Abstract
Interim immunogenicity and efficacy data for the Ad26.COV2.S vaccine for COVID-19 have recently been reported1–3. We describe here the 8-month durability of humoral and cellular immune responses in 20 individuals who received one or two doses of 5×1010 vp or 1011 vp Ad26.COV2.S and in 5 participants who received placebo2. We evaluated antibody and T cell responses on day 239, which was 8 months after the single-shot vaccine regimen (N=10) or 6 months after the two-shot vaccine regimen (N=10), although the present study was not powered to compare these regimens3. We also report neutralizing antibody responses against the parental SARS-CoV-2 WA1/2020 strain as well as against the SARS-CoV-2 variants D614G, B.1.1.7 (alpha), B.1.617.1 (kappa), B.1.617.2 (delta), P.1 (gamma), B.1.429 (epsilon), and B.1.351 (beta).
Antibody responses were detected in all vaccinees on day 239 (Fig. 1A). Median WA1/2020 receptor binding domain (RBD)-specific binding antibody titers were 645, 1772, 1962, and 1306 on days 29, 57, 71, and 239, respectively. Median WA1/2020 pseudovirus neutralizing antibody titers were 272, 169, 340, and 192 on days 29, 57, 71, and 239, respectively (Fig. 1A, upper panels), and were comparable when restricted to individuals who received the single-shot vaccine regimen (Fig. S1). Three Ad26.COV2.S vaccine recipients showed a sharp increase in antibody responses during this time period; one individual developed breakthrough SARS-CoV-2 infection and two received mRNA vaccines. Excluding these three participants, antibody responses were relatively stable over 8 months with only a 1.8-fold reduction of median neutralizing antibody titers between peak responses on day 71 and the durability timepoint day 239.
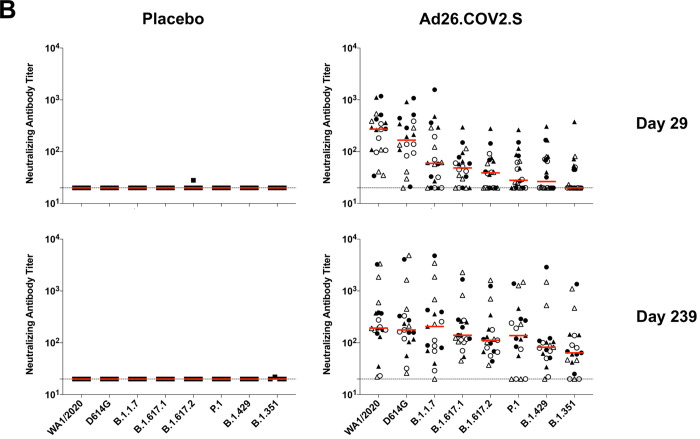
Figure 1. Durability of humoral and cellular immune responses following Ad26.COV2.S vaccination.
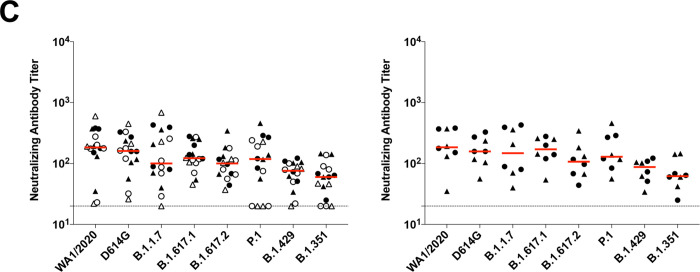
(A) SARS-CoV-2 WA1/2020 receptor binding domain (RBD)-specific binding antibodies by ELISA, pseudovirus neutralizing antibody assays, and spike-specific CD8+ and CD4+ T cell responses by intracellular cytokine staining assays on days 29, 57, 71 or 85, and 239. Red arrows highlight three individuals who developed breakthrough SARS-CoV-2 infection (filled circle; N=1) or who received mRNA vaccines (open triangles; N=2) between days 71 and 239. (B) Pseudovirus neutralizing antibody assays against the parental WA1/2020 strain as well as the SARS-CoV-2 variants D614G, B.1.1.7 (alpha), B.1.617.1 (kappa), B.1.617.2 (delta), P.1 (gamma), B.1.429 (epsilon), and B.1.351 (beta) on days 29 and 239. (C) Left, pseudovirus neutralizing antibody assays on day 239 following Ad26.COV2.S vaccination excluding the three individuals who developed breakthrough SARS-CoV-2 infection or who received mRNA vaccines. Right, pseudovirus neutralizing antibody assays on day 239 also restricted to individuals who received single-shot Ad26.COV2.S vaccination. Red bars reflect median responses. Dotted lines reflect lower limits of quantitation based on the WA1/2020 assay. Filled squares, placebo; filled circles, 1011 vp (single dose); open circles, 1011 vp (two dose); filled triangles, 5×1010 vp (single dose); open triangles, 5×1010 vp (two dose). For the two-dose vaccine, immunizations were on Day 1 and Day 57.
On day 29, median neutralizing antibody titers showed a >13-fold reduction to the B.1.351 variant compared with WA1/2020 (Fig. 1B). On day 239, however, median neutralizing antibody titers showed a more modest 3-fold reduction to the B.1.351 variant compared with WA1/2020 (Fig. 1B). Excluding the three individuals who developed breakthrough SARS-CoV-2 infection or who received mRNA vaccines, and restricted to individuals who received the single-shot vaccine regimen, median neutralizing antibody titers on day 239 were 184, 158, 147, 171, 107, 129, 87, and 62 against the SARS-COV-2 variants WA1/2020, D614G, B.1.1.7 (alpha), B.1.617.1 (kappa), B.1.617.2 (delta), P.1 (gamma), B.1.429 (epsilon), and B.1.351 (beta), respectively (Fig. 1C). These data suggest an expansion of neutralizing antibody breadth with improved coverage of SARS-CoV-2 variants over time, including increased neutralizing antibody titers against these variants of concern.
Spike-specific IFN-γ CD8+ and CD4+ T cell responses were evaluated by intracellular cytokine staining assays and also showed durability and stability over this time period (Fig. 1A, lower panels). Median CD8+ T cell responses were 0.0545%, 0.0554%, and 0.0734% on days 57, 85, and 239, respectively. Median CD4+ T cell responses were 0.0435%, 0.0322%, and 0.0176% on days 57, 85, and 239, respectively.
These data show that the Ad26.COV2.S vaccine elicited durable humoral and cellular immune responses with minimal decline for at least 8 months following immunization. In addition, we observed an expansion of neutralizing antibody breadth against SARS-CoV-2 variants over this time period, including against the more transmissible B.1.617.2 (delta) variant and the partially neutralization resistant B.1.351 (beta) and P.1 (gamma) variants, suggesting maturation of B cell responses even without further boosting. The durability of immune responses elicited by Ad26.COV2.S is consistent with the durability reported for an Ad26-based Zika vaccine4. Longitudinal antibody responses to mRNA COVID-19 vaccines have also been reported for 6 months but with more rapid decline kinetics5. The durability of humoral and cellular immune responses following Ad26.COV2.S vaccination with increased neutralizing antibody responses to SARS-CoV-2 variants over time, including after single-shot vaccination, further support the use of the Ad26.COV2.S vaccine for the global COVID-19 pandemic.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge support from Janssen Vaccines & Prevention BV, the Ragon Institute of MGH, MIT, the Massachusetts Consortium on Pathogen Readiness (MassCPR), the Musk Foundation, the National Institutes of Health (CA260476). This project has been funded in part with federal funds from the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, under Other Transaction Agreement HHSO100201700018C.
Footnotes
DISCLOSURES
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org. D.H.B. is a co-inventor on related vaccine patents. J.S., M.L.G., A.M.G., D.H., F.S., M.D., J.V.H., and H.S. are employees of Janssen Pharmaceuticals and may be co-inventors on related vaccine patents.
DATA SHARING STATEMENT
Requests for access to the study data can be submitted to D.H.B. (dbarouch@bidmc.harvard.edu).
REFERENCES
- 1.Sadoff J, Gray G, Vandebosch A, et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stephenson KE, Le Gars M, Sadoff J, et al. Immunogenicity of the Ad26.COV2.S Vaccine for COVID-19. JAMA 2021;325:1535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadoff J, Le Gars M, Shukarev G, et al. Interim Results of a Phase 1–2a Trial of Ad26.COV2.S Covid-19 Vaccine. N Engl J Med 2021;384:1824–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salisch NC, Stephenson KE, Williams K, et al. A Double-Blind, Randomized, Placebo-Controlled Phase 1 Study of Ad26.ZIKV.001, an Ad26-Vectored Anti-Zika Virus Vaccine. Ann Intern Med 2021. [DOI] [PubMed] [Google Scholar]
- 5.Doria-Rose N, Suthar MS, Makowski M, et al. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for Covid-19. N Engl J Med 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.