Abstract
Obesity is one of the foremost risk factors in coronavirus infection resulting in severe illness and mortality as the pandemic progresses. Obesity is a well-known predisposed chronic inflammatory condition. The dynamics of obesity and its impacts on immunity may change the disease severity of pneumonia, especially in acute respiratory distress syndrome, a primary cause of death from SARS-CoV-2 infection. The adipocytes of adipose tissue secret leptin in proportion to individuals’ body fat mass. An increase in circulating plasma leptin is a typical characteristic of obesity and correlates with a leptin-resistant state. Leptin is considered a pleiotropic molecule regulating appetite and immunity. In immunity, leptin functions as a cytokine and coordinates the host’s innate and adaptive responses by promoting the Th1 type of immune response. Leptin induced the proliferation and functions of antigen-presenting cells, monocytes, and T helper cells, subsequently influencing the pro-inflammatory cytokine secretion by these cells, such as TNF-α, IL-2, or IL-6. Leptin scarcity or resistance is linked with dysregulation of cytokine secretion leading to autoimmune disorders, inflammatory responses, and increased susceptibility towards infectious diseases. Therefore, leptin activity by leptin long-lasting super active antagonist’s dysregulation in patients with obesity might contribute to high mortality rates in these patients during SARS-CoV-2 infection. This review systematically discusses the interplay mechanism between leptin and inflammatory cytokines and their contribution to the fatal outcomes in COVID-19 patients with obesity.
Keywords: COVID-19, leptin, obesity, inflammation, cytokine, mortality
Introduction
Obesity is marked as redundant fat accumulation in the body. Obesity is considered an increased circulating fatty acid that is causing low-grade chronic inflammation due to macrophages’ chemoattraction and its expansion in the adipose tissue (1, 2). An individual with obesity presents with increased TNF-α cytokine, changed T-cell subset, and suppressed T-cell responses driving to a high possibilities of communicable diseases (3). Obesity has been shown to be associated with the production of dysregulated cytokines and augment the synthesis of acute-phase reactants, such as IL-6, IL-8, TNF-α, C-reactive protein (CRP), and monocyte chemotactic protein-1 (MCP-1) in patients with obesity and various animal models of obesity (4–7). Pre-conditioned chronic inflammation directly or indirectly impacts the immune response to infection in individuals with obesity (8). Several viruses, such as influenza A virus, Adenovirus Ad 36, cytomegalovirus (CMV), and HIV utilize adipocytes as cellular reservoirs for viral shedding (9). In the 2009 influenza pandemic, individuals with obesity exhibited more severe illness from the H1N1 virus and were reported as a nonpartisan threat for severe influenza infection and appended death (10). Many other reports have been confirmed the severity of disease and death from H1N1 influenza linked in a patient with obesity (11–13). Experimental studies have confirmed that obese mice show increased susceptibility to influenza, including increased lethality with H1N1 and H3N2 strains (14–17). A precise study of MERS-CoV cases reveals that diabetes and hypertension are equally predominant in 50% of the patients. In contrast, the chances of cardiac diseases and obesity are 30% and 16%, respectively. These conditions affect the pro-inflammatory cytokine responses, resulting in impairment of the host’s innate and humoral immune response among these patients (18). However, the critical mechanism for more inclusive COVID-19 severity and mortality in obesity remains unknown, despite how other viral infections can be providing some thought of COVID-19 illnesses and the severity of disease in an individual with obesity.
The connection between obesity and severity of viral infection, such as SARS and MERS, may be paramount in COVID-19 disease because of the genetic resemblance between the SARS-CoV-2 virus with SARS-CoV and MERS-CoV which is 80% and 50%, respectively (19). Patients with obesity in SARS-CoV-2 infection show severe clinical complications in the case of acute respiratory distress syndrome (ARDS), cardiac injury, thromboembolic disease, disseminated intravascular coagulation (DIC), and in severe chronic obstructive pulmonary disease (COPD) (20, 21). Acute lung injury (ALI) is another primary reason of severe illness and mortality among SARS-CoV-2–infected patients (22). Display of ARDS and ALI is cited as lung failure because of the hyper inflammatory response rather than the direct viral cytopathic injury (23, 24). Another important factor that could be contributing to the immune imbalance in COVID-19 patients with obesity is chronically higher adipokines, such as leptin. In influenza infection, leptin resistance is a major infection susceptibility factor in individuals with obesity by quashing the countenance of IFN-α, IFN-β, IFN-γ mRNAs, and memory T cells (25). It is thoroughly documented that leptin regulates the pro-inflammatory cytokines response to maintain the Th1/Th2 dichotomy. This review highlights the distinctive facts regarding the link between obesity and inflammation, specifically the interplay of leptin and inflammatory cytokine response leading severity and mortality among COVID-19 patients.
Obesity Linked COVID-19 Pathogenesis in Human
A study reported from China showed COVID-19 patients with body mass index (BMI) oF 28.0 ± 2.5 display severe signs of symptoms compared with patients with BMI of 21.2 ± 2.7. It also showed that a cohort of patients with obesity has a high frequency of getting severe symptoms and admitted to the intensive care unit (26). Two independent studies of hospitalized COVID-19 patients in New York City reported that obesity is one of the utmost shared comorbidities along with hypertension and diabetes and is independently linked to worse in-hospital outcomes and higher death (27, 28). A meta-analysis of pooled data of 22 and 35 independent studies showed that persons with obesity increase the probabilities of staying more in an intensive care unit by 74% and likely to have unfavorable fallouts with a 48% upsurge in deaths of COVID-19 patients, respectively (29). As leptin levels are vastly associated with obesity, there is the yet unanswered question of whether leptin alone is responsible for a bad prognosis. Preliminary data from various studies revealed that younger adults with obesity are more vulnerable to a higher risk for intubation or mortality in COVID-19 infection (30, 31). The morbidity and mortality in a young patient with obesity are mainly because of the visceral adiposity, leading to multiple organ failure in severe COVID-19 illness (32). A retrospective cohort study of 65 admitted patients aged 18 to 40 years in Zhongnan Hospital, Wuhan showed that obesity is a vital parameter to determine the severity of COVID-19 infection among young patients (33). The computed tomography (CT) analysis of visceral fat revealed that severity was linked to the accumulation of ectopic fat in several visceral organs, such as the liver, epicardial, and kidney (33, 34). Other studies that included a cross-sectional analysis of fat distribution showed that it is critical to consider BMI as a risk factor apart from the overall obesity in COVID-19 patients. CT-based quantification of fat showed the increment in the visceral fat area (VFA) and upper abdominal perimeter augmented the COVID-19 severity in patients (35).
The SARS-CoV-1 and SARS-CoV-2 virus entry into the adipocytes is mediated through the membrane protein angiotensin-converting enzyme 2 (ACE2) receptor (36, 37). It is vastly expressed in vascular tissues, such as the lungs and adipose tissue (38). ACE2 is expressed by a majority of cells in human at varying levels, including the intestinal enterocytes, liver, heart, and kidneys (39). The SARS-CoV-2 virus binds to the ACE2 receptors present in the host’s cell membrane. Subsequently, transmembrane serine protease (TMPRSS2) cleaves the viral capsid spike (S) protein and favoring the viral particle to infiltrates into host cells (40). The ACE2 expression in adipocytes appears to be stimulated by high-fat diets (41, 42). Adipocytes of individuals with obesity express a high level of ACE2 receptor, shedding more viral load than lean individuals, which exacerbate the susceptibility and severity of patients during COVID-19 infection (43–45). Several reports suggest that drugs used to treat patients with obesity complications (antihypertensives, statins, thiazolidinediones) could induce the overexpression of ACE2 receptor in adipocytes, leading to more viral uptake and severity of the disease (46–48) ( Figure 1 ). Similarly, various extracellular vehicles (EVs) could also carry viral particles and ACE2 receptors from infected to healthy cells that could enhance COVID-19 susceptibility (43, 49). Fatty adipocytes in the visceral depots allow the SARS-CoV-2 entry through the ACE2 receptor (50), resulting in necrotic death of adipocytes in AT, which leads to a meta-inflammatory condition called fat embolism syndrome (FES). Strikingly, lung autopsy of an overweight patient with COVID-19 infection showed fat embolism macrophages infiltration in the AT (51, 52). The significant increase of inflammatory biomarkers, such as serum amyloid A, IL-6, and CRP in severe COVID-19 patients (53), are directly or indirectly linked to adipocytes with sub-clinical low-grade inflammation that further exacerbates COVID-19 severity in individuals with obesity (54, 55). Adipocytes is known to secrete leptin, which is an imperative mark of obesity and is directly proportional to body fat mass. Leptin involves distinct endocrine, paracrine, and autocrine signaling mechanism and cytokine-mediated inflammatory changes in the body (56). Therefore, leptin could play a significant role in developing severe COVID-19 infection in patients with obesity.
Figure 1.
Schematic representation of extra pulmonary viral shedding and adipose tissue (AT) inflammation in COVID-19 patients with obesity. Angiotensin-converting enzyme 2 (ACE2) receptor in the lung epithelial cells recognizes the COVID-19 spike protein and the virus with receptor taken into the cells by receptor-mediated endocytosis. Then, viral RNA integrates into the host genome for viral replication and assembly. The free viral particles inside the host cell are released through exocytosis to infect healthy cells. Extracellular vesicles (EVs) containing viral proteins, and ACE2 receptors are also carried over to cells of extra pulmonary fatty visceral organs. The fatty adipose tissue in patients with obesity is a link to extra pulmonary viral shedding through the ACE2 expressing adipocytes. The adipocytes in the fatty AT of visceral organs (liver, heart, and kidney) act as a reservoir for viral replication and assembly. Apoptotic and necrotic death of the virus inside the adipocytes leads to meta-inflammation and subsequent fat embolism syndrome (FES) in COVID-19 patients with obesity.
Adipose Tissue Linked Inflammation
Adipose tissue is typically heeded as an inert tissue and functionally considered as an energy store house. It also deeds a central role in the immune and endocrine function that regulates inflammatory responses against infection. Visceral adiposity is associated with metabolic disorder and is recognized as a high-risk factor during SARS-CoV-2 infections (34). However, the exact mechanisms lying behind it are partly explained. Adipose tissue–linked inflammation is marked by the secretion of inflammatory cytokines, such as TNF-α, IL-6, and IL-1, from adipocytes and resident macrophages in adipose tissue (2). However, a typical feature of adipose tissue is adipocyte hypertrophy due to the accumulation of extended fat droplets. Adipocytes can reach a diameter of 150 to 200 microns in size, which limits the diffusion/exchange of gas (O2/CO2) in the adipose tissue. This produces a progressive local hypoxia by activating the hypoxia-inducible factor 1α (HIF-1α) and NF-κβ expression (57). The HIF-1α is a molecular sensor of hypoxia, and it transactivates the leptin gene expression by HIF-1α transcription-dependent activity (58). In addition to this, hundreds of immuno-inflammatory-reparative genes are also expressed, profoundly changing the adipocyte phenotype and functionality. Leptin is a product of a hypoxia-inducible gene, and hypoxia differentiates the preadipocytes into leptin secreting endocrine cells in an mTOR-dependent manner. Preadipocyte-derived leptin activates inflammatory cytokines by a specific local autocrine/paracrine signaling (57, 59) ( Figure 2 ).
Figure 2.
Schematic representation of the effects of Hypoxia and Leptin on pro-inflammatory cytokine response in adipose tissue (AT). The effect of low O2 tension on the production of adipokines in hypoxia condition. The adipocyte in the adipose tissue release leptin, which induces the release of pro-inflammatory cytokine by endocrine, paracrine, and autocrine in the AT. Leptin induces Th1 cells, and it inhibits T regulatory cells (Treg cells) and induces Th17 cells differentiation to produce IL-2 and IFN-γ. Leptin directly activates macrophages, dendritic cells (DC), and natural killer (NK) cells to produce pro-inflammatory cytokines, such as IL-6, IL-1, and TNF-α. Adipocyte-derived leptin induces the adipocytes to produce pro-inflammatory cytokines (IL-6, IL-1, and TNF-α) through autocrine signaling.
Adipocytes also secrete numerous adipocytokines, such as adiponectin, resistin, visfatin, and leptin. These molecules are believed to be linked with insulin resistance, inflammatory disorders, and obesity (60–62). Adiponectin plays a vital role in the secretion of anti-inflammatory cytokines, like IL-10 and IL-1 receptor antagonist (IL-1RA), by inducing the monocytes, macrophages, and dendritic cells (DCs). Adiponectin also plays a significant role in suppressing the interferon-γ (IFN-γ) secretion in LPS-stimulated macrophages (63, 64). Human resistin incites the secretion of the proinflammatory cytokines TNF-α, IL-1, IL-6, and IL-12 by several immune cell via NF-κB–dependent pathway (65, 66). It also upregulates the expression of vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), and CC-chemokine ligand-2(CCL-2) by human endothelial cells to release endothelin-1 (67). Serum amyloid A is another adipokines produced by adipocytes that enhances inflammatory cytokines production in individuals with obesity (68).
The primary function of leptin is to regulate appetite and energy (60). Nonetheless, leptin is also thought to be a proinflammatory cytokine, and it has a structural similarity with other inflammatory cytokines, such as IL-6, IL-12, and granulocyte colony-stimulating factor. Leptin promotes the migration of resident macrophages in the AT and stimulates their polarization toward a proinflammatory profile or a classically activated macrophage (M1) phenotype. It overturns the T helper cell phenotype by diminishing regulatory T-cells (Treg) activity and favoring Th17 polarization (69). Leptin modulates macrophage and T-cell function through the activation JAK-STAT pathway, leading to immune dysregulation and proinflammatory cytokine production (70, 71). Hyperleptinemia is another complication due to leptin resistance or leptin-impaired signaling. It interferes with the leptin receptor, contributing to the inflammatory response in obesity (72). Moreover, various other fatty tissue products have been characterized, such as TNF-α, IL-6, IL-1, CCL2, and plasminogen activator inhibitor type-1, which play a crucial role in immune dysregulation in individuals with obesity (68). Therefore, adipocyte interactions with immune cells ( Figure 2 ) could be facilitating the disease severity in COVID-19 patients linked with obesity.
Leptin Dysregulation Linked to Cytokine Storm in COVID-19 Infection
Leptin is a non-glycosylated 16-kDa protein encoded by the obese (ob) gene and mainly secreted by adipocytes to the circulation. Leptin acts as a pleiotropic protein, like hormone, as well as a cytokine. The circulatory leptin binds to the long form of the leptin receptor (Lep-R), class I receptor cytokine family mediates the phosphorylation and activation of STAT3 signaling pathway (71, 73). Lep-R is expressed in most of the immune cells, and leptin binds and triggers the immune response mainly through the JAK-STAT– and NF-kB–dependent pathway (69, 71, 74). Leptin functions in immune response have been confirmed in leptin-deficient (ob/ob) and leptin receptor-deficient (db/db) mice (75–77). The genetically leptin-deficient or leptin-resistant animals or humans develop profound obesity because of hormonal and immune system abnormalities (77–79). Leptin secretion in individuals with obesity is chronically higher than lean subjects. The higher level of pro-inflammatory leptin and lower anti-inflammatory adiponectin ratio could be contributing to the obesity-associated complication due to biased immune response (80). Leptin can tempt the stimulation and proliferation of various immune cells, including circulating monocytes, natural killer cells (NK) cells, and T helper cells by increasing the release of inflammatory cytokines, such as TNF−α, IL−1, and IL−6 (81–83). Leptin has structural homology with the long-chain helical family’s cytokines, such as IL-6, IL-11, IL-12 cytokine, and oncostatin M (84). Leptin has a shared signal-transducing peptide portion for the IL-6-related cytokines, such as IL-6, IL-11, leukocyte inhibitory factor (LIF), and granulocyte colony-stimulating factor (GCSF) (69, 85). Along with these, certain inflammatory and infectious stimuli are also enhancing the leptin expression, such as TNF-α, IL-1, IL-6, and lipopolysaccharide (LPS) (86). Therefore, the crucial question is whether or not the cytokine storm in COVID-19 patients with obesity is linked to leptin dysregulation.
Adipocytes engage in a cross-talk with various immune cells and could be directly involved in their activation and proliferation, resulting in the production of inflammatory cytokines (87, 88). Adipocytes add nearly one third of the IL-6 concentration to the circulation, along with macrophages that are recruited into the AT by leptin (89). IL-6 is the foremost cytokine in acute phase inflammation, an innate immune response elicited by infection. IL-6 also plays an indispensable role in improving acquired immunity against pathogens, including cytokine and chemokine expressions, stimulation of antibody production from B cells, macrophage regulation, and dendritic cell differentiation (90, 91). The macrophage deposition and its chronic activation cause the abnormal secretion of IL-6, TNF-α during SARS-CoV-2 infection in a patient with obesity (51, 92). An altered pro-inflammatory profile and hyperleptinemia lead to thrombotic risk in these patients that are linked to coagulation of blood, resulting in visceral organ failures (90, 93). In addition, leptin modulates the gene expression in cardiomyocytes via hypoxia-mediated HIF-1α factors, which is the leading cause of heart ischemia (94). In a recent cross-sectional study in 31 COVID-19–positive patients, interestingly, the serum leptin levels of ventilated patients were significantly higher compared with the control groups (95). Adipocyte dysfunction linked to leptin could be contributing to the expansion of ARDS in COVID-19 patients. Therefore, a strong possibility of neutralizing the circulatory leptin could control the hyper cytokine responses that are linked to obesity in COVID-19 patients.
Interplay Between Leptin and IL-6 in COVID-19 Infection
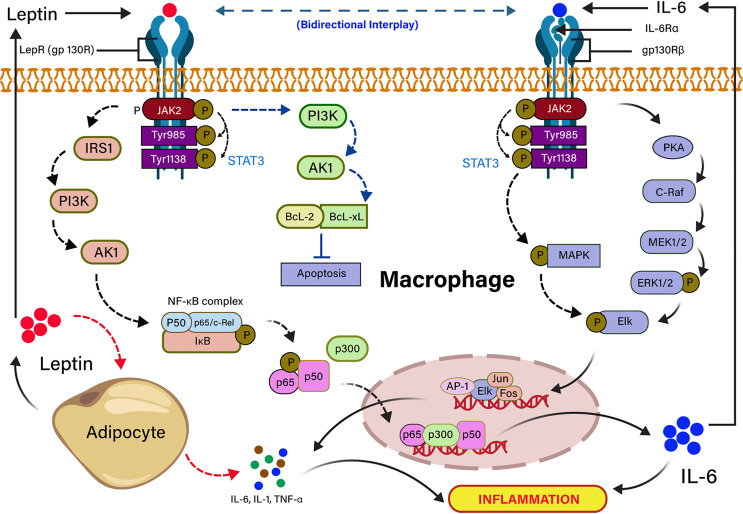
Severe COVID-19 patients show an expressively higher serum IL-6 than those with mild infection (96). High level of IL-6 is also another key signature of severe SARS-CoV, MERS-CoV, and pandemic H1N1 influenza A viral infections (97–99) In individuals with obesity, adipocytes secrete pleiotropic leptin and pleiotropic adipocyte-specific IL-6 cytokine (91, 100). IL-6–mediated inflammation in obesity is source-specific, and the IL-6 secreted by adipocytes or infection of non-resident adipocyte cells, including adipose tissue macrophage (ATM) and T cells in a non-canonical mechanism (101). Visceral adipose tissue (VAT) produces higher inflammatory cytokines (TNF-α, IL-6, and IL-1β) in comparison to subcutaneous adipose tissue (102), suggesting that cytokine storm in COVID-19 infection could be independent of obesity. IL-6 is released from adipocytes, either binding to a soluble or membrane-bound receptor, and forms an IL-6 ligand/receptor complex. IL-6 is also associated with a homodimer of glycoprotein 130 for downstream signaling in adipocytes (103, 104). Leptin and IL-6 act in agreement with partly sharing a common downstream target mediated by phosphorylation of STAT3 in ventromedial hypothalamic leptin signaling (105, 106). Furthermore, the structural and functional similarity of leptin and IL-6 receptor-mediated signaling in-vitro showed that both are activating the JAK-STAT pathway (82, 107). Leptin induces IL-6 expression through the binding to the leptin receptor (LEPR) and activates JAK2/STAT-3–dependent phosphorylation of IRS-1, PI3K, Akt signaling pathway (72). The binding of p65 and p50 transcription factor to the NF-κB element enhances the recruitment of p300 to the IL-6 promoter, resulting in up-regulation of IL-6 expression (91, 104). Conversely, IL-6 upregulates the expression of TNF-α and IL-1 through JAK/STAT-dependent phosphorylation of PKA signaling pathway (108). The dose-dependent effects of TNF-α and IL-1 cytokines on leptin gene overexpression revealed that their anorectic effects might be arbitrated in bit by expression of the leptin gene in the adipocyte (82, 109). Leptin also inhibits the apoptosis through the activation of PI3K-dependent Bcl-2 and Bcl-xL signaling pathways (110). The high leptin concentration in serum is correlated with incremental death in patients with ARDS caused by an infectious agent (111). It has also been stated that leptin induces an inflammatory phenotype in murine alveolar macrophages, hinting that a higher concentration of leptin in obesity might be potentiating the incidence of pulmonary inflammation in COVID-19 infection (112). The dysregulation of leptin in COVID-19 patients could be linked to IL-6 cytokine signaling because it shares the IL-6 receptors. The mechanism of leptin and IL-6 signaling pathways are summarized in Figure 3 .
Figure 3.
Signal transduction cascades of leptin and IL-6 interplay in inflammation. Leptin binding to the long-form leptin receptor (LRb or gp130R) results in the activation of the Janus kinase 2 (JAK2) signaling pathway by autophosphorylation, which subsequently phosphorylates tyrosine 985 and 1138 residues of the intracellular receptor tail. The phosphorylated Tyr 1138 activates the transcription factor STAT3. The activated JAK/STAT3 activates IRS1 and PI3K downstream signaling pathway, which recruits NF-kB complex (P50, P65/c-Rel, IkB), resulting in the transcription of IL-6 expressing genes in the nucleus. JAK/STAT3 directly activates PI3k inhibits apoptosis by the activation of anti-apoptotic factors (BCL-2, BCL-XL). Pleiotropic leptin and IL-6 follow similar signaling through gp130R and gp130Rβ, respectively. Leptin-induced IL-6 binding to the gp130Rβ results in the activation of JAK/STAT followed by the activation of PKA- and MAPK-mediated Elk phosphorylation. The phosphorylated Elk induces the transcription factors for TNF-α and IL-1. IL-6–mediated TNF-α and IL-1 induce the production of leptin from adipocytes.
Another interesting study showed that leptin and IL-6 induce anorexia by acting on the lateral parabrachial nucleus (lPBN) in the brain, suggesting that targeting leptin might be controlling the IL-6 signaling in individuals with obesity (113). The elevated level of IL-6 in serum due to increased production of adipokines leads to induce low-grade chronic inflammation (114). Although high IL-6 in COVID-19 infection has a prominent role in inflammation linked mortality, recent studies showed that colchicine treatment decreased the concentrations of multiple inflammatory molecules, including IL-6, CRP, and resistin in patient with obesity (115). Tocilizumab is used to treat cytokine release syndrome (CRS) in patients with cancer treated with chimeric antigen receptor-modified (CAR) T cells. It inhibits the expression of IL-6 through the blocking of the IL-6, receptor resulting in inhibitions of proinflammatory cytokine, such as TNF-α, IL-1, and IFNγ (116, 117). In an early pandemic, it was reported that IL-6 plays a significant role in the cytokine storm in COVID-19 patients, opening new hopes to treat the cytokine storm by blocking the IL-6 cytokine receptor. However, later on, multicenter studies reported that there is no significant improvement after blocking the IL-6 receptor in these patients (118). Subsequently, IL-6 is not the only cytokine, several other cytokines, such as TNF-α, IL-1, and IL-8, have also been elevated in CRS and are equally important in acute inflammatory reactions that contribute to tissue injury. TNF-α and IL-1 blockade might be another probable therapeutic approach to restrained organ damage in patients with SARS-CoV-2 infection (119, 120). In vitro studies showed that adiponectin also inhibits IL-6 expression by murine pulmonary endothelial cells and reduces lung inflammation in mice during ARDS induced by bacterial liposaccharides (121). However, data if adipokines can regulate pulmonary inflammation in COVID-19 infection are lacking so far. Despite the fact that blocking the specific cytokines could be a strategy to treat COVID-19 patients, there is a possibility it may lead to severe outcomes. Therefore, the bidirectional interplay of cytoadipokines might play a role in non-specific immune response, such as cytokine storm in obesity-related mortality in severe COVID-19 patients.
Prospective of LeptinTreatment in COVID-19 Infection
Because obesity is strongly correlated with poor prognosis in COVID-19 infection and because hyperleptinemia is characteristic of obesity, we suggest that a possibility lowering leptin activity may improve such bad prognosis. This can be achieved by blocking leptin action using leptin receptor antagonists (L39A/D40A/F41A mutants of human/mouse/rat/ovine leptins) (122) developed by our groups. The antagonistic activity of those mutants was up to 60-fold elevated by additional mutation (D23L), resulting in a respective super active D23L/L39A/D40A/F41A mutants (123). In view of the potential pharmaceutical uses of recombinant leptin mutants acting as antagonists, the basic question of how the biopotency of recombinant proteins can be elevated in vivo needs to be investigated. The capability of a hormone or hormone antagonists to elicit a biological effect in vivo depends not only on the affinity for its receptor but also on its clearance from the circulation. The measured half-life in circulation of leptin is or leptin antagonist only ~ 60 min (123, 124). As kidney-mediated clearance is mostly reliant on molecular mass and proteins greater than 70 to 80 kDa are cleared at a remarkably slower rate, increasing protein size will prolong its in vivo half-life. This can be attained by increasing the size of the hormone without affecting its activity, via attachment of polyethylene glycol (PEG) molecule. Therefore, to extend the biological activity of those mutants in animals by prolonging their in vivo half-life in circulation, they were mono-pegylated at their amino-terminus by 20-kDa PEG (123, 124) and termed PEG-SMLA (mouse) or PEG-SHLA (human) or PEG-SRLA (rat). Although the molecular weight of those analogs is ~ 36 kDa, due to the very large hydrodynamic volume of PEG, they behave like 220-kDa proteins, and their half-life in mice circulation was extended to ~ 14 to 15 h. The in vivo effect of those mono-pegylated analogs was well-documented in Prof. Gertler’s and in other laboratories (125–129). By applying different amounts of leptin antagonists, it is possible to control the leptin activity in serum.
Conclusion
The interplays between leptin and inflammatory cytokine response that regulate the immune-metabolic feats of leptin on immune cells are required to explore the insight of their relation. The past two decades of studies about leptin reveals that adipose tissues play a crucial role in regulating metabolism and immune cell function. Adipose tissue inflammation is marked by infiltration of immune cells, such as lymphocytes, macrophages, dendritic cells, and neutrophils into it. Leptin expands the monocyte, macrophage, and T-cell proliferation by enhancing inflammatory cytokines secretion. Leptin also plays a major role in the anorexia and cachexia condition of inflammatory diseases caused by pathogens, collagen vascular disease, and even cancer. However, chronic inflammation, either from autoimmune or infectious diseases, or impaired leptin response to CNS (hypothalamus)/leptin resistance, induce resistance to the weight control, which is a leading cause of obesity and anorexia. The absence of leptin triggers the immune defects that eventually translate into exaggerated death due to infections. Therefore, high circulating leptin might involve dysregulation of proinflammatory cytokine in obesity, which is the leading cause of high morbidity and mortality in patients with SARS-CoV-2 infection. Individuals with obesity suggest that metabolic consequences of obesity compromise host antiviral defenses, leading to critical outcomes from infection. This could be one of the reasons why the virus has taken its toll to damaged individuals with obesity more aggressively. Therefore, more studies in the mechanism of leptin are warranted to investigate the immune response in COVID-19 patients with obesity.
Author Contributions
RM and AG conceived and designed the study. PS, MN, and MD have done literature collection and wrote the initial version of the draft. RM and PS have prepared the figures and finalized the manuscript. All authors contributed to the article and approved the submitted version. RM is the guarantor and takes all responsibility for the integrity and accuracy of the data.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We sincerely thank Dr. David Sacks, LPD/NIAID, NIH, Bethesda, for his valuable comments and suggestions during the manuscript preparation. We acknowledge the ICMR for providing ICMR-DHR International Fellowship, DST-SERB, DST-FIST, DST-Purse, and University of Hyderabad for the subscription of scientific journal and books to compile the review.
References
- 1. Ervin RB. Prevalence of Metabolic Syndrome Among Adults 20 Years of Age and Over, by Sex, Age, Race and Ethnicity, and Body Mass Index: United States, 2003-2006. Natl Health Stat Rep (2009) (13):2003–6. [PubMed] [Google Scholar]
- 2. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity Is Associated With Macrophage Accumulation in Adipose Tissue. J Clin Invest (2003) 112(12):1796–808. 10.1172/JCI200319246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schaible UE, Kaufmann SHE. Malnutrition and Infection: Complex Mechanisms and Global Impacts. PLoS Med (2007) 4(5):0806–12. 10.1371/journal.pmed.0040115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sudhakar M, Silambanan S, Chandran AS, Prabhakaran AA, Ramakrishnan R. C-Reactive Protein (CRP) and Leptin Receptor in Obesity: Binding of Monomeric CRP to Leptin Receptor. Front Immunol (2018) 9(MAY):1–13. 10.3389/fimmu.2018.01167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hotamisligil GS. Inflammation, Metaflammation and Immunometabolic Disorders. Nat Publ Gr (2017) 542(7640):177–85. 10.1038/nature21363 [DOI] [PubMed] [Google Scholar]
- 6. Ellulu MS, Patimah I, Khaza H, Rahmat A, Abed Y, Sci AM. Obesity and Inflammation : The Linking Mechanism and the Complications. Arch Med Sci (2016) 13(4):851–63. 10.5114/aoms.2016.58928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Greenberg AS, Obin MS. Obesity and the Role of Adipose Tissue in Inflammation and Metabolism. Am J Clin Nutr (2006) 83(2):461–5. 10.1093/ajcn/83.2.461S [DOI] [PubMed] [Google Scholar]
- 8. Ramos-Nino ME. The Role of Chronic Inflammation in Obesity-Associated Cancers. ISRN Oncol (2013) 2013:697521. 10.1155/2013/697521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bourgeois C, Gorwood J, Barrail-Tran A, Lagathu C, Capeau J, Desjardins D, et al. Specific Biological Features of Adipose Tissue, and Their Impact on HIV Persistence. Front Microbiol (2019) 10:2837. 10.3389/fmicb.2019.02837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Louie JK, Acosta M, Samuel MC, Schechter R, Vugia DJ, Harriman K. Major Article A Novel Risk Factor for a Novel Virus : Obesity and 2009 Pandemic Influenza A (H1N1). Clin Infect Dis (2011) 52(3):301–12. 10.1093/cid/ciq152 [DOI] [PubMed] [Google Scholar]
- 11. Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, et al. Emergence of a Novel Swine-Origin Influenza A (H1N1) Virus in Humans. N Engl J Med (2009) 360(25):2605–15. 10.1056/NEJMoa0903810 [DOI] [PubMed] [Google Scholar]
- 12. Fuhrman C, Bonmarin I, Bitar D, Cardoso T, Duport N, Herida M, et al. Adult Intensive-Care Patients With 2009 Pandemic Influenza A(H1N1) Infection. Epidemiol Infect (2011) 139(8):1202–9. 10.1017/S0950268810002414 [DOI] [PubMed] [Google Scholar]
- 13. Vaillant L, La Ruche G, Tarantola A, Barboza P. Epidemiology of Fatal Cases Associated With Pandemic H1N1 Influenza 2009. Euro Surveill (2009) 14(33):19309. 10.2807/ese.14.33.19309-en [DOI] [PubMed] [Google Scholar]
- 14. Smith AG, Sheridan PA, Harp JB, Beck MA. Diet-Induced Obese Mice Have Increased Mortality and Altered Immune Responses When Infected With Influenza Virus. J Nutr (2007) 137(5):1236–43. 10.1093/jn/137.5.1236 [DOI] [PubMed] [Google Scholar]
- 15. Radigan KA, Morales-Nebreda L, Soberanes S, Nicholson T, Nigdelioglu R, Cho T, et al. Impaired Clearance of Influenza a Virus in Obese, Leptin Receptor Deficient Mice Is Independent of Leptin Signaling in the Lung Epithelium and Macrophages. PLoS One (2014) 9(9):1–8. 10.1371/journal.pone.0108138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Easterbrook JD, Dunfee RL, Schwartzman LM, Jagger BW, Sandouk A, Kash JC, et al. Obese Mice Have Increased Morbidity and Mortality Compared to Non-Obese Mice During Infection With the 2009 Pandemic H1N1 Influenza Virus. Influenza Other Respir Viruses (2011) 5(6):418–25. 10.1111/j.1750-2659.2011.00254.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O’Brien KB, Vogel P, Duan S, Govorkova EA, Webby RJ, McCullers JA, et al. Impaired Wound Healing Predisposes Obese Mice to Severe Influenza Virus Infection. J Infect Dis (2012) 205(2):252–61. 10.1093/infdis/jir729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Badawi A, Ryoo SG. Prevalence of Comorbidities in the Middle East Respiratory Syndrome Coronavirus (MERS-CoV): A Systematic Review and Meta-Analysis. Int J Infect Dis (2016) Aug 49:129–33. 10.1016/j.ijid.2016.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Muniyappa R, Gubbi S. COVID-19 Pandemic, Coronaviruses, and Diabetes Mellitus. Am J Physiol Endocrinol Metab (2020) 318(5):E736–41. 10.1152/ajpendo.00124.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis (2020) 71(15):762–8. 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ye Q, Wang B, Mao J. The Pathogenesis and Treatment of the ‘Cytokine Storm’’ in COVID-19. J Infect (2020) 80(6):607–13. 10.1016/j.jinf.2020.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical Predictors of Mortality Due to COVID-19 Based on an Analysis of Data of 150 Patients From Wuhan, China. Intensive Care Med (2020) 46(5):846–8. 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fanelli V, Ranieri VM. Mechanisms and Clinical Consequences of Acute Lung Injury. Ann Am Thorac Soc (2015) 12:S3–8. 10.1513/AnnalsATS.201407-340MG [DOI] [PubMed] [Google Scholar]
- 24. Bhattacharyya R, Iyer P, Phua GC, Lee JH. The Interplay Between Coagulation and Inflammation Pathways in COVID-19-Associated Respiratory Failure : A Narrative Review. Pulm Ther (2020) 6(2):215–31. 10.1007/s41030-020-00126-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hur SJ, Kim DH, Chun SC, Lee SK. Effect of Adenovirus and Influenza Virus Infection on Obesity. Life Sci (2013) 93(16):531–5. 10.1016/j.lfs.2013.08.016 [DOI] [PubMed] [Google Scholar]
- 26. Cai Q, Chen F, Wang T, Luo F, Liu X, Wu Q, et al. Obesity and COVID-19 Severity in a Designated Hospital in Shenzhen, China. Diabetes Care (2020) 43(7):1392–8. 10.2337/dc20-0576 [DOI] [PubMed] [Google Scholar]
- 27. Palaiodimos L, Kokkinidis DG, Li W, Karamanis D, Ognibene J, Arora S, et al. Severe Obesity Is Associated With Higher in-Hospital Mortality in a Cohort of Patients With COVID-19 in the Bronx, New York. Metabolism (2020) 108:154262. 10.1016/j.metabol.2020.154262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA - J Am Med Assoc (2020) 323(20):2052–9. 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Popkin BM, Du S, Green WD, Beck MA, Algaith T, Herbst CH, et al. Individuals With Obesity and COVID-19: A Global Perspective on the Epidemiology and Biological Relationships. Obes Rev (2020) 21(11):1–17. 10.1111/obr.13128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anderson MR, Geleris J, Anderson DR, Zucker J, Nobel YR, Freedberg D, et al. Body Mass Index and Risk for Intubation or Death in SARS-CoV-2 Infection. Ann Intern Med (2020) 6:782–90. 10.7326/M20-3214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lighter J, Phillips M, Hochman S, Sterling S, Johnson D, Francois F, et al. Obesity in Patients Younger Than 60 Years Is a Risk Factor for COVID-19 Hospital Admission. Clin Infect Dis (2020) 71(15):896–7. 10.1093/cid/ciaa415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nogueira-de-Almeida CA, Del Ciampo LA, Ferraz IS, Del Ciampo IRL, Contini AA, Ued F da V. COVID-19 and Obesity in Childhood and Adolescence: A Clinical Review. J Pediatr (Rio J) (2020) 96(5):546–58. 10.1016/j.jped.2020.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Deng M, Qi Y, Deng L, Wang H, Xu Y, Li Z, et al. Obesity as a Potential Predictor of Disease Severity in Young COVID-19 Patients: A Retrospective Study. Obesity (2020) 8(10):1815–25. 10.1002/oby.22943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Iacobellis G, Malavazos AE, Ferreira T. COVID-19 Rise in Younger Adults With Obesity: Visceral Adiposity Can Predict the Risk. Obesity (2020) 28(10):1795. 10.1002/oby.22951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Petersen A, Bressem K, Albrecht J, Thieß H-M, Vahldiek J, Hamm B, et al. The Role of Visceral Adiposity in the Severity of COVID-19: Highlights From a Unicenter Cross-Sectional Pilot Study in Germany. Metabolism (2020) 110:154317. 10.1016/j.metabol.2020.154317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A Crucial Role of Angiotensin Converting Enzyme 2 (ACE2) in SARS Coronavirus-Induced Lung Injury. Nat Med (2005) 11(8):875–9. 10.1038/nm1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell (2020) 181(2):271–80.e8. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kruglikov IL, Scherer PE. The Role of Adipocytes and Adipocyte-Like Cells in the Severity of COVID-19 Infections. Obesity (2020) 28(7):1187–90. 10.1002/oby.22856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 Cell Receptor Gene ACE2 in a Wide Variety of Human Tissues. Infect Dis Poverty (2020) 9(1):1–7. 10.1186/s40249-020-00662-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O’Rourke RW, Lumeng CN. Pathways to Severe COVID-19 for People With Obesity. Obesity (Silver Spring) (2020) 29(4):645–53. 10.1002/oby.23099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Méry G, Epaulard O, Borel A, Toussaint B, Lucas R. COVID-19 : Underlying Adipokine Storm and Angiotensin 1-7 Umbrella. Front Immunol (2020) 11:1–10. 10.3389/fimmu.2020.01714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gupte M, Boustany-Kari CM, Bharadwaj K, Police S, Thatcher S, Gong MC, et al. ACE2 Is Expressed in Mouse Adipocytes and Regulated by a High-Fat Diet. Am J Physiol Regul Integr Comp Physiol (2008) 295(3):781–8. 10.1152/ajpregu.00183.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kass DA, Duggal P, Cingolani O. Obesity Could Shift Severe COVID-19 Disease to Younger Ages. Lancet (2020) 395(10236):1544–5. 10.1016/S0140-6736(20)31024-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ryan PM, Caplice NM. Is Adipose Tissue a Reservoir for Viral Spread, Immune Activation, and Cytokine Amplification in Coronavirus Disease 2019? Obesity (2020) 28(7):1–4. 10.1002/oby.22843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Luzi L, Radaelli MG. Influenza and Obesity: Its Odd Relationship and the Lessons for COVID-19 Pandemic. Acta Diabetol (2020) 57(6):759–64. 10.1007/s00592-020-01522-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Romaní-Pérez M, Outeiriño-Iglesias V, Moya CM, Santisteban P, González-Matías LC, Vigo E, et al. Activation of the GLP-1 Receptor by Liraglutide Increases ACE2 Expression, Reversing Right Ventricle Hypertrophy, and Improving the Production of SP-A and SP-B in the Lungs of Type 1 Diabetes Rats. Endocrinology (2015) 156(10):3559–69. 10.1210/en.2014-1685 [DOI] [PubMed] [Google Scholar]
- 47. Tikoo K, Patel G, Kumar S, Karpe PA, Sanghavi M, Malek V, et al. Tissue Specific Up Regulation of ACE2 in Rabbit Model of Atherosclerosis by Atorvastatin: Role of Epigenetic Histone Modifications. Biochem Pharmacol (2015) 93(3):343–51. 10.1016/j.bcp.2014.11.013 [DOI] [PubMed] [Google Scholar]
- 48. Zhang W, Xu Y-Z, Liu B, Wu R, Yang Y-Y, Xiao X-Q, et al. Pioglitazone Upregulates Angiotensin Converting Enzyme 2 Expression in Insulin-Sensitive Tissues in Rats With High-Fat Diet-Induced Nonalcoholic Steatohepatitis. SciWorld J (2014) 2014:603409. 10.1155/2014/603409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang J, Chen S, Bihl J. Exosome-Mediated Transfer of ACE2 (Angiotensin-Converting Enzyme 2) From Endothelial Progenitor Cells Promotes Survival and Function of Endothelial Cell. Oxid Med Cell Longev (2020) 2020:4213541. 10.1155/2020/4213541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Al-Benna S. Association of High Level Gene Expression of ACE2 in Adipose Tissue With Mortality of COVID-19 Infection in Obese Patients. Obes Med (2020) 19:100283. 10.1016/j.obmed.2020.100283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cinti S, Graciotti L, Giordano A. COVID-19 and Fat Embolism : A Hypothesis to Explain the Severe Clinical Outcome in People With Obesity. Int J Obes (2020) 44(8):1800–2. 10.1038/s41366-020-0624-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Meng Y, Yu W, Liu Z, Zhang M, Chen Y, Li S, et al. The Inflammation Patterns of Different Inflammatory Cells in Histological Structures of Hyperplasic Prostatic Tissues. Transl Androl Urol (2020) 9(4):1639–49. 10.21037/tau-20-448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kermali M, Khalsa RK, Pillai K, Ismail Z, Harky A. The Role of Biomarkers in Diagnosis of COVID-19 - a Systematic Review. Life Sci (2020) 254:117788. 10.1016/j.lfs.2020.117788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Docea AO, Tsatsakis A, Albulescu D, Cristea O, Zlatian O, Vinceti M, et al. A New Threat From an Old Enemy: Re−Emergence of Coronavirus (Review). Int J Mol Med (2020) 45(6):1631–43. 10.3892/ijmm.2020.4555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Petrakis D, Margină D, Tsarouhas K, Kouretas D, Spandidos DA, Tsatsakis A. Obesity − A Risk Factor for Increased COVID − 19 Prevalence, Severity and Lethality (Review). Mol Med Rep (2020) 22(1):9–19. 10.3892/mmr.2020.11127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mangge H, Almer G, Truschnig-Wilders M, Schmidt A, Gasser R, Fuchs D. Inflammation, Adiponectin, Obesity and Cardiovascular Risk. Curr Med Chem (2010) 17(36):4511–20. 10.2174/092986710794183006 [DOI] [PubMed] [Google Scholar]
- 57. Trayhurn P. Hypoxia and Adipose Tissue Function and Dysfunction in Obesity. Physiol Rev (2013) 93(1):1–21. 10.1152/physrev.00017.2012 [DOI] [PubMed] [Google Scholar]
- 58. Grosfeld A, André J, De Mouzon SH, Berra E, Pouysségur J, Guerre-Millo M. Hypoxia-Inducible Factor 1 Transactivates the Human Leptin Gene Promoter. J Biol Chem (2002) 277(45):42953–7. 10.1074/jbc.M206775200 [DOI] [PubMed] [Google Scholar]
- 59. Palhinha L, Liechocki S, Hottz ED, Pereira JA da S, de Almeida CJ, Moraes-Vieira PMM, et al. Leptin Induces Proadipogenic and Proinflammatory Signaling in Adipocytes. Front Endocrinol (Lausanne) (2019) 10:841. 10.3389/fendo.2019.00841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. La Cava A, Matarese G. The Weight of Leptin in Immunity. Nat Rev Immunol (2004) 4(5):371–9. 10.1038/nri1350 [DOI] [PubMed] [Google Scholar]
- 61. Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, et al. CCR2 Modulates Inflammatory and Metabolic Effects of High-Fat Feeding Find the Latest Version: CCR2 Modulates Inflammatory and Metabolic Effects of High-Fat Feeding. J Clin Invest (2006) 1(116):115–24. 10.1172/JCI24335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kusminski CM, McTernan PG, Schraw T, Kos K, O’Hare JP, Ahima R, et al. Adiponectin Complexes in Human Cerebrospinal Fluid: Distinct Complex Distribution From Serum. Diabetologia (2007) 50(3):634–42. 10.1007/s00125-006-0577-9 [DOI] [PubMed] [Google Scholar]
- 63. Wolf AM, Wolf D, Rumpold H, Moschen AR, Kaser A, Obrist P, et al. Overexpression of Indoleamine 2,3-Dioxygenase in Human Inflammatory Bowel Disease. Clin Immunol (2004) 113(1):47–55. 10.1016/j.clim.2004.05.004 [DOI] [PubMed] [Google Scholar]
- 64. Kershaw EE, Flier JS. Adipose Tissue as an Endocrine Organ. J Clin Endocrinol Metab (2004) 89(6):2548–56. 10.1210/jc.2004-0395 [DOI] [PubMed] [Google Scholar]
- 65. Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, An Adipokine With Potent Proinflammatory Properties. J Immunol (2005) 174(9):5789–95. 10.4049/jimmunol.174.9.5789 [DOI] [PubMed] [Google Scholar]
- 66. Silswal N, Singh AK, Aruna B, Mukhopadhyay S, Ghosh S, Ehtesham NZ. Human Resistin Stimulates the Pro-Inflammatory Cytokines TNF-α and IL-12 in Macrophages by NF-κB-Dependent Pathway. Biochem Biophys Res Commun (2005) 334(4):1092–101. 10.1016/j.bbrc.2005.06.202 [DOI] [PubMed] [Google Scholar]
- 67. Verma S, Buchanan MR, Anderson TJ. Endothelial Function Testing as a Biomarker of Vascular Disease. Circulation (2003) 108(17):2054–9. 10.1161/01.CIR.0000089191.72957.ED [DOI] [PubMed] [Google Scholar]
- 68. Tilg H, Moschen AR. Adipocytokines : Mediators Linking Adipose Tissue, Inflammation and Immunity. Nat Rev Immunol (2006) 6(September):772–83. 10.1038/nri1937 [DOI] [PubMed] [Google Scholar]
- 69. Abella V, Scotece M, Conde J, Pino J, Gonzalez-Gay MA, Gómez-Reino JJ, et al. Leptin in the Interplay of Inflammation, Metabolism and Immune System Disorders. Nat Rev Rheumatol (2017) 13(2):100–9. 10.1038/nrrheum.2016.209 [DOI] [PubMed] [Google Scholar]
- 70. Rebello CJ, Kirwan JP, Greenway FL. Obesity, the Most Common Comorbidity in SARS-CoV-2: Is Leptin the Link? Int J Obes (2020) 44(9):1810–7. 10.1038/s41366-020-0640-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Maurya R, Bhattacharya P, Dey R, Nakhasi HL. Leptin Functions in Infectious Diseases. Front Immunol (2018) 9:1–15. 10.3389/fimmu.2018.02741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pérez-Pérez A, Vilariño-García T, Fernández-Riejos P, Martín-González J, Segura-Egea JJ, Sánchez-Margalet V. Role of Leptin as a Link Between Metabolism and the Immune System. Cytokine Growth Factor Rev (2017) 35(2016):71–84. 10.1016/j.cytogfr.2017.03.001 [DOI] [PubMed] [Google Scholar]
- 73. Li M-D. Leptin and Beyond: An Odyssey to the Central Control of Body Weight. Yale J Biol Med (2011) 84(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- 74. Procaccini C, Jirillo E, Matarese G. Molecular Aspects of Medicine Leptin as an Immunomodulator. Mol Aspects Med (2012) 33(1):35–45. 10.1016/j.mam.2011.10.012 [DOI] [PubMed] [Google Scholar]
- 75. Ikejima S, Sasaki S, Sashinami H, Mori F, Ogawa Y, Nakamura T, et al. Impairment of Host Resistance to Listeria Monocytogenes Infection in Liver of Db/Db and Ob/Ob Mice. Diabetes (2005) 54(1):182–9. 10.2337/diabetes.54.1.182 [DOI] [PubMed] [Google Scholar]
- 76. Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin Modulates the T-Cell Immune Response and Reverses Starvation-Induced Immunosuppression. Nature (1998) 394(6696):897–901. 10.1038/29795 [DOI] [PubMed] [Google Scholar]
- 77. Busso N, So A, Chobaz-Péclat V, Morard C, Martinez-Soria E, Talabot-Ayer D, et al. Leptin Signaling Deficiency Impairs Humoral and Cellular Immune Responses and Attenuates Experimental Arthritis. J Immunol (2002) 168(2):875–82. 10.4049/jimmunol.168.2.875 [DOI] [PubMed] [Google Scholar]
- 78. Matarese G, Moschos S, Mantzoros CS. Leptin in Immunology. J Immunol (2005) 174(6):3137–42. 10.4049/jimmunol.174.6.3137 [DOI] [PubMed] [Google Scholar]
- 79. Faggioni R, Fantuzzi G, Gabay C, Moser A, Dinarello CA, Feingold KR, et al. Leptin Deficiency Enhances Sensitivity to Endotoxin-Induced Lethality. Am J Physiol Regul Integr Comp Physiol (1999) 276(1 45-1):136–42. 10.1152/ajpregu.1999.276.1.R136 [DOI] [PubMed] [Google Scholar]
- 80. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in Inflammation and Metabolic Disease. Nat Rev Immunol (2011) 11(2):85–97. 10.1038/nri2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yong S, Hyun J, Won S, Kim M, Kim S, Kim M, et al. Biochemical and Biophysical Research Communications Preferential Effects of Leptin on CD4 T Cells in Central and Peripheral Immune System Are Critically Linked to the Expression of Leptin Receptor. Biochem Biophys Res Commun (2010) 394(3):562–8. 10.1016/j.bbrc.2010.03.019 [DOI] [PubMed] [Google Scholar]
- 82. Paz-Filho G, Mastronardi C, Franco CB, Wang KB, Wong M-L, Licinio J. Leptin: Molecular Mechanisms, Systemic Pro-Inflammatory Effects, and Clinical Implications. Arq Bras Endocrinol Metabol (2012) 56(9):597–607. 10.1590/S0004-27302012000900001 [DOI] [PubMed] [Google Scholar]
- 83. Acedo SC, Gambero S, Cunha FGP, Lorand-Metze I, Gambero A. Participation of Leptin in the Determination of the Macrophage Phenotype: An Additional Role in Adipocyte and Macrophage Crosstalk. In Vitro Cell Dev Biol Anim (2013) 49(6):473–8. 10.1007/s11626-013-9629-x [DOI] [PubMed] [Google Scholar]
- 84. Faggioni R, Feingold KR, Grunfeld C. Leptin Regulation of the Immune Response and the Immunodeficiency of Malnutrition 1. FASEB J (2001) 15(14):2565–71. 10.1096/fj.01-0431rev [DOI] [PubMed] [Google Scholar]
- 85. Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim H, et al. The Full-Length Leptin Receptor has Signaling Capabilities of Interleukin 6-Type Cytokine Receptors. Proc Natl Acad Sci USA (1996) 93(16):8374–8. 10.1073/pnas.93.16.8374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Maury E, Brichard SM. Adipokine Dysregulation, Adipose Tissue Inflammation and Metabolic Syndrome. Mol Cell Endocrinol (2010) 314:1–16. 10.1016/j.mce.2009.07.031 [DOI] [PubMed] [Google Scholar]
- 87. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose Expression of Tumor Necrosis Factor-Alpha: Direct Role in Obesity-Linked Insulin Resistance. Science (1993) 259(5091):87–91. 10.1126/science.7678183 [DOI] [PubMed] [Google Scholar]
- 88. Huh JY, Park YJ, Ham M, Kim JB. Crosstalk Between Adipocytes and Immune Cells in Adipose Tissue Inflammation and Metabolic Dysregulation in Obesity. Mol Cells (2014) 37(5):365–71. 10.14348/molcells.2014.0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sonnenberg GE, Krakower GR, Kissebah AH. Perspective A Novel Pathway to the Manifestations of Metabolic Syndrome. Obes Res (2004) 12(2):180–6. 10.1038/oby.2004.24 [DOI] [PubMed] [Google Scholar]
- 90. Mülberg J, Schooltink H, Stoyan T, Günther M, Graeve L, Buse G, et al. The Soluble Interleukin-6 Receptor Is Generated by Shedding. Eur J Immunol (1993) 23(2):473–80. 10.1002/eji.1830230226 [DOI] [PubMed] [Google Scholar]
- 91. Tang C-H, Lu D-Y, Yang R-S, Tsai H-Y, Kao M-C, Fu W-M, et al. Leptin-Induced IL-6 Production Is Mediated by Leptin Receptor, Insulin Receptor Substrate-1, Phosphatidylinositol 3-Kinase, Akt, NF-κB, and P300 Pathway in Microglia. J Immunol (2007) 179(2):1292–302. 10.4049/jimmunol.179.2.1292 [DOI] [PubMed] [Google Scholar]
- 92. Tan Y, Lim SG, Hong W. Microreview Regulation of Cell Death During Infection by the Severe Acute Respiratory Syndrome Coronavirus and Other Coronaviruses. Cell Microbiol (2007) 9:2552–61. 10.1111/j.1462-5822.2007.01034.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Konstantinides S, Schäfer K, Koschnick S, Loskutoff DJ. Leptin-Dependent Platelet Aggregation and Arterial Thrombosis Suggests a Mechanism for Atherothrombotic Disease in Obesity. Curr Pharm Des (2001) 24(9):1012–8. 10.1172/JCI13143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Abd Alkhaleq H, Kornowski R, Waldman M, Levy E, Zemel R, Nudelman V, et al. Leptin Modulates Gene Expression in the Heart and Cardiomyocytes Towards Mitigating Ischemia-Induced Damage. Exp Cell Res (2020) 397(2):112373. 10.1016/j.yexcr.2020.112373 [DOI] [PubMed] [Google Scholar]
- 95. van der Voort PHJ, Moser J, Zandstra DF, Muller Kobold AC, Knoester M, Calkhoven CF, et al. Leptin Levels in SARS-CoV-2 Infection Related Respiratory Failure: A Cross-Sectional Study and a Pathophysiological Framework on the Role of Fat Tissue. Heliyon (2020) 6(8):e04696. 10.1016/j.heliyon.2020.e04696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chiappetta S. COVID-19 and the Role of Chronic Inflammation in Patients With Obesity. Int J Obes (2020) 6(8):e04696. 10.1038/s41366-020-0597-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Paquette SG, Banner D, Zhao Z, Fang Y, Huang SSH, León AJ, et al. Interleukin-6 Is a Potential Biomarker for Severe Pandemic H1N1 Influenza a Infection. PLoS One (2012) 7(6). 10.1371/journal.pone.0038214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Fehr AR, Channappanavar R, Perlman S. Middle East Respiratory Syndrome: Emergence of a Pathogenic Human Coronavirus. Annu Rev Med (2017) 68:387–99. 10.1146/annurev-med-051215-031152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Channappanavar R, Perlman S. Pathogenic Human Coronavirus Infections: Causes and Consequences of Cytokine Storm and Immunopathology. Semin Immunopathol (2017) 39(5):529–39. 10.1007/s00281-017-0629-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fuster JJ, Walsh K. The Good, the Bad, and the Ugly of Interleukin-6 Signaling. EMBO J (2014) 33(13):1425–7. 10.15252/embj.201488856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Han MS, White A, Perry RJ, Camporez JP, Hidalgo J, Shulman GI, et al. Regulation of Adipose Tissue Inflammation by Interleukin 6. Proc Natl Acad Sci USA (2020) 117(6):2751–60. 10.1073/pnas.1920004117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the Release of Adipokines by Adipose Tissue, Adipose Tissue Matrix, and Adipocytes From Visceral and Subcutaneous Abdominal Adipose Tissues of Obese Humans. Endocrinology (2004) 145(5):2273–82. 10.1210/en.2003-1336 [DOI] [PubMed] [Google Scholar]
- 103. Wueest S, Konrad D. The Role of Adipocyte-Specific IL-6-Type Cytokine Signaling in FFA and Leptin Release. Adipocyte (2018) 7(3):226–8. 10.1080/21623945.2018.1493901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Febbraio MA. Gp130 Receptor Ligands as Potential Therapeutic Targets for Obesity. J Clin Invest (2007) 117(4):841–9. 10.1172/JCI30453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Mishra D, Richard JE, Maric I, Porteiro B, Häring M, Kooijman S, et al. Parabrachial Interleukin-6 Reduces Body Weight and Food Intake and Increases Thermogenesis to Regulate Energy Metabolism. Cell Rep (2019) 26(11):3011–26.e5. 10.1016/j.celrep.2019.02.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Dunn-Meynell AA, Le Foll C, Johnson MD, Lutz TA, Hayes MR, Levin BE. Endogenous VMH Amylin Signaling Is Required for Full Leptin Signaling and Protection From Diet-Induced Obesity. Am J Physiol Regul Integr Comp Physiol (2016) 310(4):R355–65. 10.1152/ajpregu.00462.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ghilardi N, Ziegler S, Wiestner A, Stoffel R, Heim MH, Skoda RC. Defective STAT Signaling by the Leptin Receptor in Diabetic Mice. Proc Natl Acad Sci USA (1996) 93(13):6231–5. 10.1073/pnas.93.13.6231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zhao T, Hou M, Xia M, Wang Q, Zhu H, Xiao Y, et al. Globular Adiponectin Decreases Leptin-Induced Tumor Necrosis Factor-α Expression by Murine Macrophages: Involvement of cAMP-PKA and MAPK Pathways. Cell Immunol (2005) 238(1):19–30. 10.1016/j.cellimm.2005.12.002 [DOI] [PubMed] [Google Scholar]
- 109. Sarraf P, Frederich RC, Turner EM, Ma G, Jaskowiak NT, Rivet DJ, et al. Multiple Cytokines and Acute Inflammation Raise Mouse Leptin Levels: Potential Role in Inflammatory Anorexia. J Exp Med (1997) 185(1):171–5. 10.1084/jem.185.1.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Guo X, Roberts MR, Becker SM, Podd B, Zhang Y, Chua SC, et al. Leptin Signaling in Intestinal Epithelium Mediates Resistance to Enteric Infection by Entamoeba Histolytica. Mucosal Immunol (2011) 4(3):294–303. 10.1038/mi.2010.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ubags NDJ, Stapleton RD, Vernooy JHJ, Burg E, Bement J, Hayes CM, et al. Hyperleptinemia Is Associated With Impaired Pulmonary Host Defense. JCI Insight (2016) 1(8):294–303. 10.1172/jci.insight.82101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Mancuso P, Canetti C, Gottschalk A, Tithof PK, Peters-Golden M. Leptin Augments Alveolar Macrophage Leukotriene Synthesis by Increasing Phospholipase Activity and Enhancing Group IVC iPLA2 (cPLA2gamma) Protein Expression. Am J Physiol Lung Cell Mol Physiol (2004) 287(3):L497–502. 10.1152/ajplung.00010.2004 [DOI] [PubMed] [Google Scholar]
- 113. Larsen L, Le Foll C, Dunn-Meynell AA, Levin BE. IL-6 Ameliorates Defective Leptin Sensitivity in DIO Ventromedial Hypothalamic Nucleus Neurons. Am J Physiol Regul Integr Comp Physiol (2016) 311(4):R764–70. 10.1152/ajpregu.00258.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Roytblat L, Rachinsky M, Fisher A, Greemberg L, Shapira Y, Douvdevani A, et al. Raised Interleukin-6 Levels in Obese Patients. Obes Res (2000) 8(9):673–5. 10.1038/oby.2000.86 [DOI] [PubMed] [Google Scholar]
- 115. Demidowich AP, Levine JA, Apps R, Cheung FK, Chen J, Fantoni G, et al. Colchicine ‘ s Effects on Metabolic and Inflammatory Molecules in Adults With Obesity and Metabolic Syndrome : Results From a Pilot Randomized Controlled Trial. Obes Res (2020) 8(9):673–5. 10.1038/s41366-020-0598-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and Toxicity Management of 19-28z CAR T Cell Therapy in B Cell Acute Lymphoblastic Leukemia. Sci Transl Med (2014) 6(224):224ra25. 10.1126/scitranslmed.3008226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Moore JB, June CH. Cytokine Release Syndrome in Severe COVID-19. Science (2020) 368(6490):473–4. 10.1126/science.abb8925 [DOI] [PubMed] [Google Scholar]
- 118. Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective Treatment of Severe COVID-19 Patients With Tocilizumab. Science (2020) 117(20):10970–5. 10.1073/pnas.2005615117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Del Valle DM, Kim-Schulze S, Huang H-H, Beckmann ND, Nirenberg S, Wang B, et al. An Inflammatory Cytokine Signature Predicts COVID-19 Severity and Survival. Nat Med (2020) 26(10):1636–43. 10.1038/s41591-020-1051-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Dinarello CA, Simon A, van der Meer JWM. Treating Inflammation by Blocking Interleukin-1 in a Broad Spectrum of Diseases. Nat Rev Drug Discov (2012) 11(8):633–52. 10.1038/nrd3800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Konter JM, Parker JL, Baez E, Li SZ, Ranscht B, Denzel M, et al. Adiponectin Attenuates Lipopolysaccharide-Induced Acute Lung Injury Through Suppression of Endothelial Cell Activation. J Immunol (2012) 188(2):854–63. 10.4049/jimmunol.1100426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Niv-Spector L, Gonen-Berger D, Gourdou I, Biener E, Gussakovsky EE, Benomar Y, et al. Identification of the Hydrophobia Strand in the A-B Loop of Leptin as Major Binding Site III: Implications for Large-Scale Preparation of Potent Recombinant Human and Ovine Leptin Antagonists. Biochem J (2005) 391(2):221–30. 10.1042/BJ20050457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Shpilman M, Niv-Spector L, Katz M, Varol C, Solomon G, Ayalon-Soffer M, et al. Development and Characterization of High Affinity Leptins and Leptin Antagonists. J Biol Chem (2011) 286(6):4429–42. 10.1074/jbc.M110.196402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Elinav E, Niv-Spector L, Katz M, Price TO, Ali M, Yacobovitz M, et al. Pegylated Leptin Antagonist Is a Potent Orexigenic Agent: Preparation and Mechanism of Activity. Endocrinology (2009) 150(7):3083–91. 10.1210/en.2008-1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ottaway N, Mahbod P, Rivero B, Norman LA, Gertler A, D’Alessio DA, et al. Diet-Induced Obese Mice Retain Endogenous Leptin Action. Cell Metab (2015) 21(6):877–82. 10.1016/j.cmet.2015.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Banks WA, Gertler A, Solomon G, Niv-Spector L, Shpilman M, Yi X, et al. Principles of Strategic Drug Delivery to the Brain (SDDB): Development of Anorectic and Orexigenic Analogs of Leptin. Physiol Behav (2011) 105(1):145–9. 10.1016/j.physbeh.2011.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Gertler A, Solomon G. Leptin-Activity Blockers: Development and Potential Use in Experimental Biology and Medicine. Can J Physiol Pharmacol (2013) 91(11):873–82. 10.1139/cjpp-2013-0012 [DOI] [PubMed] [Google Scholar]
- 128. Solomon G, Atkins A, Shahar R, Gertler A, Monsonego-Ornan E. Effect of Peripherally Administered Leptin Antagonist on Whole Body Metabolism and Bone Microarchitecture and Biomechanical Properties in the Mouse. Am J Physiol Endocrinol Metab (2014) 306(1):E14–27. 10.1152/ajpendo.00155.2013 [DOI] [PubMed] [Google Scholar]
- 129. Mak RH, Cheung WW, Solomon G, Gertler A. Preparation of Potent Leptin Receptor Antagonists and Their Therapeutic Use in Mouse Models of Uremic Cachexia and Kidney Fibrosis. Curr Pharm Des (2018) 24(9):1012–8. 10.2174/1381612824666180125094921 [DOI] [PubMed] [Google Scholar]