matter (blue) as well as β‐APP (red) and Bielschowsky's silver stain‐positive structures (brown) was identified by the presence of a mixed discoloration or the adjacent occurrence of the different precipitates in the same or neighboring cellular compartment.
Statistical analysis
Data obtained by the Bielschowsky's silver stain and immunohistochemistry were subjected to statistical analysis using the program “SPSS” for Windows (SPSS Inc., Chicago, IL, USA), version 13.0 employing the Mann–Whitney U‐test as group‐wise test. A P value of less than 0.05 was considered to be statistically significant. The correlation coefficient to compare p‐NF immunohistochemistry and Bielschowsky's silver stain as well as p‐NF and n‐NF immunohistochemistry was calculated by using the non‐parametric Spearman's correlation test.
RESULTS
Neuropathological findings
The brain tissue from the four healthy control dogs lacked significant pathological alterations (group 1, n = 20). A total of 210 white matter areas, including NAWM (group 2, n = 85), from 17 dogs with CDV infection were analyzed. The lesions were classified into groups 3–8 (non‐visible white matter lesions, group 3, n = 27; vacuolization, group 4, n = 20; acute lesions, group 5, n = 16; subacute non‐inflammatory areas, group 6, n = 30; subacute lesions with inflammation, group 6, n = 21; and chronic plaques, group 8, n = 11). The LFB–CV stain revealed mild to marked demyelination of the lesions of groups 6–8 (subacute with and without inflammation and chronic lesions). Only very few axonal spheroids could be detected with the HE stain in subacute inflammatory and chronic lesions (groups 7 and 8).
Axon pathology
In control animals, n‐NF‐specific antibodies revealed a positive signal in the perinuclear region of gray matter neurons only. Axons as well as other cellular processes and cell types were negative for n‐NF. The perinuclear neuronal expression of n‐NF remained unchanged in CDV‐positive animals, whereas intralesionally, round to oval, n‐NF‐positive axonal transversal sections with a diameter of 3–30 µm were found to a varying degree (Figure 1). Significant differences between controls (group 1) and NAWM (group 2) and groups 4–8, as well as between early (groups 3 and 4; median value 0) and advanced lesions (groups 6–8; median values for groups 6, 7 and 8: 13.0, 10.4 and 15.6, respectively) were observed (Figure 2A). Additionally, cross‐sections of some p‐NF‐positive axons showed a marked enlargement of their diameter (up to 50 µm) in advanced lesions of groups 6–8.
Figure 1.

Detection of n‐NF‐positive axons in cerebellar white matter distemper lesions. Subacute inflammatory lesion (group 7) with multifocal intralesional nonphosphorylated (n‐NF)‐positive axons (arrows; brown: n‐NF, avidin‐biotin‐peroxidase complex method, 3,3′‐diaminobenzidine tetrahydrochloride). Bar = 25 µm.
Figure 2.

(A–D) Detection of nonphosphorylated (n‐NF)‐positive axons (A), β‐amyloid precursor protein (β‐APP)‐positive axons (B), Bielschowsky's silver stain‐positive areas (C) and phosphorylated neurofilament (p‐NF)‐positive areas (D) in the white matter. A. Detection of n‐NF‐positive axons in the white matter of controls (group 1) and distemper dogs (groups 2–8). A significant difference between groups 4–8 compared to group 1 (P < 0.05) as detected by the Mann–Whitney groupwise testing is marked with an asterisk. B. Detection of β‐APP‐positive axons in the white matter of controls (group 1) and distemper dogs (groups 2–8). A significant difference between groups 3–8 and group 1 (P < 0.05) as detected by the Mann–Whitney groupwise testing is marked with an asterisk. C. Detection of Bielschowsky's silver stain‐positive areas in controls (group 1) and distemper dogs (groups 2–8). A significant difference between group 2 and groups 4–8 compared to group 1 (P < 0.05) as detected by the Mann–Whitney groupwise testing is marked with an asterisk. D. Detection of p‐NF‐positive areas in controls (group 1) and distemper dogs (group 2–8). A significant difference between group 2 and groups 5–8 compared to group 1 (P < 0.05) as detected by the Mann–Whitney groupwise testing is marked with an asterisk. All box‐and‐whisker plots show median value, quartiles, minimum and maximum of positive signals. Small circle: extreme value within fourth quartile; small asterisk: outlier.
β‐APP expression characterized by a finely granular cytoplasmic reaction was detected in neurons in the gray matter in the cerebellum of controls. However, there was no expression in the adjacent or distant white matter. In distemper dogs, groups 3 (detection of antigen without visible lesion) to 5 (acute lesions) lesions revealed the presence of small, β‐APP‐positive, dot‐like to ovoid axonal structures in transversal and longitudinal sections. The neuronal expression was similar to controls (Figure 3A,B). The highest expression of β‐APP‐positive axons was found in advanced lesions of groups 6 and 7 (median value 7.6 and 23.4, respectively). Significant differences between controls (group 1, median value 0) and NAWM (group 2, median value 0) and groups 3–8 (median value groups 3, 4, 5 and 8: 0, 6.3, 5.3 and 3.1, respectively) were detected (Figure 2B). Using the combination of β‐APP immunohistochemistry and LFB stain, β‐APP‐positive axons could be shown in areas of complete myelination in groups 3–5 (Figure 3E). The number of β‐APP‐positive axons showed a fourfold increase in advanced lesions with demyelination compared to acute lesions (Figure 3F).
Figure 3.

Detection of β‐amyloid precursor protein (β‐APP)‐positive axons (A,B) in cerebellar white matter distemper lesions without (C,E) or with demyelination (D,F). A. Area of antigen detection without visible lesion (group 3) with single β‐APP‐positive axons in the cerebellar white matter [arrows; brown: β‐APP, avidin‐biotin‐peroxidase complex (ABC) method, 3,3′‐diaminobenzidine tetrahydrochloride (DAB)]. B. Subacute non‐inflammatory lesion (group 6) with numerous β‐APP‐positive axons in the cerebellar white matter (arrows; brown: β‐APP, ABC method, DAB). C. Serial section of A. Lesion of group 3 with complete myelination [asterisk; luxol fast blue (LFB) stain]. D. Serial section of B. Subacute non‐inflammatory lesion (group 6) with moderate to severe demyelination in the cerebellar white matter (asterisk; LFB stain). E. Serial section of A and C. Lesion of group 3 with single, black‐colored, β‐APP‐positive axons (arrows) within an area of complete myelination (asterisk; black: β‐APP, ABC method, DAB in combination with LFB stain). F. Serial section of B and D. Subacute non‐inflammatory lesion (group 6) with numerous, black‐colored, β‐APP‐positive axons (arrows) in an area with moderate to severe demyelination (asterisk; black: β‐APP, ABC method, DAB in combination with LFB stain). A,B, E,F. Bar = 25 µm. C,D. Bar = 50 µm.
In order to further identify the cellular localization of β‐APP immunoreactivity, a simultaneous staining with the Bielschowsky's silver method has been performed and revealed a colocalization of the red β‐APP‐positive signal with argyrophilic axons (Figure 4).
Figure 4.
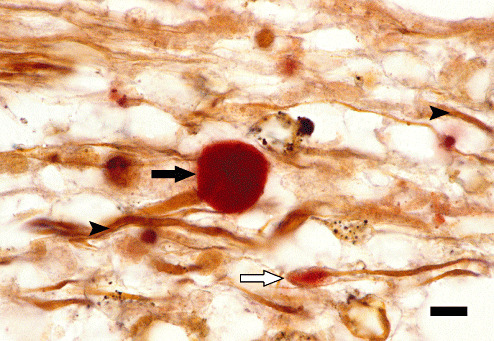
Colocalization of β‐amyloid precursor protein (β‐APP) and argyrophilic axonal structures. Subacute non‐inflammatory distemper lesion (group 6) in the cerebellar white matter with colocalization of a β‐APP‐positive (black arrow; red; avidin‐biotin‐peroxidase complex method, 3‐amino‐9‐ethylcarbazole) and argyrophilic axonal structures (arrowheads; Bielschowsky's silver stain). Note moderately enlarged axon with β‐APP‐positive axonal bulb (white arrow). Bar = 13 µm.
All control dogs (group 1) and NAWM areas of distemper dogs lacked CDV‐NP expression, whereas NP antigen was demonstrated in groups 3–8 lesions in distemper dogs in varying degrees. The strongest signal was detected in fibrous astrocytes of subacute white matter lesions (groups 6 and 7), whereas chronic plaques of group 8 showed a moderate decrease of CDV‐positive cells with almost complete elimination of virus antigen within the center of the lesions. CDV‐NP antigen could not be detected in axonal structures in any type of lesion.
Axonal density
Bielschowsky's silver stain revealed a dark brown to black coloration of densely packed, filamentous, axonal structures, 1–2 µm in diameter with a homogenous thickness in the white matter of control animals with a median value of 82.0% Bielschowsky's silver stain‐positive area per total white matter region. A mild significant reduction of the axonal density was detected in NAWM areas and early lesions of distemper dogs (groups 2–5) within a range of median values from 73.2% to 69.3%. Advanced distemper lesions (groups 6–8) showed a moderate to severe significant reduction of the axonal density. The subacute lesions without and with inflammation (groups 6 and 7) exhibited significantly reduced Bielschowsky's silver stain‐positive areas of 42.7% and 52.6%, respectively. Chronic plaques showed a markedly reduced axonal density with a value of 29.2% (Figure 5A–D).
Figure 5.
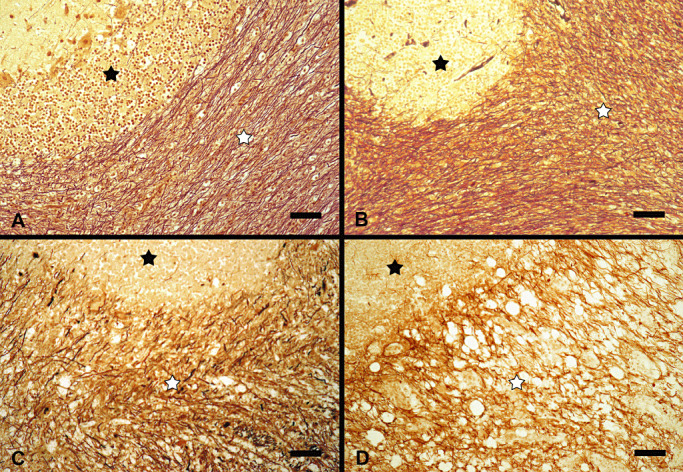
(A–D) Axonal density in controls (A) and distemper dogs (B–D) in the cerebellar white matter. A. Cerebellar white matter region (white asterisk) in a control dog (group 1) with normal axonal density. B. Normal appearing white matter region of a distemper dog (white asterisk, group 2) with mild decrease in axonal density. C. Acute white matter lesion in a distemper dog (group 5, white asterisk) with a mild to moderate decrease in axonal density. D. Chronic distemper lesion (group 8, white asterisk) with a severe decrease in axonal density. A–D. black asterisk: Stratum granulosum of cerebellar cortex. Bielschowsky's silver stain. Bars = 50 µm.
A similar observation was made for p‐NF. A significant reduction of the percentage of p‐NF‐positive axonal structures from 79.7% in controls (group 1) to 25.7% in chronic lesions in group 8 was noted.
Statistical analysis of Bielschowsky's silver stain and p‐NF immunohistochemistry showed a decreased axonal density in groups 5–8 compared to the control group (group 1; Figure 2C,D). Additionally, a significant difference between the control group and NAWM (group 2) was detected. Spearman's correlation coefficient showed a moderate correlation between Bielschowsky's silver stain values and p‐NF immunoreactivity of 0.596 (P < 0.01). A moderately inverse correlation was found between values of p‐NF and n‐NF immunoreactivity (−0.456; P < 0.01).
DISCUSSION
The aim of this study was to investigate the spatiotemporal occurrence of axonal damage in demyelinating distemper leukoencephalomyelitis (DL), a spontaneously occurring animal model for MS. The question was raised whether axonal damage triggers myelin loss or represents a secondary process. The present investigation revealed for the first time the occurrence of axonal pathology in early still myelinated and chronic plaques with myelin loss. In addition, a decreased axonal density during DL was noticed. The reduced density in the NAWM and different lesion types in MS patients was thought to be due to a diffuse axonopathy or a Wallerian‐like degeneration of axons, representing a potential early change that might trigger myelin loss 6, 15, 33. In a localized EAE rat model, marked axonal loss was detected in early as well as in late disease stages (25). In the present study, an immunohistochemical marker for axonal integrity and density, p‐NF, confirmed the results obtained by the Bielschowsky's silver stain and, in addition, a positive statistical correlation between both methods was demonstrated. The segmental increase in size of single p‐NF‐positive axonal diameters, as seen in the present study, was also detected in humans with cerebral infarction or traumatic spinal cord injury (8). In addition to “empty” myelin sheaths without p‐NF immunoreactivity in the NAWM, SJL/J mice, infected with the Daniel's strain of TMEV (DA), showed a reduction of p‐NF‐positive axons during the chronic demyelinating phase (47) similar to the chronic distemper lesions in the present study.
Loss of the myelin sheath is known to mobilize the stationary pool of p‐NF to the more easily degradable moving pool of n‐NF (29). Accordingly, n‐NF‐positive axons showed a significant increase in distemper groups 4–8 compared to controls and NAWM (groups 1 and 2). Similar findings were observed in active and chronic‐active MS lesions (43). It was assumed that de‐ or dysmyelination caused a decrease in neurofilament phosphorylation and an increase in n‐NFs, which might explain the occurrence of n‐NF‐positive axons in acute distemper lesion and progression of accumulation of affected axons in subacute and chronic lesions in the present study. The detection of destroyed axons by n‐NF‐immunohistochemistry was also possible in mice infected with mouse hepatitis virus as early as 10 days postinfection prior to the onset of demyelination (12). Similarly, TMEV‐infected SJL/J mice possessed n‐NF‐positive axons as early as 7 days postinfection in the NAWM of the ventral and lateral funiculi of the spinal cord, regions in which subsequently demyelination occurred (47). The increase of n‐NF‐positive axons often correlates with a decrease in p‐NF immunoreactivity. Likewise, a negative correlation between values of p‐NF and n‐NF immunoreactivity was observed in the present study.
Following axonal transection there is a disruption of the axonal transport and β‐APP accumulates in the proximal axonal ends (30). Interestingly, β‐APP‐positive axonal spheroids are formed and persist for less than 30 days 27, 30. In the present study, β‐APP‐positive axons were already found in areas expressing CDV antigen and still lacking visible lesions. However, the amount of β‐APP‐positive axons reached its maximum in subacute lesions with myelin loss. Similarly, β‐APP‐positive axons can be detected in acute and to a lesser extent at the periphery of chronic MS lesions 16, 26, 27. β‐APP was found also in T cells, gitter cells, activated microglia and reactive astrocytes in MS patients (19). In contrast, a colocalization of glial fibrillary acidic protein or the lectin Bandeiraea simplicifolia as a marker for macrophages/microglia with β‐APP could not be noticed in distemper dogs of the present study (data not shown).
Whether axonal damage in early distemper lesions is directly virus‐induced as suspected in some animal models of MS‐like TMEV infection (44) remains to be investigated. Though CDV antigen expression in neurons seems to be an early regular event in nervous distemper, CDV‐NP antigen was not detected in axons in the present study. Similar results were obtained in TMEV‐infected mice in which viral antigen showed neither a colocalization with p‐NF‐ nor n‐NF‐positive axons (47). In contrast to acute distemper lesions, in advanced DL lesions, immunophenotypical changes are indicative of a delayed‐type hypersensitivity reaction with reduced amounts or absence of virus antigen (51). Thus the pathogenetic hypothesis that axonopathy represents a “self‐destructive” program to prevent axonal spread of a virus (44) seems to be less applicable for canine distemper. However, the viral antigen‐induced inflammatory response may create an axon‐hostile microenvironment that includes pro‐inflammatory cytokines, reactive oxygen species and cytotoxic CD8‐positive cells as well as an accumulation of glutamate and Ca2+, which may cause axonal injury 2, 23, 40. In MS, factors contributing to the progression of chronic demyelinating lesions include adenosine‐triphosphate (ATP) depletion (35) and ionic imbalances (31).
Early axonal damage with subsequent secondary demyelination could be interpreted as an inside to outside lesion development (46). Additionally, in advanced DL lesions, primary demyelination of initially preserved axons may be caused by a “bystander” mechanism or by immunopathological processes as an outside to inside lesion. Subsequently, accumulation of primarily and secondarily damaged axons in subacute and chronic DL lesions would explain the high amount of n‐NF‐ and β‐APP‐positive axons.
Summarized, the provided data indicated that axonal damage occurred early in canine distemper DL and could be detected before myelin loss appeared. Using immunohistochemistry, β‐APP seemed to be the most sensitive protein for early detection of axonal damage in DL. A directly or indirectly virus‐induced primary axonal damage in early distemper lesions without myelin loss seems to represent the most likely pathogenetic mechanism. In addition, demyelination as well as primary and secondary axonal damage seems to occur simultaneously in late distemper lesions. A similar mechanism was proposed for axonal injury in Theiler's murine encephalomyelitis 44, 46. Based on the present findings the paradigm of canine distemper leukoencephalitis as an only primary demyelinating disorder should be reconsidered. It seems that both primary axonopathies and primary demyelination play an important contributing role during nervous distemper. Furthermore, it can be concluded that axonal damage represents a key event in the initial phase and during the progression of canine distemper DL.
ACKNOWLEDGMENTS
The authors wish to thank Petra Grünig for excellent technical assistance.
Dr. Frauke Seehusen received a scholarship from Bayer HealthCare, Division Animal Health, Leverkusen, Germany.
REFERENCES
- 1. Alldinger S, Baumgärtner W, Örvell C (1993) Restricted expression of viral surface proteins in canine distemper encephalitis. Acta Neuropathol 85:635–645. [DOI] [PubMed] [Google Scholar]
- 2. Babbe H, Roers A, Waisman A, Lassmann H, Goebels N, Hohlfeld R et al (2000) Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med 192:393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baumgärtner W, Alldinger S (2005) The pathogenesis of canine distemper virus induced demyelination—a biphasic process. In: Experimental Models of Multiple Sclerosis. Lavi E, Constantinescu CS (eds), pp. 871–887. Springer: New York. [Google Scholar]
- 4. Beineke A, Markus S, Borlak J, Thum T, Baumgärtner W (2008) Increase of pro‐inflammatory cytokine expression in non‐demyelinating early cerebral lesions in nervous canine distemper. Viral Immunol 21:401–410. [DOI] [PubMed] [Google Scholar]
- 5. Beineke A, Puff C, Seehusen F, Baumgärtner W (2009) Pathogenesis and immunopathology of systemic and nervous canine distemper. Vet Immunol Immunopathol 127:1–18. [DOI] [PubMed] [Google Scholar]
- 6. Bitsch A, Schuchardt J, Bunkowski S, Kuhlmann T, Brück W (2000) Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain 123:1174–1183. [DOI] [PubMed] [Google Scholar]
- 7. Brück W (2005) Inflammatory demyelination is not central to the pathogenesis of multiple sclerosis. J Neurol 252(Suppl. 5):v10–v15. [DOI] [PubMed] [Google Scholar]
- 8. Buss A, Brook GA, Kakulas B, Martin D, Franzen R, Schoenen J et al (2004) Gradual loss of myelin and formation of an astrocytic scar during Wallerian degeneration in the human spinal cord. Brain 127:34–44. [DOI] [PubMed] [Google Scholar]
- 9. Chao CC, Hu S (1994) Tumor necrosis factor‐alpha potentiates glutamate neurotoxicity in human fetal brain cell cultures. Dev Neurosci 16:172–179. [DOI] [PubMed] [Google Scholar]
- 10. Czasch S, Paul S, Baumgärtner W (2006) A comparison of immunohistochemical and silver staining methods for the detection of diffuse plaques in the aged canine brain. Neurobiol Aging 27:293–305. [DOI] [PubMed] [Google Scholar]
- 11. Dal Canto MC, Rabinowitz SG (1982) Experimental models of virus‐induced demyelination of the central nervous system. Ann Neurol 11:109–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dandekar AA, Wu GF, Pewe L, Perlman S (2001) Axonal damage is T cell mediated and occurs concomitantly with demyelination in mice infected with a neurotropic coronavirus. J Virol 75:6115–6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Stefano N, Matthews PM, Fu L, Narayanan S, Stanley J, Francis GS et al (1998) Axonal damage correlates with disability in patients with relapsing‐remitting multiple sclerosis. Results of a longitudinal magnetic resonance spectroscopy study. Brain 121:1469–1477. [DOI] [PubMed] [Google Scholar]
- 14. De Vos KJ, Grierson AJ, Ackerley S, Miller CC (2008) Role of axonal transport in neurodegenerative diseases. Annu Rev Neurosci 31:151–173. [DOI] [PubMed] [Google Scholar]
- 15. Evangelou N, Konz D, Esiri MM, Smith S, Palace J, Matthews PM (2000) Regional loss in the corpus callosum correlates with cerebral white matter lesion volume and distribution in multiple sclerosis. Brain 123:1845–1849. [DOI] [PubMed] [Google Scholar]
- 16. Ferguson B, Matyszak MK, Esiri MM, Perry VH (1997) Axonal damage in acute multiple sclerosis lesions. Brain 120:393–399. [DOI] [PubMed] [Google Scholar]
- 17. Frisk AL, Baumgärtner W, Gröne A (1999) Dominating interleukin‐10 mRNA expression induction in cerebrospinal fluid cells of dogs with natural canine distemper virus induced demyelinating and non‐demyelinating CNS lesions. J Neuroimmunol 97:102–109. [DOI] [PubMed] [Google Scholar]
- 18. Gaedke K, Zurbriggen A, Baumgärtner W (1999) Lack of correlation between virus nucleoprotein and mRNA expression and the inflammatory response in demyelinating distemper encephalitis indicates a biphasic disease progress. Eur Vet Pathol 5:9–20. [Google Scholar]
- 19. Gehrmann J, Banati RB, Cuzner ML, Kreutzberg GW, Newcombe J (1995) Amyloid precursor protein (APP) expression in multiple sclerosis lesions. Glia 15:141–151. [DOI] [PubMed] [Google Scholar]
- 20. Gröne A, Alldinger S, Baumgärtner W (2000) Interleukin‐1β, ‐6, ‐12 and tumor necrosis factor‐α expression in brains of dogs with canine distemper virus infection. J Neuroimmunol 110:20–30. [DOI] [PubMed] [Google Scholar]
- 21. Gröters S, Alldinger S, Baumgärtner W (2005) Up‐regulation of mRNA for matrix metalloproteinases‐9 and ‐14 in advanced lesions of demyelinating canine distemper leukoencephalitis. Acta Neuropathol 110:369–382. [DOI] [PubMed] [Google Scholar]
- 22. Higgins RJ, Krakowka SG, Metzler AE, Koestner A (1982) Experimental canine distemper encephalomyelitis in neonatal gnotobiotic dogs. A sequential ultrastructural study. Acta Neuropathol 57:287–295. [DOI] [PubMed] [Google Scholar]
- 23. Hohlfeld R (1997) Biotechnological agents for the immunotherapy of multiple sclerosis. Principles, problems and perspectives. Brain 120:865–916. [DOI] [PubMed] [Google Scholar]
- 24. Julien JP, Mushynski WE (1998) Neurofilaments in health and disease. Prog Nucleic Acid Res Mol Biol 61:1–23. [DOI] [PubMed] [Google Scholar]
- 25. Kerschensteiner M, Stadelmann C, Buddeberg BS, Merkler D, Bareyre FM, Anthony DC et al (2004) Targeting experimental autoimmune encephalomyelitis lesions to a predetermined axonal tract system allows for refined behavioral testing in an animal model of multiple sclerosis. Am J Pathol 164:1455–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kornek B, Storch MK, Weissert R, Wallstroem E, Stefferl A, Olsson T et al (2000) Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am J Pathol 157:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuhlmann T, Lingfeld G, Bitsch A, Schuchardt J, Brück W (2002) Acute axonal damage in multiple sclerosis is most extensive in early disease stages and decreases over time. Brain 125:2202–2212. [DOI] [PubMed] [Google Scholar]
- 28. Kutzelnigg A, Faber‐Rod JC, Bauer J, Lucchinetti CF, Sorensen PS, Laursen H et al (2007) Widespread demyelination in the cerebellar cortex in multiple sclerosis. Brain Pathol 17:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee MK, Cleveland DW (1996) Neurofilament function and dysfunction: involvement in axonal growth and neuronal disease. Curr Opin Cell Biol 6:34–40. [DOI] [PubMed] [Google Scholar]
- 30. Li GL, Farooque M, Holtz A, Olsson Y (1995) Changes of beta‐amyloid precursor protein after compression trauma to the spinal cord: an experimental study in the rat using immunohistochemistry. J Neurotrauma 12:269–277. [DOI] [PubMed] [Google Scholar]
- 31. Li S, Stys PK (2000) Mechanisms of ionotropic glutamate receptor‐mediated excitotoxicity in isolated spinal cord white matter. J Neurosci 20:1190–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lindner M, Fokuhl J, Linsmeier F, Trebst C, Stangel M (2009) Chronic toxic demyelination in the central nervous system leads to axonal damage despite remyelination. Neurosci Lett 453:120–125. [DOI] [PubMed] [Google Scholar]
- 33. Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H (1999) A quantitative analysis of oligodendrocytes in multiple sclerosis lesions. A study of 113 cases. Brain 122:2279–2295. [DOI] [PubMed] [Google Scholar]
- 34. Mancardi G, Hart B, Roccatagliata L, Brok H, Giunti D, Bontrop R et al (2001) Demyelination and axonal damage in a non‐human primate model of multiple sclerosis. J Neurol Sci 184:41–49. [DOI] [PubMed] [Google Scholar]
- 35. Nave KA, Trapp BD (2008) Axon‐glial signaling and the glial support of axon function. Annu Rev Neurosci 31:535–561. [DOI] [PubMed] [Google Scholar]
- 36. Neumann H, Medana IM, Bauer J, Lassmann H (2002) Cytotoxic T lymphocytes in autoimmune and degenerative CNS diseases. Trends Neurosci 25:313–319. [DOI] [PubMed] [Google Scholar]
- 37. Nimnual AS, Taylor LJ, Bar‐Sagi D (2003) Redox‐dependent down‐regulation of Rho by Rac. Nat Cell Biol 5:236–241. [DOI] [PubMed] [Google Scholar]
- 38. Oehmichen M, Meissner C, Schmidt V, Pedal I, König HG, Saternus KS (1998) Axonal injury—a diagnostic tool in forensic neuropathology? A review. Forensic Sci Int 95:67–83. [DOI] [PubMed] [Google Scholar]
- 39. Ozawa K, Suchanek G, Breitschopf H, Brück W, Budka H, Jellinger K, Lassmann H (1994) Patterns of oligodendroglia pathology in multiple sclerosis. Brain 117:1311–1322. [DOI] [PubMed] [Google Scholar]
- 40. Pitt D, Werner P, Raine CS (2000) Glutamate excitotoxicity in a model of multiple sclerosis. Nat Med 6:67–70. [DOI] [PubMed] [Google Scholar]
- 41. Seehusen F, Orlando EA, Wewetzer K, Baumgärtner W (2007) Vimentin‐positive astrocytes in canine distemper: a target for canine distemper virus especially in chronic demyelinating lesions? Acta Neuropathol 114:597–608. [DOI] [PubMed] [Google Scholar]
- 42. Summers BA, Appel MJ (1994) Aspects of canine distemper virus and measles encephalomyelitis. Neuropathol Appl Neurobiol 20:525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L (1998) Axonal transection in the lesions of multiple sclerosis. N Engl J Med 338:278–285. [DOI] [PubMed] [Google Scholar]
- 44. Tsunoda I (2008) Axonal degeneration as a self‐destructive defense mechanism against neurotropic virus infection. Future Virol 3:579–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tsunoda I, Fujinami RS (1996) Two models for multiple sclerosis: experimental allergic encephalomyelitis and Theiler's murine encephalomyelitis virus. J Neuropathol Exp Neurol 55:673–686. [DOI] [PubMed] [Google Scholar]
- 46. Tsunoda I, Fujinami RS (2002) Inside‐out versus outside‐in models for virus induced demyelination: axonal damage triggering demyelination. Springer Semin Immunopathol 24:105–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tsunoda I, Kuang LQ, Libbey JE, Fujinami RS (2003) Axonal injury heralds virus‐induced demyelination. Am J Pathol 162:1259–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ulrich R, Seeliger F, Kreutzer M, Germann PG, Baumgärtner W (2008) Limited remyelination in Theiler's murine encephalomyelitis due to insufficient oligodendroglial differentiation of nerve/glial antigen 2 (NG2)‐positive putative oligodendroglial progenitor cells. Neuropathol Appl Neurobiol 34:603–620. [DOI] [PubMed] [Google Scholar]
- 49. Vandevelde M, Zurbriggen A (2005) Demyelination in canine distemper virus infection: a review. Acta Neuropathol 109:56–68. [DOI] [PubMed] [Google Scholar]
- 50. Wang D, Ayers MM, Catmull DV, Hazelwood LJ, Bernard CC, Orian JM (2005) Astrocyte‐associated axonal damage in pre‐onset stages of experimental autoimmune encephalomyelitis. Glia 51:235–240. [DOI] [PubMed] [Google Scholar]
- 51. Wünschmann A, Alldinger S, Kremmer E, Baumgärtner W (1999) Identification of CD4+ and CD8+ cell subsets and B cells in the brain of dogs with spontaneous acute, subacute‐, and chronic‐demyelinating distemper encephalitis. Vet Immunol Immunopathol 67:101–116. [DOI] [PubMed] [Google Scholar]


