Abstract
Purpose
To explore how the assisted reproductive technology (ART) laboratories can be optimized and standardized to enhance embryo culture and selection, to bridge the gap between standard practice and the new concept of shortening time to healthy singleton birth.
Methods
A Delphi consensus was conducted (January to July 2018) to assess how the ART laboratory could be optimized, in conjunction with existing guidelines, to reduce the time to a healthy singleton birth. Eight experts plus the coordinator discussed and refined statements proposed by the coordinator. The statements were distributed via an online survey to 29 participants (including the eight experts from step 1), who voted on their agreement/disagreement with each statement. Consensus was reached if ≥ 66% of participants agreed/disagreed with a statement. If consensus was not achieved for any statement, that statement was revised and the process repeated until consensus was achieved. Details of statements achieving consensus were communicated to the participants.
Results
Consensus was achieved for all 13 statements, which underlined the need for professional guidelines and standardization of lab processes to increase laboratory competency and quality. The most important points identified were the improvement of embryo culture and embryo assessment to shorten time to live birth through the availability of more high-quality embryos, priority selection of the most viable embryos and improved cryosurvival.
Conclusion
The efficiency of the ART laboratory can be improved through professional guidelines on standardized practices and optimized embryo culture environment, assessment, selection and cryopreservation methodologies, thereby reducing the time to a healthy singleton delivery.
Keywords: ART laboratory, Time to live birth, Process optimization, Consensus, Expert opinion
Introduction
Time to live birth is becoming increasingly important for the evaluation of assisted reproductive technologies (ART) [1]. In general, women are likely to be in their mid- to late-thirties or early forties before they are diagnosed with infertility [2, 3]. As female fertility declines with age, especially after the age of 35, this results in decreasing cumulative live birth rates [4]. It has been recommended that treatment is commenced and optimized as rapidly as possible [5–7]. Any increase in treatment duration reduces the likelihood of a successful outcome and further increases the risk of discontinuation. An analysis has shown that a course of three ART cycles (including both fresh and frozen embryo transfers) can take up to 2 years to complete [8], and considerations of age and treatment duration [9] emphasize the need to ensure that effective treatment proceeds as promptly as possible. Furthermore, ART protocols should be optimized to ensure maximum efficacy while minimizing the risk for complications, including ovarian hyperstimulation syndrome (OHSS) [1].
A reduction in the time taken for any aspect of ART treatment, particularly in optimizing the work up, controlled ovarian stimulation and laboratory procedures, and regardless of the outcome, is therefore an important factor when personalizing ART treatment [1, 10]. This is particularly pertinent as clinical practice has shifted from multiple to single embryo transfer (SET), owing to the concerns about the risks associated with multiple pregnancy, despite multiple embryo transfer potentially increasing the likelihood of successful pregnancy [2, 5, 8, 11, 12]. There is, however, a lack of homogeneity and consensus on the definitions of time-related outcomes for evaluation of fertility treatments, and time to achieve a meaningful obstetric outcome has not been evaluated in many clinical studies [13]. To date, the only proposed definition of a time-related treatment measure is the International Committee Monitoring Assisted Reproductive Technologies (ICMART) glossary definition of time to pregnancy: “The time taken to establish a pregnancy, measured in months or in numbers of menstrual cycles” [14]. However, this definition does not provide details of the start and end points for evaluation of this outcome [14]. There is, therefore, an unmet need for a more relevant definition of the time to achieve a clinically meaningful obstetric outcome (i.e. live birth) to guide treatment selection decisions and patient counselling. Furthermore, live birth is infrequently reported as an outcome in clinical trials [15]; surprisingly, when assessing the effectiveness of a treatment, clinical pregnancy and live birth are often regarded as comparable outcomes [16].
The Delphi method comprises a series of structured group processes (rounds) to survey expert opinion, organized in a systematic fashion that focuses primarily on consensus, to reach a response [17], and has previously been successfully used to help inform decision-making in relation to ART [1, 18]. Indeed, a previous Delphi consensus on how time to healthy singleton delivery could affect decision-making during infertility treatment concluded that timely and individualized care of patients is central to the success of ART treatment [1]. Furthermore, primary care physicians and the general population should be educated about age-related fertility decline, patients should be informed about lower in vitro fertilization (IVF) success rates at older ages, and physicians should ensure prompt referral, according to age and duration of infertility. In particular, targeted treatment with accelerated treatment pathways and optimized, streamlined protocols should be considered for older women with reduced or sub-optimal reproductive potential. Procedures that might reduce time to healthy singleton delivery should also be evaluated for these patients [1]. A number of these procedures will be performed by the ART laboratory, which is, therefore, integral to the success of ART treatment, as the technologies used therein can significantly affect treatment outcomes [1, 19–21]. There are many baseline and treatment-related factors not associated with the ART laboratory that can influence the success of procedures conducted in the laboratory, including patient medical history, clinical protocol and practice, and the quality and quantity of the gametes provided to the laboratory. Therefore, safety, efficacy, efficiency, medical history and diagnosis should all be considered when evaluating ART laboratory technologies.
Owing to the high demand for ART and technical progress in hardware and software, laboratory technologies are rapidly developing and there is frequently a variety of different methods available for each laboratory procedure. For example, embryos may be cultured using single-step or sequential media, they may be assessed using either conventional morphological assessment at isolated time points or by evaluating embryo development with continuous embryo monitoring using time-lapse technology, various different grading systems can be used for embryo assessment and selection, and gametes or embryos can be cryopreserved, either by slow freezing or vitrification, at different stages.
Some of these are new methods or technologies with variable levels of implementation in IVF laboratories but whose benefits require further confirmation. For example, time-lapse monitoring; measurement of glucose, lactate, pyruvate or amino acid levels in the media; oxygen consumption; closed versus open culture; genomic, proteomic and metabolomics analyses; and non-invasive pre-implantation viability assessments [22–26]. Other methods (such as standard morphological assessment of embryos using inverted light microscopy, culture with sequential media, blastocyst culture to day 5/6, and controlled rate freezing or manual vitrification methods) are now well established, even though not all of these techniques were introduced after rigorous testing.
To the best of our knowledge, there has been no synthesis on the effect of new technologies in ART on time to pregnancy leading to healthy singleton delivery. Therefore, a Delphi consensus was initiated in 2018 to focus solely on the technologies used within ART laboratories and gather expert opinions on how these technologies could be optimized to reduce the time to pregnancy resulting in a healthy singleton delivery. The experts were also asked to provide guidance on which of the older technologies have been optimized and whether any of the newer technologies that are less widely used should be considered by ART laboratories.
Materials and methods
Role of the sponsor
Each step was coordinated by a healthcare consulting and training company (Sanitanova Srl, Milan, Italy). The consensus concept was initiated and funded by Merck KGaA, Darmstadt, Germany. The sponsor was involved early in the process, defining the overarching topic to be discussed, but did not participate in the development of the statements or in any of the meetings or discussions involved in developing the Delphi consensus. The statements were, therefore, developed independently of the industry sponsor. The authors from Merck KGaA were only involved in the development of the manuscript, critically revising it for important intellectual content, especially in the “Introduction”, “Results” and “Discussion” sections, but could not alter the consensus statements in any way.
Participants
The initial panel comprised a coordinator (GC) and eight experts in clinical embryology and ART laboratory practice (Table 1). The experts were selected and invited to participate by Sanitanova Srl on the basis of their publication record and relevant contributions to international medical congresses and meetings, in addition to global representation and independence from commercial interests (see the conflict of interest statement). Each expert, with the exception of the coordinator, proposed four additional experts, who were invited to participate in the second and third steps of the consensus process. Of the 32 additional experts invited to participate, 21 accepted; therefore, in total, 29 experts were involved in the Delphi consensus in addition to the coordinator (Table 1).
Table 1.
Participants involved in step 1, step 2 and step 3 of the consensus
| Name | Country | Step 1 (WebEx meeting) | Step 2 (online survey) | Step 3 (WebEx meeting) | |||
|---|---|---|---|---|---|---|---|
| 15 Jan 2018 | 24 Jan 2018 | 27 Jun 2018 | 11 Jul 2018 | ||||
| Giovanni Coticchioa | Italy | X | X | X | X | ||
| Valerio Pisaturob | Italy | X | X | X | X | ||
| Experts | Barry Behr | USA | X | X | X | ||
| Alison Campbell | UK | X | X | X | |||
| Kersti Lundin | Sweden | X | X | X | |||
| Marcos Meseguer | Spain | X | X | X | X | ||
| Dean Morbeck | New Zealand | X | X | X | |||
| Carlos Plancha | Portugal | X | X | X | |||
| Denny Sakkas | USA | X | X | X | |||
| Alison Bartolucci | USA | X | |||||
| Montse Boada | Spain | X | X | ||||
| Sonia Correia | Portugal | X | |||||
| Thorir Hardarson | Sweden | X | |||||
| Ciara Hughes | Ireland | X | |||||
| Ge Lin | China | X | |||||
| Cristina Magli | Italy | X | |||||
| Maria Giulia Minasi | Italy | X | X | ||||
| Zsolt Peter Nagy | USA | X | X | ||||
| Rocio Nuñez Calogne | Spain | X | X | ||||
| Soraia Pinto | Portugal | X | X | ||||
| Thomas ‘Rusty’ Pool | USA | X | |||||
| Eugenia Rocafort Curia | Spain | X | X | ||||
| Maria José de los Santos | Spain | X | X | ||||
| Catello Scarica | Italy | X | X | ||||
| Ioannis Sfontouris | Greece | X | X | ||||
| Amy Sparks | USA | X | |||||
| Jason Swain | USA | X | |||||
| Riccardo Talevi | Italy | X | |||||
| Matthew VerMilyea | USA | X | |||||
| Yanwen Xu | China | X | X | X | |||
| Sören Ziebe | Denmark | X | |||||
aCoordinator
bNon-voting member, contributed to project coordination and assisted with editing and reviewing manuscript content including approving version for publication
The Delphi consensus process
The importance of the concept of reducing time to healthy singleton delivery when making treatment decisions for all patients undergoing infertility treatment was initially proposed following a Delphi consensus process [1]. On the basis of this, Sanitanova Srl developed a proposal on the contribution of the ART laboratory to achieve this goal. With input from the sponsor, this proposal was refined to encompass five main focus areas: (1) standardization/objectivity/automation; (2) optimal embryo culture conditions; (3) optimal embryo assessment and selection; (4) optimal cryopreservation; and (5) improving laboratory workflow and management tools to increase efficacy, safety and efficiency.
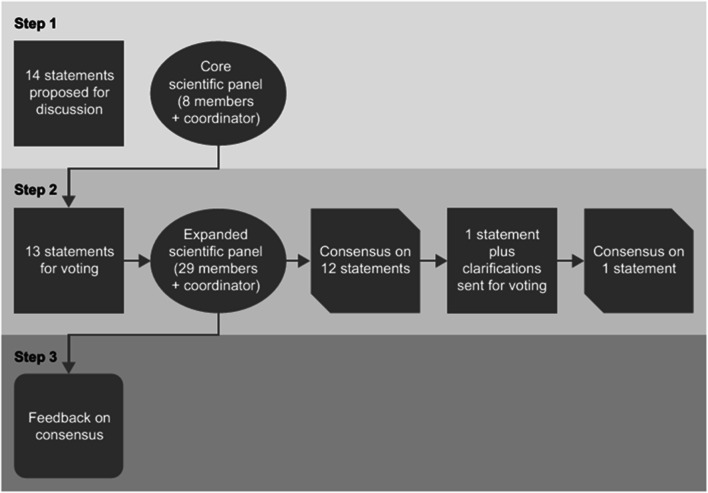
The Delphi consensus process comprised three steps (Fig. 1). The coordinator (GC) proposed initial statements based on the five focus areas, relating to how the ART laboratory and associated procedures might be optimized to reduce the time to healthy singleton delivery, together with scientific references supporting these statements, with the intention to drive discussion among the panel. In step 1, these statements were discussed with the initial eight experts during two web conferences, each attending one of the two, to facilitate global time zones, and both led by the coordinator. There was no predefined number of statements that could be included and, during the discussion, participants were allowed to combine, merge or amend statements or suggest sub-statements, with the addition of supporting references. The statements were refined by the coordinator and the experts, based on these discussions, and then circulated for further comment and approval.
Fig. 1.
The three steps of the Delphi consensus process. The consensus process comprised three steps. In step 1, a core panel of 8 experts and the coordinator developed 12 statements relating to IVF technologies. In step 2, an expanded panel of 29 experts and the coordinator voted via an online survey on their level of agreement. In step 3, full details of the final agreed statements were communicated to the participants
Step 2 was initiated once the initial panel had approved the statements to be voted on. The aim of this step was to achieve consensus on the statements developed during step 1. An online survey containing the final statements developed in step 1, including the supporting references, was circulated to the 29 participants (the coordinator did not vote during step 2). Participants rated their level of agreement with each statement using a 5-item Likert scale: 1 = absolutely disagree, 2 = disagree, 3 = agree, 4 = more than agree, 5 = absolutely agree [1, 27, 28]. Participants were also asked to provide the main reason (free text) for their chosen level of agreement or disagreement. Consensus was considered to have been achieved if the proportion of participants either disagreeing with the statement (responding 1 or 2) or agreeing with the statement (responding 3, 4 or 5) exceeded 66% [1, 27, 28]. If the proportion of participants either agreeing or disagreeing with a statement did not exceed 66%, that statement would be revised according to the feedback received and another survey, including only the statements not reaching consensus, was circulated. This process would be repeated, with the statements being revised until, if possible, consensus was reached for every statement. Individual input was anonymized to better enable open discussion and critique.
In step 3, web conferences were arranged to communicate the outcome of step 2 to all participants (i.e., to report on the level of consensus with each statement). Attendance at the web conferences was not compulsory. The statements could not be amended at this stage.
Results
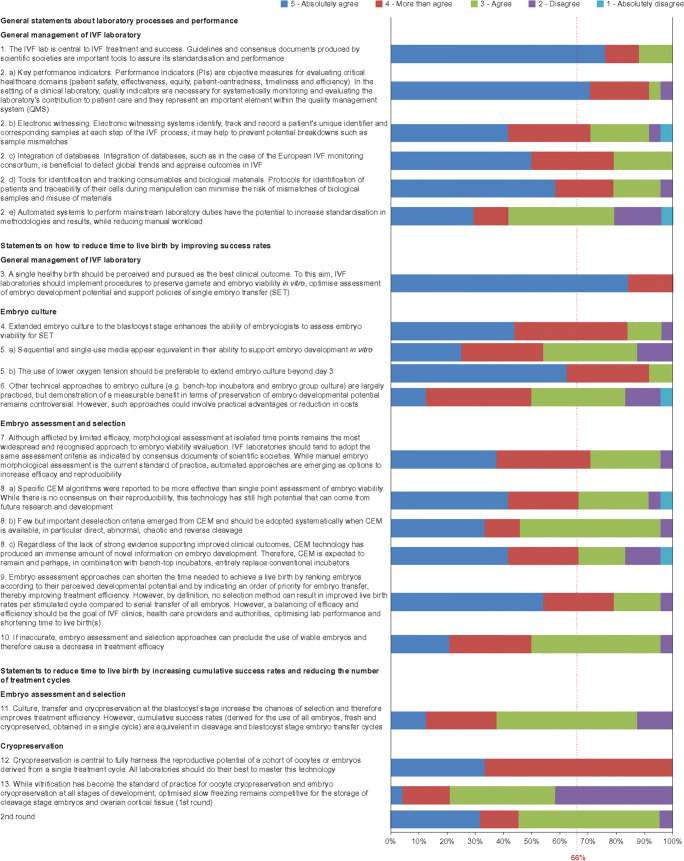
The coordinator proposed 14 statements for discussion with the initial expert panel. During step 1, three of the 14 statements were agreed on without modification, nine were agreed on with modification, and two were combined into a single statement owing to their similar focus. The statements voted on during step 2 are shown in Table 2 (some statements included sub-statements that were voted on separately). Consensus was achieved during the first round of voting on all statements except statement 13 (Fig. 2). The majority of participants disagreed with a single section of statement 13 that suggested slow freezing should be considered to be as good as vitrification for cleavage stage embryo cryopreservation and ovarian cortical tissue cryopreservation. In response to this disagreement, the coordinator provided the following statement:
Table 2.
Consensus statements
| General statements about laboratory processes and performance | |
| General management of the ART laboratory and procedures | |
| 1. | The IVF laboratory is central to IVF treatment and success. Guidelines and consensus documents produced by scientific societies are important tools to assure its standardization and performance. |
| 2. | Technology and enhanced management systems can improve laboratory efficacy, efficiency and safety.a |
| (a) Key performance indicators. Performance indicators (PIs) are objective measures for evaluating critical healthcare domains (patient safety, effectiveness, equity, patient-centredness, timeliness and efficiency). In the setting of a clinical laboratory, quality indicators are necessary for systematically monitoring and evaluating the laboratory’s contribution to patient care and they represent an important element within the quality management system (QMS). | |
| (b) Electronic witnessing. Electronic witnessing systems identify, track and record a patient’s unique identifier and corresponding samples at each step of the IVF process; it may help to prevent potential breakdowns such as sample mismatches. | |
| (c) Integration of databases. Integration of databases, such as in the case of the European IVF monitoring consortium, is beneficial to detect global trends and appraise outcomes in IVF. | |
| (d) Tools for identification and tracking consumables and biological materials. Protocols for identification of patients and traceability of their cells during manipulation can minimize the risk of mismatches of biological samples and misuse of materials. | |
| (e) Automated systems to perform mainstream laboratory duties have the potential to increase standardization in methodologies and results, while reducing manual workload. | |
| Statements on how to reduce time to live birth by improving success rates | |
| General management of the ART laboratory and procedures | |
| 3. | A single healthy birth should be perceived and pursued as the best clinical outcome. To this aim, IVF laboratories should implement procedures to preserve gamete and embryo viability in vitro, optimize assessment of embryo development potential and support policies of single embryo transfer (SET). |
| Embryo culture | |
| 4. | Extended embryo culture to the blastocyst stage enhances the ability of embryologists to assess embryo viability for SET. |
| 5. | (a) Sequential and single-use media appear equivalent in their ability to support embryo development in vitro. |
| (b) The use of lower oxygen tension should be preferable to extend embryo culture beyond day 3. | |
| 6. | Other technical approaches to embryo culture (e.g., bench-top incubators and embryo group culture) are largely practiced, but demonstration of a measurable benefit in terms of preservation of embryo developmental potential remains controversial. However, such approaches could involve practical advantages or reduction in costs. |
| Embryo assessment and selection | |
| 7. | Although afflicted by limited efficacy, morphological assessment at isolated time points remains the most widespread and recognized approach to embryo viability evaluation. IVF laboratories should tend to adopt the same assessment criteria as indicated by consensus documents of scientific societies. While manual embryo morphological assessment is the current standard of practice, automated approaches are emerging as options to increase efficacy and reproducibility. |
| 8. | Time-lapse microscopy (TLM) allows continuous embryo monitoring and observation of the morphokinetics of preimplantation development:a |
| (a) Specific continuous embryo monitoring algorithms have been reported to be more effective than single point assessment of embryo viability. While there is no consensus on their reproducibility, this technology has still high potential that can come from future research and development. | |
| (b) Several important deselection criteria have emerged from the introduction of continuous embryo monitoring, such as direct, abnormal, chaotic and reverse cleavage of embryonic cells. As time-lapse systems become available, these criteria should be adopted in a systematic manner. | |
| (c) Regardless of the lack of strong evidence supporting improved clinical outcomes, continuous embryo monitoring technology has produced an immense amount of novel information on embryo development. Therefore, continuous embryo monitoring is expected to remain and perhaps, in combination with bench-top incubators, entirely replace chamber/box incubators. | |
| 9. | Embryo assessment approaches can shorten the time needed to achieve a live birth by ranking embryos according to their perceived developmental potential and by indicating an order of priority for embryo transfer, thereby improving treatment efficiency. However, by definition, no selection method can result in improved live birth rates per stimulated cycle compared to serial transfer of all embryos. However, a balancing of efficacy and efficiency should be the goal of IVF clinics, health care providers and authorities, optimizing laboratory performance and shortening time to live birth(s). |
| 10. | Inaccuracies in embryo assessment and selection can preclude the use of viable embryos, leading to reduced treatment efficacy. |
| Statements to reduce time to live birth by increasing cumulative success rates and reducing the number of treatment cycles | |
| Embryo assessment and selection—cumulative live birth rates | |
| 11. | Culture, transfer and cryopreservation at the blastocyst stage increase the chances of selection and therefore improves treatment efficiency. However, cumulative success rates (derived for the use of all embryos, fresh and cryopreserved, obtained in a single cycle) are equivalent in cleavage and blastocyst stage embryo transfer cycles. |
| Cryopreservation | |
| 12. | Cryopreservation is central to fully harness the reproductive potential of a cohort of oocytes or embryos derived from a single treatment cycle. All laboratories should do their best to master this technology. |
| 13. | While vitrification has become the standard of practice for oocyte cryopreservation and embryo cryopreservation at all stages of development, optimized slow freezing remains competitive for the storage of cleavage stage embryos and ovarian cortical tissue.b |
The panel were initially provided with key references related to each statement. As the Delphi consensus process evolved and the manuscript developed, additional references were added by the group to support the discussion and consensus
aThis statement was an introductory statement to sub-statements; therefore, it was not voted on
bThis statement did not meet the criteria for consensus at the first voting. The scientific coordinator, Giovanni Coticchio, decided to give an appropriate clarification, illustrated below:
1. The recognition of the supremacy of vitrification is recognized in the statement (“vitrification has become the standard of practice for oocyte cryopreservation and embryo cryopreservation”)
2. Slow freezing is considered competitive—i.e. as good as, NOT outperforming—in only two areas of application:
i. Cleavage stage embryo cryopreservation
ii. Ovarian cortical tissue cryopreservation
Points 2i and 2ii do not reflect personal opinions of the board members. Rather, they derive from established evidence:
• Point 2i: most comparisons between slow freezing and vitrification are based on “traditional” slow freezing protocols (including 0.1 M sucrose in their formulations), which indeed are inferior to most or all vitrification protocols (Edgar et al. Reprod Biomed Online 2009;19:521–5). However, if an optimized slow freezing protocol (0.2 M sucrose) is used (Edgar et al. Reprod Biomed Online 2009;19:521–5), survival rates are above 90%, with clinical outcomes similar to those obtained from equivalent fresh embryos. This makes the optimized slow freezing approach “competitive” with vitrification protocols: “There is no basis for supporting the preferential use of either technique in this context” (Edgar et al. Reprod Biomed Online 2009;19:521–5)
• Point 2ii: it appears that of the > 100 births derived from cryopreserved and re-implanted ovarian tissue, almost all of them were achieved using the slow freezing approach
After this comment was shared with the other opinion leaders, the panel was invited to vote again on statement 13
Fig. 2.
Agreement/disagreement with the consensus statements. Results of the participants’ agreement with the 13 statements (some with sub-statements), divided into four categories, using a 5-item Likert scale (5 [dark blue], absolutely agree; 4 [red], more than agree; 3 [green], agree; 2 [purple], disagree; 1 [aqua blue], absolutely disagree). Consensus was defined as > 66% participants agreeing (responding 3, 4 or 5) or disagreeing (responding 1 or 2) with a statement (red line). Consensus was reached on all statements, except statement 13, during the first round voting. After the second round of voting, consensus was achieved for all statements
The coordinator acknowledges that the supremacy of vitrification was recognized in the statement; i.e. “vitrification has become the standard of practice for oocyte cryopreservation and embryo cryopreservation” and that slow freezing was considered competitive—i.e. as good as, not outperforming—in only two areas of application: (a) cleavage stage embryo cryopreservation and (b) ovarian cortical tissue cryopreservation.
The coordinator also provided further explanation on why an optimal slow freezing protocol was considered competitive for cleavage stage embryos:
Most comparisons between slow freezing and vitrification are based on “traditional” slow freezing protocols (including 0.1 M sucrose in the method proposed by Lassalle et al., which employs 1,2-propanediol as the permeating cryoprotectant in conjunction with sucrose as non-permeating cryoprotectant [29] ), which are inferior to most or all vitrification protocols. However, when an optimized slow freezing protocol (0.2 M sucrose) is used on cleavage stage embryos, survival rates are above 90%, with clinical outcomes similar to those obtained from equivalent fresh embryos [30]. This makes the optimized slow freezing approach “competitive” with vitrification protocols: “There is no basis for supporting the preferential use of either technique in this context.”
After this explanation was provided, a second round of voting took place and consensus was reached for statement 13.
During manuscript development, the statements were re-classified into three main categories: general statements about laboratory processes and performance; statements on how to reduce time to live birth by improving success rates; statements to reduce time to live birth by increasing cumulative success rates and reducing the number of treatment cycles. Within these three main categories, statements were further grouped, where applicable, according to four general focus areas (general management of the ART laboratory and procedures; embryo culture; embryo assessment and selection; cryopreservation). The original focus areas 1 (standardization/objectivity/automation) and 5 (improving laboratory workflow and management tools to increase efficacy, safety and efficiency) defined at the inception of the Delphi process (and detailed in the “Materials and methods” section) were combined for simplicity into focus area 1 (general management of the ART laboratory and procedures).
General statements about laboratory processes and performance
These statements concern procedures that will not, per se, directly reduce the time to a healthy singleton birth but will contribute to the improvement and streamlining of performance and workflow processes, thus enabling a reduction in time to live birth.
General management of the ART laboratory and procedures
1. The IVF laboratory is central to IVF treatment and success. Guidelines and consensus documents produced by scientific societies are important tools to assure its standardization and good performance.
The guidelines for good practice in ART laboratories were revised in 2015 by the European Society of Human Reproduction and Embryology (ESHRE) Guideline Group on Good Practice in IVF Labs, which consisted of ten embryologists representing different European countries, settings and expertise [19]. These revised ESHRE guidelines provide a comprehensive overview of the key procedures and general organization of the ART laboratory. Furthermore, they emphasize the importance of the personnel working in the laboratory and provide guidance on training, education, quality management and communication between staff, with the aim of ensuring that all personnel are qualified and competent [19]. Another international consensus, including the Alpha Scientists in Reproductive Medicine Executive and ESHRE Special Interest Group of Embryology, focused on defining the minimum criteria for embryo morphology assessment and grading [31]. The intention of this document was to provide a common terminology and enable standardization of laboratory practice, which should result in more effective comparison of treatment outcomes between centres [31]. In addition, this document discusses the minimum data set required for the accurate description of embryo development and standardized timing of observations of key developmental events. An international consensus expert opinion-based guideline on IVF culture formulated more than 50 points based on the strengths and weaknesses of currently available options for equipment and procedures [22]. These can be incorporated according to fitness for purpose among individual laboratories and operating environments, rather than to define exactly what should or should not be done in the IVF laboratory. International accreditation systems also exist and are often applied in IVF clinics to improve standardization, quality and competency. Accreditation systems aimed at improving standardization include the Quality Management Systems ISO 90011:2015 (applicable to general organizations) [32], ISO 17025:2017 (general requirements for competency in testing and calibration laboratories) [33] and ISO 15189:2012 (requirements for quality and competence in medical laboratories) [34]. In the USA, the main body is the College of American Pathologists (CAP), which provides accreditation and proficiency testing to medical laboratories through its laboratory quality solution programmes [35]. Other agencies that oversee different aspects of IVF in the USA are separate state and federal authorities (e.g. the New York Department of Health), the Clinical Laboratory Improvement Amendment (CLIA [https://www.cdc.gov/clia/index.html]) and the Food and Drug Administration (FDA [https://www.fda.gov/home]). In Europe, the European Tissues and Cells Directive 2004/23/EC [36] sets out the legal framework defining safety and quality standards for human tissues and cells. To help execute this mother directive, several implementing directives were adopted in close cooperation with member countries: 2006/17/EC [37] (amended in 2012 to 2012/39/EU [38]), detailing specific technical requirements; 2006/86/EC detailing traceability, serious adverse reactions/events and specific technical requirements [39]. This was amended in 2015 to 2015/565 to define specific coding requirements for tissues and cells [40]. Increasing trends in reproductive tourism and movement of gametes/embryos around the world prompted an implementing directive (2015/566) to address standards of quality and safety of imported tissues and cells [41].
2. Technology and enhanced management systems can improve laboratory efficacy, efficiency and safety.
-
Key performance indicators. Performance indicators (PIs) are objective measures for evaluating critical healthcare domains (patient safety, effectiveness, equity, patient-centredness, timeliness and efficiency). In the setting of a clinical laboratory, quality indicators are necessary for systematically monitoring and evaluating the laboratory’s contribution to patient care and they represent an important element within the quality management system (QMS).
Monitoring of key performance indicators for laboratory processes enables the early detection and correction of problems that could have a clinical impact, thus helping patients to achieve a healthy live birth and potentially reducing the time to achieve this outcome [42]. Minimum criteria for oocyte and embryo morphology assessment and guidelines on good practice in clinical embryology laboratories have been recommended [31, 43]. Objective performance measures are also necessary to monitor the proficiency of the ART laboratory. An international consensus meeting, supported by ESHRE and Alpha Scientists in Reproductive Medicine, aimed to identify and establish consensus definitions and recommend minimal performance-level values (competency) and aspirational values (benchmark) for PIs for ART laboratories. The PIs were mostly for fresh IVF and intracytoplasmic sperm injection (ICSI) cycles, and were established to estimate the competency profiles for clinical embryologists, with the gap between competency and benchmark as the desirable range [44].
-
Electronic witnessing. Electronic witnessing are systems to identify, track and record a patient’s unique identifier and corresponding samples at each step of the IVF process, helping to prevent potential errors such as sample mismatches.
Electronic witnessing systems have been suggested as an aid to sample identification, witnessing and tracking, with the aim of avoiding potentially critical errors in the ART laboratory (e.g., sample mix-ups). These systems may facilitate critical consumable and workflow tracking and reduce manual workload. Electronic witnessing may also reduce patient concerns about sample mix-ups and increase patient satisfaction of sample traceability [45].
-
Integration of databases. Integration of databases, such as in the case of the European IVF monitoring consortium, is beneficial to detect global trends and appraise outcomes in IVF.
The integration of databases potentially enables broad trends to be identified and tracked by facilitating large-scale retrospective analyses and could include cycle-by-cycle-based registries from individual national treatment centres that are used in an aggregated form on international basis. For example, results generated by ESHRE included data collected from national registries and personal information that was provided on a voluntary basis was used in the European IVF-monitoring Consortium [46]. Furthermore, the mandatory publication of the clinic-specific success rate for ART procedures in the USA was achieved through collaboration between the Society for Assisted Reproductive Technology (SART) and the American Society for Reproductive Medicine (ASRM) [47], and the global utilization, outcomes and practices in ART (2008–2010) were surveyed by the International Committee for Monitoring Assisted Reproductive Technologies (ICMART) [48]. Such integrated databases should be able to provide evidence for the benefits and harms of one approach compared with another; however, this may be hampered by centre effects, which have been observed to be a source of variability in multicentre studies of fertility treatment [49].
-
Tools for identification and tracking consumables and biological materials. Protocols for identification of patients and traceability of their cells during manipulation can minimize the risk of mismatches of biological samples and misuse of materials.
Failure mode and effects analysis (FMEA) methodology has been used to identify the ART phases most susceptible to mismatch errors, to pinpoint possible causes of such mistakes and to suggest corrective measures [50]. All laboratory phases, from oocyte and sperm collection to embryo transfer to cryopreservation, were susceptible for sample mismatch. Investigation of errors and continuous improvements to sample identification, witnessing and tracking protocols and procedures will further minimize risk of critical mismatch errors.
-
Automated systems to perform mainstream laboratory duties have the potential to increase standardization in methodologies and results, while reducing manual workload.
The introduction of automated cryopreservation and embryo assessment systems may help to standardize processes, reduce inter- and intra-operator variability and reduce the volume of time-consuming manual work in an ART laboratory.
The first publication of a semi-automated vitrification system reported a reduction in vitrification time with similar laboratory outcomes to those seen for mouse and human blastocysts vitrified using a manual method [51]. The first European report of two ongoing pregnancies, from blastocysts cryopreserved with a semi-automated vitrification system, was recently published [52]. The authors conclude that the semi-automated vitrification may contribute to improving outcomes and laboratory logistics but further specifically designed studies are required to assess its impact on clinical outcomes.
The use of automated approaches to embryo assessment is increasing through the use of machine learning/artificial intelligence (AI) in conjunction with time-lapse imaging. The potential of time-lapse technology to evolve into a full-blown embryo selection modality in conjunction with AI has been appraised by the ESHRE working group on time-lapse technology as becoming a versatile and resourceful tool that embryologists and IVF laboratories should harness [53].
Unlike other imaging fields, human embryology and IVF have not fully leveraged machine learning/AI for unbiased, automated embryo assessment [54]. Machine learning/AI is discussed in more detail in the “Embryo assessment and selection” Section.
Statements on how to reduce time to live birth by improving success rates
These statements concern optimization of laboratory procedures that will contribute to increases in the implantation and pregnancy rates, thereby shortening time to live birth.
General management of the ART laboratory and procedures
3. A single healthy birth should be perceived and pursued as the best clinical outcome. To this aim, IVF laboratories should implement procedures to preserve gamete and embryo viability in vitro, optimize assessment of embryo development potential and support policies of single embryo transfer.
SET is the best way to reduce the risk of multiple pregnancies and births and increase the chance of a healthy singleton birth at term [11, 55, 56]. In laboratories that have optimized the processes for assessing embryo development and preserving embryo viability (e.g., those that have a high rate of good-quality embryos and have a high cryosurvival rate) there will potentially be fewer instances where a fresh IVF cycle will need to be started for each transfer and the cumulative pregnancy and cumulative live birth rates per aspirated cycle will be higher. Therefore, the laboratory has a crucial role by optimizing embryo culture and selection in support of a SET policy.
An update to the UK Best Practice Guidelines on elective SET provided by the Association Of Clinical Embryologists (ACE) and the British Fertility Society (BFS) supported the implementation of elective SET to substantially reduce the clinical risks associated with multiple, premature births and low birth weight [11]. In women under 36 years, the transfer of a single fresh embryo, followed by transfer of a frozen–thawed embryo if necessary, reduced the rate of multiple birth (multiple birth rates 33.1 vs 0.8, p < 0.001) but did not substantially lower the rate of live births (difference in at least one live birth was 4.1%, 95% CI − 3.4 to 11.6) [56]. A meta-analysis including 1367 patients showed that multiple live births were reduced after an elective SET cycle (3/181 [2%]) compared with a double embryo transfer cycle (84/285 [29%]) [55]. Even though the live birth rate was lower after SET (181/683 [27%]) compared with double embryo transfer (285/683 [42%]), a subsequent frozen SET cycle resulted in a cumulative live birth rate similar to that after a single fresh double embryo transfer (132/350 [38%] vs 149/353 [42%]) [55]. These studies suggest that, when combined with frozen embryo transfer, SET results in similar live birth rates to those observed with multiple embryo transfer, with a decreased risk for multiple pregnancy and its associated risks. Double embryo transfer may be considered in some cases; for example, previous failed implantation, in women > 40 years, or when only low-quality embryos are available [57].
Embryo culture
4. Extended embryo culture to the blastocyst stage enhances the ability of embryologists to assess embryo viability for SET.
With the optimization of culture systems, there has been a move towards culture to the blastocyst stage [58, 59]. Embryo transfer at the blastocyst, rather than the cleavage stage, is considered to allow embryologists to better assess embryo viability and select the embryos with the highest potential for implantation [60]. Transfer at the blastocyst stage is also reported to improve synchronicity with the endometrium [61]. This should, in turn, increase the implantation and pregnancy rates and reduce the time to healthy singleton delivery, as fewer cycles should be required. In a multicentre, open-label, and randomised controlled trial of 458 patients ≤ 37 years, cumulative pregnancy rate was significantly higher with personalized day 5/6 embryo transfer guided by endometrial receptivity analysis (93.6%) compared with frozen embryo transfer (79.7%; p = 0.0005) and fresh embryo transfer (80.7%; p = 0.0013), and in per-protocol analyses, statistically significant improvements for first embryo transfer were reported for pregnancy and implantation rates and for cumulative live birth rates up to 12 months with personalized embryo transfer with endometrial receptivity analysis compared with fresh or frozen fresh embryo transfer [62]. In a Cochrane review to determine whether blastocyst stage embryo transfer results in higher live birth rates compared with cleavage stage embryo transfer, a higher live birth rate was observed following fresh blastocyst transfer compared with cleavage stage transfer [63]. A meta-analysis of 27 randomized clinical trials including 4031 women showed an odds ratio (OR) of 1.48 (95% confidence interval [CI] 1.20, 1.82) in favour of blastocyst transfer. The evidence was, however, considered to be of low-quality and there was no evidence of a difference in cumulative pregnancy rates between the groups [63].
In order to have a successful blastocyst programme, an optimal system for extended culture is essential, as well as appropriately trained and skilled staff. In addition, although culture to blastocyst stage enables self-selection of viable embryos, it also increases the risk of having no embryos to transfer, especially for patients with poor prognoses [64]. Therefore, to reduce this risk, the number of oocytes/zygotes/good-quality embryos that should be available to proceed to blastocyst culture needs to be optimized. Candidate biomarkers that could be used when counselling patients include age and ovarian reserve (AMH levels) [65] and morphokinetic parameters (time of morula formation, tM and the time of transition from a 5-blastomere embryo to an 8-blastomere embryo; receiver operating characteristic curve value 0.849, 95% CI0.835–0.854) [66]. Furthermore, the Patient-Oriented Strategies Encompassing IndividualizeD Oocyte Number (POSEIDON) group developed an ART calculator to predict the POSEIDON marker (the ability to retrieve the number of oocytes needed to achieve at least one euploid embryo for transfer): the predictors identified were female age, sperm source used for ICSI and the number of mature (metaphase II) oocytes (p < 0.0001) [67]. Appropriate counselling concerning the risk of no transfer occurring should always be provided and treatment decisions should be personalized according to patient characteristics [63].
5 a). Sequential and single-step media appear equivalent in their ability to support embryo development in vitro.
Sequential media uses different formulations for each stage of embryo development (days 1–3 and days 3–5/6) based on the changing metabolic and nutritional requirements of the developing embryo, whereas single-step media contains all the nutrients needed for all stages of embryo development to blastocyst stage, where appropriate. In a recent meta-analysis, culture with single-step media compared with sequential media was associated with higher blastocyst formation rates per oocyte/zygote randomized (ten studies; 7455 oocytes/zygotes; relative distribution 0.06; 95% CI 0.01, 0.12) but similar clinical pregnancy rates per woman randomized (one study; 100 women; relative risk 1.0; 95% CI 0.7, 1.4). Similar ongoing pregnancy rates per woman randomized were also reported (two studies; 246 women; relative risk 0.9; 95% CI 0.7, 1.3) [64]. The authors concluded that there was insufficient evidence available to support the superiority of either single-step or sequential media and that the interpretation of findings is inevitably confounded by heterogeneity relating to the ART laboratory and the included patient populations. In addition, one brand of single-step medium or sequential medium may not be comparable to another brand regarding embryo development and success rates. However, there may be practical, non-clinical advantages relating to single-step media, including simplification of laboratory procedures, which could result in a reduction in the risk for unintentional handling errors, and cost savings owing to reductions in labour and consumables used. An additional benefit of single-step media is its compatibility with time-lapse systems. An important consideration when using single-step media is whether it should be refreshed on day 3 or days 5–6 due to concerns about ammonia building-up from the breakdown of amino acids, in particular glutamine [68].
5 b). The use of lower oxygen tension should be preferable to extend embryo culture beyond day 3.
A Cochrane meta-analysis based on four included studies with a total of 1382 participants showed evidence of a beneficial effect for live birth rate when culturing embryos in low oxygen concentration (5%) compared with exposure to an atmospheric oxygen concentration (20%) (OR 1.39; 95% CI 1.11 to 1.76; p = 0.005) [69]; the methodological quality of the included trials was, however, relatively low. A similarly modest increase in live births/ongoing pregnancy was reported in a systematic review and meta-analysis of 21 studies (> 16,000 participants) of low-quality evidence: RR 1.1, 95% CI 1.0 to 1.3) [70].
Animal studies have also suggested that the use of oxygen at an atmospheric concentration can have a negative impact on the development of embryos [71]. Therefore, efforts should be made to ensure that the embryo is exposed to an oxygen concentration that more closely resembles the one in the fallopian tubes and uterus [72].
6. Other technical approaches to embryo culture (e.g., bench-top incubators and embryo group culture) are largely practiced, but demonstration of a measurable benefit in terms of preservation of embryo developmental potential remains controversial. However, such approaches could involve practical advantages or reduction in costs.
Culturing of embryos in a closed continuous embryo monitoring system allows embryos to be assessed without removing them from the incubator, reducing the time during which embryos are exposed to the external environment. A randomized controlled trial comparing the number of good-quality embryos after incubation in a conventional incubator system (box/chamber) compared with a closed system showed no significant difference in the number of good-quality embryos obtained (difference 0.23; 95% CI 0.69;–0.24) [73, 74]. However, in this study, the embryos were only cultured to day 2, and any advantage of undisturbed culture to days 3 or 5 needs to be determined in further studies.
The grouping of embryos in culture may improve preimplantation development. A prospective study including 936 zygotes showed that, compared with individual culture, group culture was superior in terms of compaction (p < 0.01) and blastulation (p < 0.001) [75]. A study by Rebollar-Lazaro also assessed the advantages of culturing cleavage stage embryos in a group compared with culturing embryos individually and reported that a significantly higher number of blastocysts suitable for freezing and thawing were observed when embryos were cultured together [76]. In a post hoc analysis of these data, this difference was only observed for women aged < 35 years old (i.e. not those aged ≥ 35 years old); further studies are, therefore, needed to explore clinical outcomes in different age subgroups [76]. It should also be noted that group culture does not allow the continuous monitoring of individual embryos and will, thus, influence our knowledge regarding the impact of embryo behaviour during development on success rates.
Embryo assessment and selection
7. Although afflicted by limited efficacy, morphological assessment at isolated time points remains the most widespread and recognized approach to embryo viability evaluation. IVF laboratories should tend to adopt the same assessment criteria as indicated by consensus documents of scientific societies. While manual embryo morphological assessment is the current standard of practice, automated approaches are emerging as options to increase efficacy and reproducibility.
Several embryo assessment methods have been developed that aim to select embryos with the highest developmental potential for transfer [31]. The most widely used method is conventional morphological assessment, in which embryo development is observed and graded at isolated time points by trained embryologists. However, there are no commonly used criteria or terminology for embryo grading at different developmental stages. Agreed-upon criteria, as well as standardized timings of observations (relative to the time of insemination), are required, to allow results to be compared between laboratories [31].
The Society for Assisted Reproductive Technology (SART) developed a single, standardized grading system that accounts for both cleavage stage and blastocyst stage assessment and can be easily implemented in any ART laboratory: day 3 embryos are graded according to cell number, fragmentation and symmetry; day 5 embryos are assessed by developmental stage and the quality of the inner cell mass and trophectoderm [77]. Having common criteria for embryo assessment and related nomenclature enables collaborative efforts and prospective studies of the relation between embryo morphology, cycle outcomes and the long-term health of the offspring [77]. The Alpha Executive and ESHRE Embryology Special Interest Group have developed a more detailed grading scheme, which introduces checks for oocyte, fertilization, syngamy, early cleavage, and the morula and blastocyst stages [31].
Automated methods of morphological assessment have also been developed, but these require further research to confirm their reliability and safety [78, 79]. Time-lapse evaluation enables embryo assessment at frequent intervals (5–20 min), as well as observation of the timings of key developmental stages. In a study by Conaghan et al. (2013), embryos assessed using morphology only and those assessed with morphology combined with time-lapse morphokinetics showed that, in this particular setting, the combination of the two methods increased the ability to identify which embryos would reach the blastocyst stage and which would not. Adjunctive use of morphology and an automated time-lapse imaging test has also been observed to reduce inter-individual variability in embryo selection [80]. However, more research is needed to demonstrate the benefits on clinical outcomes.
The success of manual embryo assessment and selection increases with the experience and expertise of the embryologist performing the assessment, and this can reduce the effectiveness of ART clinics with only a few experienced embryologists [80]. Furthermore, manual embryo assessment and selection is limited to single-point subjective observations, which can lead to intra- and inter-observer variabilities. Continuous embryo monitoring enabled by time-lapse microscopy (TLM) and/or automated monitoring systems help to avoid this variability [81]. Another possible approach that is currently being evaluated is to use machine learning, or “artificial intelligence” (AI) to aid the scoring and selection of embryos [82]. AI, which classifies oocytes/embryos by applying a pattern recognition algorithm to images at different stages of embryo development, has shown promising results compared with traditional morphological assessment for the identification of embryos with high implantation potential [82]. For example, a recent study by Tran et al. (2019) described a deep learning model that was able to predict foetal heart pregnancy from time-lapse videos with an AUC of 0.93 (95% CI 0.92–0.94) in fivefold stratified cross-validation [83]. A machine learning/AI framework using deep learning was reported to predict blastocyst quality (good, fair or poor) with an AUC > 0.98, outperforming individual embryologists [54]. A decision tree integrating patient age and automated blastocyst quality was used to predict chances of pregnancy from individual embryos, ranging from 13.8% (> 41 years and poor quality) to 66.3% (37 years and good quality), uncovering new, personalized strategies to select embryos.
8. Time-lapse microscopy (TLM) allows continuous embryo monitoring and observation of the morphokinetics of preimplantation development:
Specific continuous embryo monitoring algorithms have been reported to be more effective than single point assessment of embryo viability. While there is no consensus on their reproducibility, this technology still has high potential that can come from future research and development.
The evaluation of continuous embryo monitoring data requires a different approach to the evaluation of static images. A hierarchical model based on the timing of three events has been reported to be more effective than morphological assessment, in terms of predicting which embryos are more likely to implant [84]. The three events evaluated are time of division to five cells (t5; OR 3.31, 95% CI 1.65–6.66), time between division to three cells and subsequent division to four cells (t4–t3 = S2; OR 2.04, 95% CI 1.07–4.07) and duration of second cell cycle (t3–t2 or CC2; OR 1.84, 95% CI 0.95–3.58) [84].
Although consensus is still lacking, time-lapse markers, defined by time-lapse imaging and correlated with clinical outcomes, potentially provide embryologists with new opportunities for embryo selection. They may also lead to an increase in both the day 3 implantation (30.2 vs 19.0%) and clinical pregnancy (46.0 vs 32.1%) rates compared with morphological assessment alone [85]. In one retrospective study of 3002 embryos from 626 IVF cycles, an automatic time-lapse imaging test in conjunction with standard morphological assessment was superior to single point morphological assessment for predicting which embryos would most likely result in ongoing pregnancy: the inclusion of at least one day 3 embryo classified as high in the imaging test increased ongoing pregnancy rate by 2.567 times compared with cycles in which no embryos classified as high were transferred [86].
One study that compared selection using time-lapse microscopy with a multivariate morphokinetic model—with selection based exclusively on morphology for several reproductive outcomes—observed that the implantation rate (44.9% [41.4–48.4] vs 37.1% [33.6–40.7]) and ongoing pregnancy rate per cycle (51.4% [46.7–56.0] vs 41.7% [36.9–46.5]) and per transfer (54.5% [49.6–59.2] vs 45.3% [40.3–50.4]) were increased with time-lapse microscopy [87]. In addition, early pregnancy loss was decreased with time-lapse microscopy compared with morphological assessment alone (16.6% [12.6–21.4] vs 25.8% [20.6–31.9]) [87]. However, a single-centre external validation of the model failed to replicate the previously reported implantation rates, suggesting that further work was needed on this model for embryo selection in different settings [88].
In a retrospective study using a dataset of transferred blastocysts with known outcome, embryos were categorized according to the timing model by Conaghan et al. (i.e. as either a usable blastocyst [t2–t1 9.33–11.45 h and t3–t2 0–1.73 h] or an unusable blastocyst [t2–t1 > 9.33–11.45 h and t3–t2 > 0–1.73 h]). The actual implantation rate for the blastocysts classified as usable was 22.7%, whereas the rate for blastocysts classified as unusable was 14.2% [89], showing an increase of 30% in implantation rate for embryos grouped as usable compared with the entire data cohort. It also showed that 50% of the implanted embryos were categorized as unusable in the model, indicating that a large proportion of embryos may be incorrectly classified using this model. However, the Conaghan model did not suggest the embryo categories (high and low) to be termed “usable” or “unusable” but rather to be used in conjunction with standard morphology to improve the chances of selecting an embryo with a high potential to develop into a useable blastocyst.
A retrospective study by Basile et al. (2015), which aimed to identify key morphokinetic marker variables, found that the time to three cells (t3: 34–40 h), the length of the second cell cycle (cc2 9–12 h) and the time to five cells (t5: 45–55 h) predicted with the highest accuracy the embryos most likely to implant [90].
-
(b)
Several important deselection criteria have emerged from the introduction of continuous embryo monitoring, such as direct, abnormal, chaotic and reverse cleavage of embryonic cells. As time-lapse systems become available, these criteria should be adopted in a systematic manner.
The 2020 ESHRE Good Practice Recommendations provided 11 recommendations on how to introduce time-lapse technology into the IVF laboratory, which are expected to have a significant impact on future developments of clinical embryology, considering the increasing role and impact of time-lapse technology [53].
Continuous embryo monitoring can be used to identify several atypical phenotypes that might reduce the implantation potential of embryos. In one retrospective study, there was a high prevalence of atypical embryos: abnormal syngamy (25.1% [163/649]), abnormal first cytokinesis (18% [115/639]), abnormal cleavage 18% [115/639]) and chaotic cleavage (15% [96/639]). Although a high proportion of embryos with atypical phenotypes were judged to be of good or fair quality on day 3, the blastocyst formation rates were lower for these embryos than for embryos in the control groups, suggesting that embryos with atypical phenotypes have low developmental potential [91].
Embryos with reverse cleavage assessed at day 3, which were classified as poor quality when assessed by conventional grading or morphokinetics, were reported to have poor implantation potential [92]. Embryos that underwent direct cleavage from 1 to 3 cells or that stayed at the 2-cell stage were observed to be less likely to implant than embryos with a normal cleavage pattern [93]. However, in one study, 21.6% of embryos with irregular cleavage, where one blastomere divided directly into three or more daughter cells, continued development to the blastocyst stage. Seventy-five percent of these embryos (i.e. 16.2% in total) were shown to have a euploid chromosomal constitution [94].
In line with this, Zhan et al., reported that blastocyst formation, implantation potential and euploid rate were decreased in embryos with direct unequal cleavages (defined by Zhan et al. as extremely short cell cycles with incomplete DNA replication and possible unequal distribution of DNA to blastomeres) and recommended that these embryos should be deselected for transfer at day 3, but should be cultured to the blastocyst stage [95]. These results highlight the importance of not relying solely on technology, as false positives are inherent with all diagnostics tests and demonstrate why current time-lapse systems are not designed to predict absolute viability.
-
(c)
Regardless of the lack of strong evidence supporting improved clinical outcomes, continuous embryo monitoring technology has produced an immense amount of novel information on embryo development. Therefore, continuous embryo monitoring is expected to remain and perhaps, in combination with bench-top incubators, entirely replace conventional (box/chamber) incubators.
A review by Basile et al. looked at how assessment of embryo morphokinetics could be used in combination with standard morphological assessment to improve IVF outcomes [20]. They reported that even though there are several randomized controlled trials comparing clinical outcomes after selection based on morphokinetics or standard morphology, only one observed a significant increase in implantation and ongoing pregnancy rates after selection based on these parameters. Nevertheless, the authors concluded that assessment of embryo morphokinetics may be used as a complement to standard morphological assessment to provide the best treatment outcomes.
An example of how continuous embryo monitoring may provide novel information on the effect of environmental factors on embryo development was reported by Freour et al. [96]. This study compared embryo morphokinetics from smoking and non-smoking women and observed cleavage events occurring later in smokers. The authors argued that such differences would be missed using conventional embryo assessment [97].
9. Embryo assessment approaches can shorten the time needed to achieve a live birth by ranking embryos according to their perceived developmental potential and by indicating an order of priority for embryo transfer, thereby improving treatment efficiency. However, by definition, no selection method can result in improved live birth rates per stimulated cycle compared to serial transfer of all embryos. A balancing of efficacy and efficiency should be the goal of IVF clinics, health care providers and authorities, optimizing laboratory performance and shortening time to live birth(s).
The primary aim of embryo selection is to identify and transfer the embryo(s) that are most likely to implant and result in a healthy live birth. However, as previously discussed, defining the variables that accurately predict ART success at the early stages of embryo development remains challenging. Furthermore, although it might shorten the time to achieve a live birth, there is a lack of evidence that embryo selection results in higher live birth rates compared with cryopreservation and subsequent transfer of all available embryos [98, 99]. Nevertheless, different embryo assessment approaches provide valuable information on the events occurring at different stages of embryo development. These data can be used to identify the relationship between those events and clinical outcomes and define predictive factors and models that could further assist embryo selection. Incorporation of this strategy is key to shortening the time to live birth for couples undergoing ART.
10. Inaccuracies in embryo assessment and selection can preclude the use of viable embryos, leading to reduced treatment efficacy.
In a review published in 2011, Mastenbroek et al. argued that embryo selection techniques could potentially lower ART treatment success rates compared with transfer of all embryos. The authors were of the opinion that the only parameter that can be improved if embryos with the highest potential are transferred first is time to pregnancy [98]. They proposed that any embryos not transferred should be cryopreserved and transferred in subsequent cycles [98]. This may ensure that both treatment efficacy and efficiency are maintained. In a later review by Wong et al., the authors were of the opinion that, with the success rates of frozen–thawed embryo transfers now reaching the same level as fresh embryo transfers, success rates could be optimized by adopting a freeze-all strategy, rather than employing embryo assessment and selection approaches, and they called for randomized trials to corroborate their proposal [99]. However, the relevance of any intervention that effectively shortens time to live birth should not be underestimated. Indeed, infertility and ART treatment can be accompanied by high levels of psychological, emotional, physical and financial stress for couples. This combined burden of treatment is often underestimated and is the primary reason for women to abandon IVF treatment. A study by Domar et al. observed that after a first failed fresh treatment cycle and within 1 year of this cycle, 65% of women did not return for treatment or seek care elsewhere, 40% citing stress as the main reason [9]. By improving treatment efficiency and shortening time to live birth, the burden of care and associated high drop-out rates can be reduced.
Statements to reduce time to live birth by increasing cumulative success rates and reducing the number of treatment cycles
These statements include procedures that will contribute to shorten time to live birth by reducing the number of total treatment cycles needed for a live birth.
Embryo assessment and selection
11. Culture, transfer and cryopreservation at the blastocyst stage increase the chances of selection and therefore improve treatment efficiency. However, cumulative success rates (derived from the use of all embryos, fresh and cryopreserved, obtained in a single cycle) are equivalent in cleavage and blastocyst stage embryo transfer cycles.
Fresh embryo transfer at the blastocyst stage can lead to better ART outcomes compared with fresh embryo transfer at the cleavage stage [63]. However, cleavage stage transfer is associated with greater numbers of embryos for freezing, whereas blastocyst transfer is associated with an increased number of cycles with no embryos to transfer [60]. Therefore, blastocyst transfer is recommended only when there is a good chance of an embryo reaching the blastocyst stage. Furthermore, as there is no way to predict whether an embryo that did not survive to blastocyst stage would have survived if it were transferred at the cleavage stage, early cleavage stage transfer may in some cases be more efficient. Such decisions can be facilitated by embryo assessment and ranking, to ensure that the best-quality embryos are transferred earlier in the treatment process; and optimization of these processes will reduce the time to live birth through the need for fewer treatment cycles.
In a Cochrane review, the live birth rate following fresh transfer was higher in the blastocyst transfer group than the cleavage transfer group (OR 1.48, 95% CI 1.20–1.82; 13 randomized controlled trials of 1630 women) [63]. However, there were no differences in the rates of cumulative pregnancy following either fresh or frozen–thawed transfer after one oocyte retrieval cycle (OR 0.89, 95% CI 0.64–1.22; five randomized controlled trials of 632 women). The evidence for both outcomes was judged to be of low quality, owing to a serious risk of bias in the constituent trials [63]. In a retrospective study by de Vos et al. (2016), it was shown that day 5 transfer plus frozen–thawed blastocyst transfers resulted in a cumulative live birth rate of 52.5%, while day 3 transfer plus frozen–thawed cleavage stage transfers resulted in a cumulative live birth rate of 52.6%, although a higher number of cleavage stage transfers was needed [100]. The increased number of cleavage stage embryo transfers required to achieve similar cumulative live birth rate as that reported for blastocyst transfers may, however, prolong the time taken to achieve a healthy singleton birth as more couples will achieve live birth at the first attempt after transfer of a blastocyst embryo that has been assessed to be good quality.
Cryopreservation
12. Cryopreservation is central to fully harness the reproductive potential of a cohort of oocytes or embryos derived from a single treatment cycle. All laboratories should do their best to master this technology.
Cryopreservation of oocytes or embryos helps to avoid the need for another stimulation cycle, allows successive transfers of single embryos and is an important factor in achieving higher cumulative pregnancy rates [99]. The two most widely used methods for cryopreservation are slow freezing and vitrification [101]. Slow freezing is based on gradual reduction in temperature with the use of low concentrations of cryoprotectants. Vitrification employs high cryoprotectant concentrations, rapid reduction in temperature and immediate exposure to liquid nitrogen. Several parameters, including day of cryopreservation, freezing method and selection method, can vary from laboratory to laboratory, and more work is needed to develop optimal cryopreservation methods [101]. The loss of embryos after cryopreservation will inevitably reduce the cumulative live birth rate and increase the time to live birth. There are published consensus recommendations to benchmark the key performance indicators and best practice goals for oocyte and embryo cryopreservation using either slow freezing or vitrification, with the aim to improve efficacy [102]. However, these recommendations are not put into the broader context of how improvements in cryopreservation and vitrification can impact on treatment efficacy/efficiency or time to live birth.
13. While vitrification has become the standard of practice for oocyte and embryo cryopreservation, especially at the later stages of development, optimized slow freezing remains competitive for the cryopreservation of cleavage stage embryos and ovarian cortical tissue.
The scientific evidence comparing vitrification with slow freezing remains controversial. One study by Edgar et al. showed that the application of an optimized slow freezing protocol to cleavage stage embryos led to increased rates of surviving embryos (92.6 vs 78.5%), blastomeres (91.1 vs 74.1%) and fully intact embryos (80.4 vs 54.6%) compared with a conventional freezing protocol [30]. This improvement was achieved by increasing the concentration of the non-permeating cryoprotectant sucrose from 0.1 to 0.2 mol/L; post-thaw resumption of mitosis in vitro and implantation were not adversely affected by the increased sucrose concentration. By contrast, a randomized controlled trial assessing live birth rates after cleavage stage embryo transfer showed that better clinical outcomes were achieved using vitrification rather than slow freezing (implantation rate per embryo: relative risk 2.76, 95% CI 1.59–4.81; live birth rate: 3.23, 95% CI 1.64–6.35) [103]. However, it is important to note that 0.1 mol/L sucrose was used in this study, and improved clinical outcomes might have been observed with an optimized slow freezing protocol, as suggested by Edgar et al.
Rienzi et al. (2017) performed a meta-analysis comparing clinical outcomes after slow freezing or vitrification of oocytes and embryos [104]. Although the results were inconclusive and only a small number of trials met the inclusion criteria for each outcome, a secondary analysis showed an improvement in cryosurvival following vitrification compared with slow freezing (RR 1.59, 95% CI 1.30–1.93; seven studies, 3615 embryos). Both cleavage stage and blastocyst stage embryos were included in this analysis, and the outcomes for both groups were analysed collectively and results were pooled.
Although vitrification of embryos and oocytes can be successful in experienced hands, manual vitrification is currently performed using complex and technically challenging manual procedures and a variety of devices and protocols, which are dependent on operator skills [105]. Several factors can influence recovery, survival, clinical outcomes, miscarriages and ultimately the time needed to achieve a healthy live birth. Although many laboratories achieve very high standards of performance, this is not the case for all laboratories, and wide differences in survival rates among operators and laboratories are commonly seen. Future technology may reduce this dependence on the skills of the embryologist, facilitating standardization of the vitrification procedure and possibly raising success rates and reducing the time to live birth.
Discussion
The aim of this consensus was to consider the benefits and limitations of available ART laboratory technologies and, taking into consideration existing guidelines from international societies, provide guidance on how the technologies used in the ART laboratory could be optimized to reduce the time to a healthy singleton birth. This guidance should be considered in the context of other key factors that could be optimized, including access to care, ovarian stimulation, ovulation triggering and luteal phase support, with the aim of reducing drop-out rates among women undergoing ART. In addition, there is limited mention in the published literature of the investigation of laboratory technologies in relation to reducing the time to live birth, as this is a relatively new endpoint. Appropriate time-related outcome measures are considered worthy of discussion, selection and inclusion in future study designs, when evaluating fertility treatment [13].
General management of the ART laboratory and procedures
The Delphi consensus identified the laboratory as playing a central role in the success of ART treatment and provided guidance on how to integrate current knowledge on laboratory practice to achieve a high number of good-quality embryos from the perspective of how the key position of the laboratory can be harnessed to shorten the time to live birth. An important message that emerged from this consensus was that, although evidence-based studies are often unavailable in this field, professional body guidelines and traditional well-researched technologies are needed. These could be used to harmonize and benchmark laboratory performance (e.g. in gamete processing; embryo culture, assessment, selection and cryopreservation; and on the standardization of laboratory processes to increase competency and quality) and to improve success rates and shorten the time to live birth. As we have identified, optimization of several laboratory processes may influence this endpoint but it is still not clear how to bridge the gap between standard practices and the introduction of new technologies to achieve this goal.
In addition, laboratories should be equipped with the most up-to-date integrated quality management and audit systems, covering all aspects of the function of the laboratory, including assessment of laboratory performance indicators; recording/reporting of non-conformance and corrective actions/preventative actions (CAPA); identification, witnessing and tracking of patients and their samples; and the traceability of critical consumables. Developing technologies, including continuous embryo monitoring/time-lapse technology and possibly future implementations of AI, may be considered and employed in combination with traditional methods to optimize laboratory performance and potentially reduce the time taken to achieve a healthy singleton delivery.
The adoption of novel technologies in ART laboratories may be impeded by the lack of sufficient evidence supporting their use, and the studies used to evaluate them might be conducted in a manner that does not allow inter-study comparison. This has resulted in some of the technologies discussed in this Delphi consensus that were only developed in the past decade being introduced into the ART laboratory without robust evidence from randomized controlled trials. This may partly be the result of the difficulties inherent in conducting randomized controlled trials on laboratory interventions for ART: the source materials (e.g. the gametes) are not uniform; a successful outcome depends on multiple factors (e.g. patient history and stimulation protocols); and the attainment of many of the clinical endpoints evaluated (e.g. implantation rates, clinical pregnancy rates and live birth rates) depends on many factors outside of the laboratory. It is, therefore, challenging to determine whether the reasons for unsuccessful treatment cycles are related to laboratory factors, to factors external to the laboratory or to a mix of external and laboratory factors. Because there is a wide variation among laboratories and countries in success rates following ART treatment, and the benefits on reducing time to live birth from novel ART laboratory interventions may be small yet clinically relevant, [6], future multicentre trials should be designed to minimize variability from centre effects, in a similar manner to those for personalized ART treatment. Proof-of-principle evidence of the benefits of using a specific laboratory innovation can also be obtained from separate trials using the same study protocol in different centres. If separate studies are to be conducted at multiple centres, these centres should be selected based upon their established interest in the process being evaluated and experience addressing the primary study question, their commitment to research, the quality of the ART fertility laboratory and their experience conducting clinical trials [6]. The selection of centres needs to be as fair as possible, and efforts would be needed to ensure that selection is not influenced by economic, political or academic bias [6]. Studies evaluating the effect of laboratory interventions may first aim to demonstrate proportional and timely improvements in intermediate outcomes (i.e. number and quality of oocytes/embryos, total embryo utilization rate) that are closely related to the intervention and which may ultimately contribute to improved implantation rates, clinical pregnancy rates and live birth rates, all of which can reduce the time to live birth.
Embryo culture
Extending embryo culture to the blastocyst stage provides embryologists time to further assess the development of the embryo. Blastocyst transfer also more closely reflects the natural timing for implantation and blastocyst–endometrium synchronicity. Indeed, in light of the influence of factors external to the laboratory (discussed in the previous section), good-quality blastocyst formation rates may be the best endpoint for evaluation of laboratory performance. However, embryos are more likely to arrest naturally with extended culture, which introduces increased risk of no embryo being available for transfer. Blastocyst culture to day 7 is also being adopted; however, data indicate that even euploid day 7 blastocysts have a lower implantation potential [106]. In addition, extending culture to day 5/6 may expose the embryo to sub-optimal conditions and it is, therefore, important to optimize the culture environment and minimize embryo stressors (e.g. pH, temperature, oxygen tension and toxin build up) to reduce the risk of prolonged culture affecting the development of the embryo [107, 108]. Blastocyst culture is usually considered for patients with a good prognosis and when at least three good-quality day 3 embryos or a large number of fertilized eggs are available [6], as blastocyst culture compliments a successful elective SET policy in these patients. In a retrospective study, Racowsky et al. reported the number of 8-cell embryos as a key determinant for selecting day 3 or day 5 transfer: a day 3 transfer is warranted when no 8-cell embryos are available on day 3, with one or two 8-cell embryos on day 3, any advantage of a day 5 transfer appears to be equivocal and with three or more 8-cell embryos, a day 5 transfer is recommended [109]. In older women, when only one or two early cleavage embryos are available, it may be more acceptable for the couple to transfer on day 2/3, rather than risk having no embryos available to transfer on day 5/6 [60]. Appropriate counselling is advised, with the benefits and risks assessed and the couple’s preference taken into consideration.
The introduction of integrated systems that combine both undisturbed embryo culture and continuous embryo monitoring reduces embryo handling and sudden changes in environment (e.g. culture media pH and temperature, as well as exposure to the broader laboratory environment). In a retrospective study, McEvoy et al. reported an improvement in live birth rates (42 vs 31%, p = 0.006), in addition to a significant reduction in miscarriage rate (4.4 vs 11.1%, p = 0.001), when a closed system was used rather than a standard incubator [110]. Zhang et al. (2010) reported that a reduction in observation frequency/embryo exposure outside the incubator (six openings [control] vs four openings [test]) enhanced embryo quality and blastulation rate [111]. The total blastocyst formation rate, day 5 blastocyst formation rate, proportion of good blastocysts and number of cryopreserved blastocysts per patient were significantly lower in the control group compared with the test group (42.5%, 31.4%, 50.7%, 1.72 ± 1.55 and 52.6%, 40.7%, 60.1%, 2.64 ± 2.59, respectively, p < 0.05).
Closed systems may also provide large amounts of additional information on the timing of developmental events when used in conjunction with time-lapse monitoring, which can assist in the ranking of embryos and decision-making (e.g. how many embryos to transfer). Further research is required to validate the efficacy of these methods and assessment models; these should be validated internally in conjunction with standard morphological assessment, quality assurance practices and professional body guidelines.
Embryo assessment and selection
Assessment, grading and selection of embryos with the highest implantation potential have been reported to increase pregnancy rates, and ensuring that embryos for transfer/cryopreservation are prioritized in order of quality may reduce the number of treatment cycles required and thereby reduce the time to live birth. Despite the high level of intra- and inter-user variability [112] and problems with standardization, conventional morphological assessment is still the most commonly used form of embryo grading. Therefore, novel embryo grading systems should only be applied in combination with standard morphological assessment, and the grading should be correlated with laboratory and clinical outcomes.
It is important to note that selection of the “best” (i.e. with highest potential for implantation and live birth) embryos does not necessarily result in higher cumulative live birth rates compared with sequential transfer of all available embryos. However, it may improve time to healthy live birth and patient retention rates, which are also markers of ART treatment success. Furthermore, infertility and ART treatment are accompanied by high levels of psychological, emotional, physical and financial stress, and treatment burden is often underestimated, despite being the primary reason for couples abandoning IVF treatment [9]. By improving treatment efficiency and shortening time to live birth, treatment burden and associated high drop-out rates should be reduced and the birth rate per initiated couple/woman increased.
In a previous Delphi consensus of expert-selected evidence, around 80% of the expert panel agreed that preimplantation genetic testing for aneuploidy (PGT-A) could decrease the aneuploidy rate and could also increase clinical and sustained pregnancy rates, reduce multiple pregnancy rates in combination with single embryo transfer and reduce the times to pregnancy and healthy singleton delivery. These benefits were mostly attributed to increased pregnancy rates and decreased miscarriage rates per transfer compared with untested embryos, including patients who were classified as low or poor responders [1].
PGT-A might shorten time to achieve a live birth. A retrospective observational analysis of 569 frozen–thawed embryo transfers in patients undergoing PGT for monogenic disorders (131 transfers in patients who underwent PGT-A and 438 transfers in patients who underwent PGT without screening for aneuploidy) observed a higher implantation rate with PGT-A compared with PGT without aneuploidy testing (64.29% vs 50.38%) [113]. In addition, live birth was twice as likely in the first transfer after PGT-A in young women, although no difference was found in cumulative pregnancy outcomes. PGT-A may, therefore, shorten time to achieve a live birth in some patients.
However, in a randomized controlled trial of 661 women aged 25–40 years who were randomly assigned to single frozen–thawed embryo transfer with embryo selection based on PGT-A euploid status versus morphology, there was no difference in ongoing pregnancy rates per embryo transfer (50% [137/274] vs 46% [143/313]) or per intention to treat at randomization 42% [138/330] vs 44% [144/331]). A significant increase was seen per embryo transfer in a post hoc analysis of women aged 35–40 years (51% [62/122] vs 37% [54/154]) where at least two embryos were available for biopsy [114].
Increasingly, research has used novel methods of embryo assessment to define criteria that identify less-viable embryos (e.g. those with abnormal, reverse or chaotic cleavage) for deselection. However, indiscriminate use of deselection increases the risk of embryo wastage, and deselection criteria should be used to define the order in which embryos are transferred and not automatically lead to them being discarded [93]. In this context, embryos identified as having low implantation potential should be cultured to blastocyst stage, where possible, and/or cryopreserved for subsequent cycles [91, 95]. However, the benefit of the retention and possible use of such deselected embryos should be weighed against any potential gains from reducing treatment burden (from failed cycles and increased cost if there is a low chance of implantation when using these deselected embryos, which could lead to treatment discontinuation before a live birth is achieved [9]). More evidence is needed to enable the use of continuous embryo monitoring as the basis for embryo exclusion, and the algorithms, models and the terminology should be standardized with the intention of selecting and ranking embryos, rather than discarding them.
Cryopreservation
The European IVF Monitoring Consortium for ESHRE, in its ART in Europe Report 2014, reported that the rate of frozen–thawed embryo transfer is rapidly increasing [115]. Recent data from the Centers for Disease Control and Prevention (CDC) in the USA has also demonstrated that the number of frozen–thawed embryo transfers conducted has surpassed the number of fresh embryo transfers [116]. The rapid growth in frozen–thawed transfer cycles can be explained by the recent systematic application of cryopreservation for newer indications, including elective SET, cycle segmentation (planned “freeze-all”), oocyte banking (social and medical) and PGT at blastocyst stage [104]. It is also important to note the rapidly increasing success rates for frozen–thawed embryo transfers, which now achieve the same delivery rates as fresh transfers [115]. The central role that cryopreservation now plays in ART highlights the critical importance of cryopreservation techniques and the potential for cryopreservation to reduce the number of fresh treatment cycles required and consequently shorten the time to live birth. However, optimization and standardization of the cryopreservation processes is a key factor in reducing the time to a healthy singleton birth and in reducing the treatment burden to the patient.
Vitrification has become standard practice for oocyte cryopreservation and, in some cases, better clinical outcomes have been observed compared with slow freezing. It is important to note, however, that vitrification is, for the most part, a manual, rather than automated, process [104], performed with complex and technically difficult manual procedures and a variety of devices and protocols [105]. Its effectiveness is therefore dependent on laboratory protocols and on the experience and technical skills of the embryologists [104]. Future technology is likely to reduce this dependence on experience and skills, standardize processes and raise vitrification success rates [105]. Such improvements in vitrification techniques means than more embryos will survive the vitrification process, leading to fewer fresh cycles and a reduction in the time to live birth.
Limitations
This consensus has a number of limitations. Firstly, it only represents the collective opinion of the experts who participated in this consensus and does not represent the individual opinions of the participants, who individually might not have agreed with all of the statements. Secondly, the majority of the experts were from Europe and the USA, and their views may not represent those held by experts in other areas of the world. Thirdly, the concept of time to live birth is not addressed in many studies referenced in the consensus.
Conclusions
In conclusion, time to healthy singleton birth is of increasing importance when making decisions about fertility treatment, as the mean age of women seeking infertility treatment is increasing with a concomitant decline in fertility. The ART laboratory can contribute significantly to reducing the time taken to achieve a healthy singleton delivery through standardized practices and optimal embryo culture environment, embryo assessment and selection, and cryopreservation methodologies. These measures may potentially reduce the number of embryo transfers and fresh treatment cycles required by selecting and ranking the optimal embryos for transfer in fresh and subsequent frozen–thawed cycles. Traditional methods should be retained and should complement novel methods to optimize treatment outcomes from as few treatment cycles as possible. Careful consideration of the study design is needed, to enable demonstration of the added value of novel ART laboratory techniques. This should include selection of meaningful primary outcomes that would demonstrate the possibility of improved treatment outcomes.
Acknowledgments
Medical writing support was provided by Evelina Matekonyte and Steven Goodrick, inScience Communications, Springer Healthcare Ltd, UK, and funded by Merck Healthcare KGaA, an affiliate of Merck KGaA, Darmstadt, Germany. The Delphi consensus process was coordinated by Sanitanova Srl.
Authors’ contributions
GC contributed to project direction and coordination and provided the final approval of the version to be published. BB contributed to the consensus design and data analysis, assisted with drafting the article and revising it critically for important intellectual content, and provided the final approval of the version to be published. AC was an expert panel member and survey participant and assisted with editing and reviewing the manuscript and provided the final approval of the version to be published. MM was an expert panel member and survey participant and provided the final approval of the version to be published. DEM was an expert panel member and survey participant and edited and reviewed the manuscript. VP contributed to project coordination and assisted with editing and reviewing the manuscript and provided the final approval of the version to be published. CEP participated in all stages of manuscript preparation and provided final approval of the version to be published. DS participated in the construction of the consensus statements, survey and manuscript preparation and provided the final approval of the version to be published. YX was a survey participant and reviewed the manuscript and provided approval of the final version to be published. TDH contributed to overall concept and design, assisted with manuscript drafting and revision and provided the final approval of the version to be published. EC contributed to overall concept and design, assisted with manuscript drafting and revision and provided the final approval of the version to be published. KL was an expert panel member and survey participant and edited and reviewed the manuscript. All authors read and approved the final manuscript.
Funding
The work was funded by Merck KGaA, Darmstadt, Germany.
Materials availability
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to Merck KGaA’s Data Sharing Policy. All requests should be submitted in writing to Merck KGaA’s data sharing portal https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html. When Merck KGaA has a co-research, co-development, or co-marketing or co-promotion agreement, or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, Merck KGaA will endeavour to gain agreement to share data in response to requests.
Code availability
Not applicable
Declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Conflict of interest
VP, BB and YX have nothing to disclose.
EC and TDH are employees of Merck KGaA, Darmstadt, Germany.
AC has received consultancy fees from Merck KGaA, Darmstadt, Germany, and is a minor shareholder in the CARE Fertility Group, a private company offering fertility treatment.
DM is a consultant to Cook Medical, Irvine Scientific and Cooper Surgical and received personal fees from Sanitanova during the conduct of this study.
GC reports personal fees from Merck KGaA, Darmstadt, Germany, during the conduct of this study, and personal fees from IBSA and Excemed outside of this submitted work.
MM reports speaker fees from Merck KGaA, Darmstadt, Germany, during the conduct of this study, and personal fees from MSD and Ferring outside of this work.
KL reports personal fees from Sanitanova during the conduct of the study.
DS has nothing to disclose in relation to the submitted work.
CP reports personal fees from Sanitanova during the course of the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bosch E, Bulletti C, Copperman AB, Fanchin R, Yarali H, Petta CA, Polyzos NP, Shapiro D, Ubaldi FM, Garcia Velasco JA, Longobardi S, D'Hooghe T, Humaidan P, Delphi TTP Consensus Group How time to healthy singleton delivery could affect decision-making during infertility treatment: a Delphi consensus. Reprod BioMed Online. 2019;38(1):118–130. doi: 10.1016/j.rbmo.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 2.ESHRE Capri Workshop Group Europe the continent with the lowest fertility. Hum Reprod Update. 2010;16(6):590–602. doi: 10.1093/humupd/dmq023. [DOI] [PubMed] [Google Scholar]
- 3.Farquhar CM, Bhattacharya S, Repping S, Mastenbroek S, Kamath MS, Marjoribanks J, Boivin J. Female subfertility. Nat Rev Dis Primers. 2019;5(1):7. doi: 10.1038/s41572-018-0058-8. [DOI] [PubMed] [Google Scholar]
- 4.American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril. 2014;101(3):633–634. doi: 10.1016/j.fertnstert.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 5.American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Practice Committee of the American Society for Reproductive Medicine Female age-related fertility decline. Committee Opinion No. 589. Obstet Gynecol. 2014;123(3):719–721. doi: 10.1097/01.AOG.0000444440.96486.61. [DOI] [PubMed] [Google Scholar]
- 6.Mol BW, Bossuyt PM, Sunkara SK, Garcia Velasco JA, Venetis C, Sakkas D, Lundin K, Simón C, Taylor HS, Wan R, Longobardi S, Cottell E, D'Hooghe T. Personalized ovarian stimulation for assisted reproductive technology: study design considerations to move from hype to added value for patients. Fertil Steril. 2018;109(6):968–979. doi: 10.1016/j.fertnstert.2018.04.037. [DOI] [PubMed] [Google Scholar]
- 7.Annual Capri Workshop Group Towards a more pragmatic and wiser approach to infertility care. Human Reprod. 2019;34(7):1165–1172. doi: 10.1093/humrep/dez101. [DOI] [PubMed] [Google Scholar]
- 8.Goswami M, Hyslop LA, Murdoch AP. NHS-funded IVF: consequences of NICE implementation. Hum Fertil. 2013;16(2):121–127. doi: 10.3109/14647273.2013.786840. [DOI] [PubMed] [Google Scholar]
- 9.Domar AD, Rooney K, Hacker MR, Sakkas D, Dodge LE. Burden of care is the primary reason why insured women terminate in vitro fertilization treatment. Fertil Steril. 2018;109(6):1121–1126. doi: 10.1016/j.fertnstert.2018.02.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrick L, Lee YSL, Gardner DK. Reducing time to pregnancy and facilitating the birth of healthy children through functional analysis of embryo physiology. Biol Reprod. 2019;101(6):1124–1139. doi: 10.1093/biolre/ioz005. [DOI] [PubMed] [Google Scholar]
- 11.Harbottle S, Hughes C, Cutting R, Roberts S, Brison D. Elective single embryo transfer: an update to UK Best Practice Guidelines. Hum Fertil. 2015;18(3):165–183. doi: 10.3109/14647273.2015.1083144. [DOI] [PubMed] [Google Scholar]
- 12.Hughes EG. Singleton birth at term: an old alarm or a new debate? Human Reprod. 2015;30(10):2254–2256. doi: 10.1093/humrep/dev205. [DOI] [PubMed] [Google Scholar]
- 13.Sunkara SK, Zheng W, D’Hooghe T, Longobardi S, Boivin J. Time as an outcome measure in fertility-related clinical studies: long-awaited. Human Reprod. 2020;35(8):1732–1739. doi: 10.1093/humrep/deaa138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke ID, Simpson JL, van der Poel S. The international glossary on infertility and fertility care, 2017. Fertil Steril. 2017;108(3):393–406. doi: 10.1016/j.fertnstert.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Barnhart KT. Live birth is the correct outcome for clinical trials evaluating therapy for the infertile couple. Fertil Steril. 2014;101(5):1205–1208. doi: 10.1016/j.fertnstert.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke JF, van Rumste MM, Farquhar CM, Johnson NP, Mol BW, Herbison P. Measuring outcomes in fertility trials: can we rely on clinical pregnancy rates? Fertil Steril. 2010;94(5):1647–1651. doi: 10.1016/j.fertnstert.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 17.Steurer J. The Delphi method: an efficient procedure to generate knowledge. Skelet Radiol. 2011;40(8):959–961. doi: 10.1007/s00256-011-1145-z. [DOI] [PubMed] [Google Scholar]
- 18.Bulletti C, Allegra A, Mignini Renzini M, Vaiarelli A. How fixed versus variable gonadotropin dose during controlled ovarian stimulation could influence the management of infertility patients undergoing IVF treatment: a national Delphi consensus. Gynecol Endocrinol. 2020:1–9. 10.1080/09513590.2020.1770214. [DOI] [PubMed]
- 19.De los Santos MJ, Apter S, Coticchio G, Debrock S, Lundin K, Plancha CE, et al. Revised guidelines for good practice in IVF laboratories (2015) Human Reprod. 2016;31(4):685–686. doi: 10.1093/humrep/dew016. [DOI] [PubMed] [Google Scholar]
- 20.Basile N, Caiazzo M, Meseguer M. What does morphokinetics add to embryo selection and in-vitro fertilization outcomes? Curr Opin Obstet Gynecol. 2015;27(3):193–200. doi: 10.1097/gco.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 21.Meseguer M, Rubio I, Cruz M, Basile N, Marcos J, Requena A. Embryo incubation and selection in a time-lapse monitoring system improves pregnancy outcome compared with a standard incubator: a retrospective cohort study. Fertil Steril. 2012;98(6):1481–1489. doi: 10.1016/j.fertnstert.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Cairo Consensus Group ‘There is only one thing that is truly important in an IVF laboratory: everything’ Cairo Consensus Guidelines on IVF Culture Conditions. Reprod BioMed Online. 2020;40:33–60. doi: 10.1016/j.rbmo.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Katz-Jaffe MG, McReynolds S, Gardner DK, Schoolcraft WB. The role of proteomics in defining the human embryonic secretome. Mol Hum Reprod. 2009;15(5):271–277. doi: 10.1093/molehr/gap012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Botros L, Sakkas D, Seli E. Metabolomics and its application for non-invasive embryo assessment in IVF. Mol Hum Reprod. 2008;14(12):679–690. doi: 10.1093/molehr/gan066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovacs GL, Montsko G, Zrinyi Z, Farkas N, Varnagy A, Bodis J. Non-invasive assessment of viability in human embryos fertilized in vitro. EJIFCC. 2016;27(2):112–121. [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy NM, Samarasekera TS, Macaskill L, Mullen J, Rombauts LJF. Genome sequencing of human in vitro fertilisation embryos for pathogenic variation screening. Sci Rep. 2020;10(1):3795. doi: 10.1038/s41598-020-60704-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Concolino D, Degennaro E, Parini R. Delphi consensus on the current clinical and therapeutic knowledge on Anderson-Fabry disease. Eur J Intern Med. 2014;25(8):751–756. doi: 10.1016/j.ejim.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Girolomoni G, Altomare G, Ayala F, Berardesca E, Calzavara Pinton P, Chimenti S, Martini P, Peserico A, Puglisi Guerra A, Antonio Vena G, on behalf on the Delphi working group Differential management of mild-to-severe psoriasis with biologic drugs: an Italian Delphi consensus expert panel. J Dermatolog Treat. 2015;26(2):128–133. doi: 10.3109/09546634.2014.907466. [DOI] [PubMed] [Google Scholar]
- 29.Lassalle B, Testart J, Renard JP. Human embryo features that influence the success of cryopreservation with the use of 1,2 propanediol. Fertil Steril. 1985;44(5):645–651. doi: 10.1016/S0015-0282(16)48981-8. [DOI] [PubMed] [Google Scholar]
- 30.Edgar DH, Karani J, Gook DA. Increasing dehydration of human cleavage-stage embryos prior to slow cooling significantly increases cryosurvival. Reprod BioMed Online. 2009;19(4):521–525. doi: 10.1016/j.rbmo.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Alpha Scientists In Reproductive Medicine and ESHRE Special Interest Group of Embryology The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Human Reprod. 2011;26(6):1270–1283. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 32.ISO . Quality management systems – requirements. Geneva: International Standards Organisation; 2015. [Google Scholar]
- 33.ISO . General requirements for the competence of testing and calibration laboratories. Geneva: International Standards Organisation; 2017. [Google Scholar]
- 34.ISO . Medical laboratories – requirements for quality and competence. Geneva: International Standards Organisation; 2012. [Google Scholar]
- 35.College of American Pathologists. Laboratory accreditation program. College of American Pathologists, Northfiled, IL. 2019. https://www.cap.org/laboratory-improvement/accreditation/laboratory-accreditation-program. Accessed 12 September 2019.
- 36.European Union . Directive 2004/23/EC of the European Parliament and of the Council of 31 March 2004 on setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells. European Union: Strasbourg; 2004. [Google Scholar]
- 37.European Union. COMMISSION DIRECTIVE 2006/17/EC of 8 February 2006 implementing Directive 2004/23/EC of the European Parliament and of the Council as regards certain technical requirements for the donation, procurement and testing of human tissues and cells. In: The Commission of the European Communities, editor. Brussels: European Commission; 2006.
- 38.European Union . COMMISSION DIRECTIVE 2012/39/EU of 26 November 2012 amending Directive 2006/17/EC as regards certain technical requirements for the testing of human tissues and cells Text with EEA relevance. European Commission: Brussels; 2012. [Google Scholar]
- 39.European Union. COMMISSION DIRECTIVE 2006/86/EC of 24 October 2006 implementing Directive 2004/23/EC of the European Parliament and of the Council as regards traceability requirements, notification of serious adverse reactions and events and certain technical requirements for the coding, processing, preservation, storage and distribution of human tissues and cells. Brussels: European Commission; 2006.
- 40.European Union . COMMISSION DIRECTIVE (EU) 2015/565 of 8 April 2015 amending Directive 2006/86/EC as regards certain technical requirements for the coding of human tissues and cells Text with EEA relevance. European Commission: Brussels; 2015. [Google Scholar]
- 41.European Union . Commission Directive (EU) 2015/566 of 8 April 2015 implementing Directive 2004/23/EC as regards the procedures for verifying the equivalent standards of quality and safety of imported tissues and cells. Text with EEA relevance. European Commission: Brussels; 2015. [Google Scholar]
- 42.Fabozzi G, Cimadomo D, Maggiulli R, Vaiarelli A, Ubaldi FM, Rienzi L. Which key performance indicators are most effective in evaluating and managing an in vitro fertilization laboratory? Fertil Steril. 2020;114(1):9–15. doi: 10.1016/j.fertnstert.2020.04.054. [DOI] [PubMed] [Google Scholar]
- 43.Hughes C. Association of clinical embryologists - guidelines on good practice in clinical embryology laboratories 2012. Hum Fertil. 2012;15(4):174–189. doi: 10.3109/14647273.2012.747891. [DOI] [PubMed] [Google Scholar]
- 44.ESHRE Special Interest Group of Embryology and Alpha Scientists in Reproductive Medicine. The Vienna consensus: report of an expert meeting on the development of ART laboratory performance indicators. Reprod BioMed Online. 2017;35(5):494–510. doi: 10.1016/j.rbmo.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 45.Forte M, Faustini F, Maggiulli R, Scarica C, Romano S, Ottolini C, Farcomeni A, Palagiano A, Capalbo A, Ubaldi FM, Rienzi L. Electronic witness system in IVF-patients perspective. J Assist Reprod Genet. 2016;33(9):1215–1222. doi: 10.1007/s10815-016-0759-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calhaz-Jorge C, De Geyter C, Kupka MS, de Mouzon J, Erb K, Mocanu E, et al. Assisted reproductive technology in Europe, 2013: results generated from European registers by ESHRE. Human Reprod. 2017;32(10):1957–1973. doi: 10.1093/humrep/dex264. [DOI] [PubMed] [Google Scholar]
- 47.Society for Assisted Reproductive T, American Society for Reproductive M Assisted reproductive technology in the United States: 2000 results generated from the American Society for Reproductive Medicine/Society for Assisted Reproductive Technology Registry. Fertil Steril. 2004;81(5):1207–1220. doi: 10.1016/j.fertnstert.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 48.Dyer S, Chambers GM, de Mouzon J, Nygren KG, Zegers-Hochschild F, Mansour R, Ishihara O, Banker M, Adamson GD. International Committee for Monitoring Assisted Reproductive Technologies world report: assisted reproductive technology 2008, 2009 and 2010. Hum Reprod. 2016;31(7):1588–1609. doi: 10.1093/humrep/dew082. [DOI] [PubMed] [Google Scholar]
- 49.Arvis P, Lehert P, Guivarc’h-Leveque A. Simple adaptations to the Templeton model for IVF outcome prediction make it current and clinically useful. Human Reprod. 2012;27(10):2971–2978. doi: 10.1093/humrep/des283. [DOI] [PubMed] [Google Scholar]
- 50.Rienzi L, Bariani F, Dalla Zorza M, Romano S, Scarica C, Maggiulli R, Nanni Costa A, Ubaldi FM. Failure mode and effects analysis of witnessing protocols for ensuring traceability during IVF. Reprod BioMed Online. 2015;31(4):516–522. doi: 10.1016/j.rbmo.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 51.Roy TK, Brandi S, Tappe NM, Bradley CK, Vom E, Henderson C, et al. Embryo vitrification using a novel semi-automated closed system yields in vitro outcomes equivalent to the manual Cryotop method. Human Reprod. 2014;29(11):2431–2438. doi: 10.1093/humrep/deu214. [DOI] [PubMed] [Google Scholar]
- 52.Dal Canto M, Moutier C, Brambillasca F, Guglielmo MC, Bartolacci A, Fadini R, Renzini MM, Buratini J. The first report of pregnancies following blastocyst automated vitrification in Europe. J Gynecol Obstet Human Reprod. 2019;48(7):537–540. doi: 10.1016/j.jogoh.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 53.ESHRE Working Group on Time-lapse Technology Good practice recommendations for the use of time-lapse technology. Hum Reprod Open. 2020;2:1–26. doi: 10.1093/hropen/hoaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khosravi P, Kazemi E, Zhan Q, Malmsten JE, Toschi M, Zisimopoulos P, et al. Deep learning enables robust assessment and selection of human blastocysts after in vitro fertilization. NPJ Digit Med. 2019;2(1):21. doi: 10.1038/s41746-019-0096-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McLernon DJ, Harrild K, Bergh C, Davies MJ, de Neubourg D, Dumoulin JC, et al. Clinical effectiveness of elective single versus double embryo transfer: meta-analysis of individual patient data from randomised trials. BMJ. 2010;341:c6945. doi: 10.1136/bmj.c6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thurin A, Hausken J, Hillensjo T, Jablonowska B, Pinborg A, Strandell A, et al. Elective single-embryo transfer versus double-embryo transfer in in vitro fertilization. N Engl J Med. 2004;351(23):2392–2402. doi: 10.1056/NEJMoa041032. [DOI] [PubMed] [Google Scholar]
- 57.Park DS, Kim JW, Chang EM, Lee WS, Yoon TK, Lyu SW. Strategies in the transfer of varying grades of vitrified-warmed blastocysts in women aged over 35 years: a propensity-matched analysis. J Obstet Gynaecol Res. 2018;45(4):849–857. doi: 10.1111/jog.13897. [DOI] [PubMed] [Google Scholar]
- 58.Zech NH, Lejeune B, Puissant F, Vanderzwalmen S, Zech H, Vanderzwalmen P. Prospective evaluation of the optimal time for selecting a single embryo for transfer: day 3 versus day 5. Fertil Steril. 2007;88(1):244–246. doi: 10.1016/j.fertnstert.2006.11.070. [DOI] [PubMed] [Google Scholar]
- 59.Papanikolaou EG, Kolibianakis EM, Tournaye H, Venetis CA, Fatemi H, Tarlatzis B, et al. Live birth rates after transfer of equal number of blastocysts or cleavage-stage embryos in IVF. A systematic review and meta-analysis. Human Reprod. 2008;23(1):91–99. doi: 10.1093/humrep/dem339. [DOI] [PubMed] [Google Scholar]
- 60.Glujovsky D, Farquhar C. Cleavage-stage or blastocyst transfer: what are the benefits and harms? Fertil Steril. 2016;106(2):244–250. doi: 10.1016/j.fertnstert.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 61.Robinson RD. Success rates and pregnancy outcomes in thawed embryos transferred after extended culture: cryopreserved embryos versus cleavage stage cryopreserved embryos. Fertil Steril. 2018;110(1):59–60. doi: 10.1016/j.fertnstert.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 62.Simón C, Gómez C, Cabanillas S, Vladimirov I, Castillón G, Giles J, Boynukalin K, Findikli N, Bahçeci M, Ortega I, Vidal C, Funabiki M, Izquierdo A, López L, Portela S, Frantz N, Kulmann M, Taguchi S, Labarta E, Colucci F, Mackens S, Santamaría X, Muñoz E, Barrera S, García-Velasco JA, Fernández M, Ferrando M, Ruiz M, Mol BW, Valbuena D, ERA-RCT Study Consortium Group A 5-year multicentre randomized controlled trial comparing personalized, frozen and fresh blastocyst transfer in IVF. Reprod BioMed Online. 2020;41:402–415. doi: 10.1016/j.rbmo.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 63.Glujovsky D, Farquhar C, Quinteiro Retamar AM, Alvarez Sedo CR, Blake D. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. 2016;6:CD002118. doi: 10.1002/14651858.CD002118.pub5. [DOI] [PubMed] [Google Scholar]
- 64.Sfontouris IA, Martins WP, Nastri CO, Viana IG, Navarro PA, Raine-Fenning N, et al. Blastocyst culture using single versus sequential media in clinical IVF: a systematic review and meta-analysis of randomized controlled trials. J Assist Reprod Genet. 2016;33(10):1261–1272. doi: 10.1007/s10815-016-0774-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.La Marca A, Minasi MG, Sighinolfi G, Greco P, Argento C, Grisendi V, et al. Female age, serum antimullerian hormone level, and number of oocytes affect the rate and number of euploid blastocysts in in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil Steril. 2017;108(5):777–783. doi: 10.1016/j.fertnstert.2017.08.029. [DOI] [PubMed] [Google Scholar]
- 66.Motato Y, de los Santos MJ, Escriba MJ, Ruiz BA, Remohi J, Meseguer M. Morphokinetic analysis and embryonic prediction for blastocyst formation through an integrated time-lapse system. Fertil Steril. 2016;105(2):376–384. doi: 10.1016/j.fertnstert.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 67.Esteves SC, Carvalho JF, Bento FC, Santos J. A novel predictive model to estimate the number of mature oocytes required for obtaining at least one euploid blastocyst for transfer in couples undergoing in vitro fertilization/intracytoplasmic sperm injection: the ART calculator. Front Endocrinol (Lausanne) 2019;10:99. doi: 10.3389/fendo.2019.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reed ML, Hamic A, Thompson DJ, Caperton CL. Continuous uninterrupted single medium culture without medium renewal versus sequential media culture: a sibling embryo study. Fertil Steril. 2009;92(5):1783–1786. doi: 10.1016/j.fertnstert.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 69.Mantikou E, Bontekoe S, van Wely M, Seshadri S, Repping S, Mastenbroek S. Low oxygen concentrations for embryo culture in assisted reproductive technologies. Hum Reprod Update. 2013;19(3):209. doi: 10.1093/humupd/dms055. [DOI] [PubMed] [Google Scholar]
- 70.Nastri CO, Nobrega BN, Teixeira DM, Amorim J, Diniz LMM, Barbosa MWP, et al. Low versus atmospheric oxygen tension for embryo culture in assisted reproduction: a systematic review and meta-analysis. Fertil Steril. 2016;106(1):95–104. doi: 10.1016/j.fertnstert.2016.02.037. [DOI] [PubMed] [Google Scholar]
- 71.Garcia-Martinez S, Sanchez Hurtado MA, Gutierrez H, Sanchez Margallo FM, Romar R, Latorre R, et al. Mimicking physiological O2 tension in the female reproductive tract improves assisted reproduction outcomes in pig. Mol Hum Reprod. 2018;24(5):260–270. doi: 10.1093/molehr/gay008. [DOI] [PubMed] [Google Scholar]
- 72.Morin SJ. Oxygen tension in embryo culture: does a shift to 2% O2 in extended culture represent the most physiologic system? J Assist Reprod Genet. 2017;34(3):309–314. doi: 10.1007/s10815-017-0880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park H, Bergh C, Selleskog U, Thurin-Kjellberg A, Lundin K. No benefit of culturing embryos in a closed system compared with a conventional incubator in terms of number of good quality embryos: results from an RCT. Human Reprod. 2015;30(2):268–275. doi: 10.1093/humrep/deu316. [DOI] [PubMed] [Google Scholar]
- 74.Paulson RJ, Reichman DE, Zaninovic N, Goodman LR, Racowsky C. Time-lapse imaging: clearly useful to both laboratory personnel and patient outcomes versus just because we can doesn't mean we should. Fertil Steril. 2018;109(4):584–591. doi: 10.1016/j.fertnstert.2018.01.042. [DOI] [PubMed] [Google Scholar]
- 75.Ebner T, Shebl O, Moser M, Mayer RB, Arzt W, Tews G. Group culture of human zygotes is superior to individual culture in terms of blastulation, implantation and life birth. Reprod BioMed Online. 2010;21(6):762–768. doi: 10.1016/j.rbmo.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 76.Rebollar-Lazaro I, Matson P. The culture of human cleavage stage embryos alone or in groups: effect upon blastocyst utilization rates and implantation. Reprod Biol. 2010;10(3):227–234. doi: 10.1016/S1642-431X(12)60042-4. [DOI] [PubMed] [Google Scholar]
- 77.Racowsky C, Vernon M, Mayer J, Ball GD, Behr B, Pomeroy KO, Wininger D, Gibbons W, Conaghan J, Stern JE. Standardization of grading embryo morphology. Fertil Steril. 2010;94(3):1152–1153. doi: 10.1016/j.fertnstert.2010.05.042. [DOI] [PubMed] [Google Scholar]
- 78.Wong CC, Loewke KE, Bossert NL, Behr B, De Jonge CJ, Baer TM, et al. Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat Biotechnol. 2010;28(10):1115–1121. doi: 10.1038/nbt.1686. [DOI] [PubMed] [Google Scholar]
- 79.Santos Filho E, Noble JA, Poli M, Griffiths T, Emerson G, Wells D. A method for semi-automatic grading of human blastocyst microscope images. Human Reprod. 2012;27(9):2641–2648. doi: 10.1093/humrep/des219. [DOI] [PubMed] [Google Scholar]
- 80.Conaghan J, Chen AA, Willman SP, Ivani K, Chenette PE, Boostanfar R, Baker VL, Adamson GD, Abusief ME, Gvakharia M, Loewke KE, Shen S. Improving embryo selection using a computer-automated time-lapse image analysis test plus day 3 morphology: results from a prospective multicenter trial. Fertil Steril. 2013;100(2):412–419. doi: 10.1016/j.fertnstert.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 81.Machtinger R, Racowsky C. Morphological systems of human embryo assessment and clinical evidence. Reprod BioMed Online. 2013;26(3):210–221. doi: 10.1016/j.rbmo.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 82.Manna C, Nanni L, Lumini A, Pappalardo S. Artificial intelligence techniques for embryo and oocyte classification. Reprod BioMed Online. 2013;26(1):42–49. doi: 10.1016/j.rbmo.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 83.Tran D, Cooke S, Illingworth PJ, Gardner DK. Deep learning as a predictive tool for fetal heart pregnancy following time-lapse incubation and blastocyst transfer. Human Reprod. 2019;34(6):1011–1018. doi: 10.1093/humrep/dez064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meseguer M, Herrero J, Tejera A, Hilligsoe KM, Ramsing NB, Remohi J. The use of morphokinetics as a predictor of embryo implantation. Human Reprod. 2011;26(10):2658–2671. doi: 10.1093/humrep/der256. [DOI] [PubMed] [Google Scholar]
- 85.Adamson GD, Abusief ME, Palao L, Witmer J, Palao LM, Gvakharia M. Improved implantation rates of day 3 embryo transfers with the use of an automated time-lapse-enabled test to aid in embryo selection. Fertil Steril. 2016;105(2):369–375. doi: 10.1016/j.fertnstert.2015.10.030. [DOI] [PubMed] [Google Scholar]
- 86.Aparicio-Ruiz B, Basile N, Perez Albala S, Bronet F, Remohi J, Meseguer M. Automatic time-lapse instrument is superior to single-point morphology observation for selecting viable embryos: retrospective study in oocyte donation. Fertil Steril. 2016;106(6):1379–1385. doi: 10.1016/j.fertnstert.2016.07.1117. [DOI] [PubMed] [Google Scholar]
- 87.Rubio I, Galan A, Larreategui Z, Ayerdi F, Bellver J, Herrero J, et al. Clinical validation of embryo culture and selection by morphokinetic analysis: a randomized, controlled trial of the EmbryoScope. Fertil Steril. 2014;102(5):1287–1294. doi: 10.1016/j.fertnstert.2014.07.738. [DOI] [PubMed] [Google Scholar]
- 88.Freour T, Le Fleuter N, Lammers J, Splingart C, Reignier A, Barriere P. External validation of a time-lapse prediction model. Fertil Steril. 2015;103(4):917–922. doi: 10.1016/j.fertnstert.2014.12.111. [DOI] [PubMed] [Google Scholar]
- 89.Kirkegaard K, Campbell A, Agerholm I, Bentin-Ley U, Gabrielsen A, Kirk J, Sayed S, Ingerslev HJ. Limitations of a time-lapse blastocyst prediction model: a large multicentre outcome analysis. Reprod BioMed Online. 2014;29(2):156–158. doi: 10.1016/j.rbmo.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 90.Basile N, Vime P, Florensa M, Aparicio Ruiz B, Garcia Velasco JA, Remohi J, et al. The use of morphokinetics as a predictor of implantation: a multicentric study to define and validate an algorithm for embryo selection. Human Reprod. 2015;30(2):276–283. doi: 10.1093/humrep/deu331. [DOI] [PubMed] [Google Scholar]
- 91.Athayde Wirka K, Chen AA, Conaghan J, Ivani K, Gvakharia M, Behr B, et al. Atypical embryo phenotypes identified by time-lapse microscopy: high prevalence and association with embryo development. Fertil Steril. 2014;101(6):1637–48 e1-5. doi: 10.1016/j.fertnstert.2014.02.050. [DOI] [PubMed] [Google Scholar]
- 92.Liu Y, Chapple V, Roberts P, Matson P. Prevalence, consequence, and significance of reverse cleavage by human embryos viewed with the use of the Embryoscope time-lapse video system. Fertil Steril. 2014;102(5):1295–1300. doi: 10.1016/j.fertnstert.2014.07.1235. [DOI] [PubMed] [Google Scholar]
- 93.Rubio I, Kuhlmann R, Agerholm I, Kirk J, Herrero J, Escriba MJ, et al. Limited implantation success of direct-cleaved human zygotes: a time-lapse study. Fertil Steril. 2012;98(6):1458–1463. doi: 10.1016/j.fertnstert.2012.07.1135. [DOI] [PubMed] [Google Scholar]
- 94.Lagalla C, Tarozzi N, Sciajno R, Wells D, Di Santo M, Nadalini M, et al. Embryos with morphokinetic abnormalities may develop into euploid blastocysts. Reprod BioMed Online. 2017;34(2):137–146. doi: 10.1016/j.rbmo.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 95.Zhan Q, Ye Z, Clarke R, Rosenwaks Z, Zaninovic N. Direct unequal cleavages: embryo developmental competence, genetic constitution and clinical outcome. PLoS One. 2016;11(12):e0166398. doi: 10.1371/journal.pone.0166398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Freour T, Dessolle L, Lammers J, Lattes S, Barriere P. Comparison of embryo morphokinetics after in vitro fertilization-intracytoplasmic sperm injection in smoking and nonsmoking women. Fertil Steril. 2013;99(7):1944–1950. doi: 10.1016/j.fertnstert.2013.01.136. [DOI] [PubMed] [Google Scholar]
- 97.Wolff HS, Fredrickson JR, Walker DL, Morbeck DE. Advances in quality control: mouse embryo morphokinetics are sensitive markers of in vitro stress. Human Reprod. 2013;28(7):1776–1782. doi: 10.1093/humrep/det102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mastenbroek S, van der Veen F, Aflatoonian A, Shapiro B, Bossuyt P, Repping S. Embryo selection in IVF. Human Reprod. 2011;26(5):964–966. doi: 10.1093/humrep/der050. [DOI] [PubMed] [Google Scholar]
- 99.Wong KM, Mastenbroek S, Repping S. Cryopreservation of human embryos and its contribution to in vitro fertilization success rates. Fertil Steril. 2014;102(1):19–26. doi: 10.1016/j.fertnstert.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 100.De Vos A, Van Landuyt L, Santos-Ribeiro S, Camus M, Van de Velde H, Tournaye H, et al. Cumulative live birth rates after fresh and vitrified cleavage-stage versus blastocyst-stage embryo transfer in the first treatment cycle. Human Reprod. 2016;31(11):2442–2449. doi: 10.1093/humrep/dew219. [DOI] [PubMed] [Google Scholar]
- 101.Edgar DH, Gook DA. A critical appraisal of cryopreservation (slow cooling versus vitrification) of human oocytes and embryos. Hum Reprod Update. 2012;18(5):536–554. doi: 10.1093/humupd/dms016. [DOI] [PubMed] [Google Scholar]
- 102.Alpha Scientists In Reproductive M The Alpha consensus meeting on cryopreservation key performance indicators and benchmarks: proceedings of an expert meeting. Reprod BioMed Online. 2012;25(2):146–167. doi: 10.1016/j.rbmo.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 103.Debrock S, Peeraer K, Fernandez Gallardo E, De Neubourg D, Spiessens C, D’Hooghe TM. Vitrification of cleavage stage day 3 embryos results in higher live birth rates than conventional slow freezing: a RCT. Human Reprod. 2015;30(8):1820–1830. doi: 10.1093/humrep/dev134. [DOI] [PubMed] [Google Scholar]
- 104.Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, Ubaldi FM, et al. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update. 2017;23(2):139–155. doi: 10.1093/humupd/dmw038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gosden R. Cryopreservation: a cold look at technology for fertility preservation. Fertil Steril. 2011;96(2):264–268. doi: 10.1016/j.fertnstert.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 106.Tiegs AW, Sun L, Neal SA, Morin SJ, Werner MD, Scott RT., Jr Worth the wait? Findings from culturing embryos through day 7. Fertil Steril. 2018;109(3):e9. doi: 10.1016/j.fertnstert.2018.02.026. [DOI] [Google Scholar]
- 107.Swain JE, Carrell D, Cobo A, Meseguer M, Rubio C, Smith GD. Optimizing the culture environment and embryo manipulation to help maintain embryo developmental potential. Fertil Steril. 2016;105(3):571–587. doi: 10.1016/j.fertnstert.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 108.Wale PL, Gardner DK. The effects of chemical and physical factors on mammalian embryo culture and their importance for the practice of assisted human reproduction. Hum Reprod Update. 2016;22(1):2–22. doi: 10.1093/humupd/dmv034. [DOI] [PubMed] [Google Scholar]
- 109.Racowsky C, Jackson KV, Cekleniak NA, Fox JH, Hornstein MD, Ginsburg ES. The number of eight-cell embryos is a key determinant for selecting day 3 or day 5 transfer. Fertil Steril. 2000;73(3):558–564. doi: 10.1016/s0015-0282(99)00565-8. [DOI] [PubMed] [Google Scholar]
- 110.McEvoy K B D, Roberts S, Turner C, Adeniyi T, Wood L, Wilson Y, Lloyd A, Critchlow D, Hunter H, Horne G. A one year retrospective analysis comparing live birth outcomes from embryos grown and transferred from an undisturbed time-lapse culture system with a conventional culture system. 32 Annual Meeting of ESHRE; Helsinki, Finland 2016. p. P-106.
- 111.Zhang JQ, Li XL, Peng Y, Guo X, Heng BC, Tong GQ. Reduction in exposure of human embryos outside the incubator enhances embryo quality and blastulation rate. Reprod BioMed Online. 2010;20(4):510–515. doi: 10.1016/j.rbmo.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 112.Sundvall L, Ingerslev HJ, Breth Knudsen U, Kirkegaard K. Inter- and intra-observer variability of time-lapse annotations. Human Reprod. 2013;28(12):3215–3221. doi: 10.1093/humrep/det366. [DOI] [PubMed] [Google Scholar]
- 113.Hou W, Xu Y, Li R, Song J, Wang J, Zeng Y, Pan J, Zhou C, Xu Y. Role of aneuploidy screening in preimplantation genetic testing for monogenic diseases in young women. Fertil Steril. 2019;111(5):928–935. doi: 10.1016/j.fertnstert.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 114.Munne S, Kaplan B, Frattarelli JL, Child T, Nakhuda G, Shamma FN, et al. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112:1071–1079.e7. doi: 10.1016/j.fertnstert.2019.07.1346. [DOI] [PubMed] [Google Scholar]
- 115.De Geyter C, Calhaz-Jorge C, Kupka MS, Wyns C, Mocanu E, Motrenko T, et al. ART in Europe, 2014: results generated from European registries by ESHRE: the European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE) Human Reprod. 2018;33(9):1586–1601. doi: 10.1093/humrep/dey242. [DOI] [PubMed] [Google Scholar]
- 116.Centers for Disease Control and Prevention. 2016 assisted reproductive technology - national summary report. 2018. https://www.cdc.gov/art/pdf/2016-report/ART-2016-National-Summary-Report.pdf. Accessed 5 Aug 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to Merck KGaA’s Data Sharing Policy. All requests should be submitted in writing to Merck KGaA’s data sharing portal https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html. When Merck KGaA has a co-research, co-development, or co-marketing or co-promotion agreement, or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, Merck KGaA will endeavour to gain agreement to share data in response to requests.
Not applicable