Abstract
Ceruloplasmin (Cp) is an acute-phase protein with ferroxidase, amine oxidase, and pro- and antioxidant activities. The primary site of Cp synthesis in human adults is the liver, but it is also synthesized by cells of monocytic origin. We have shown that gamma interferon (IFN-γ) induces the synthesis of Cp mRNA and protein in monocytic cells. We now report that the induced synthesis of Cp is terminated by a mechanism involving transcript-specific translational repression. Cp protein synthesis in U937 cells ceased after 16 h even in the presence of abundant Cp mRNA. RNA isolated from cells treated with IFN-γ for 24 h exhibited a high in vitro translation rate, suggesting that the transcript was not defective. Ribosomal association of Cp mRNA was examined by sucrose centrifugation. When Cp synthesis was high, i.e., after 8 h of IFN-γ treatment, Cp mRNA was primarily associated with polyribosomes. However, after 24 h, when Cp synthesis was low, Cp mRNA was primarily in the nonpolyribosomal fraction. Cytosolic extracts from cells treated with IFN-γ for 24 h, but not for 8 h, contained a factor which blocked in vitro Cp translation. Inhibitor expression was cell type specific and present in extracts of human cells of myeloid origin, but not in several nonmyeloid cells. The inhibitory factor bound to the 3′ untranslated region (3′-UTR) of Cp mRNA, as shown by restoration of in vitro translation by synthetic 3′-UTR added as a “decoy” and detection of a binding complex by RNA gel shift analysis. Deletion mapping of the Cp 3′-UTR indicated an internal 100-nucleotide region of the Cp 3′-UTR that was required for complex formation as well as for silencing of translation. Although transcript-specific translational control is common during development and differentiation and global translational control occurs during responses to cytokines and stress, to our knowledge, this is the first report of translational silencing of a specific transcript following cytokine activation.
Translational control of protein synthesis has specific advantages compared to transcriptional regulation; notably, it offers both rapid induction and rapid reversibility. Translational control is also efficient, since it offers a range of response levels even in the presence of a constant amount of mRNA, thus avoiding energetically inefficient cycling of mRNA by synthesis and degradation. Translational regulation is particularly important during organismal development and cell differentiation and in cells responding to changes in nutrient status or stress (50).
Translational control can be loosely divided into two classes: global and transcript-specific control. In global control, the synthesis of many proteins is simultaneously regulated, often in response to stress. In mammalian cells, two protein kinases, double-stranded RNA-dependent protein kinase (PKR) and hemin-regulated inhibitor, globally inhibit protein synthesis by phosphorylation of the eukaryotic translation initiation factor 2 α-subunit (eIF2α) (10). In transcript-specific translational control, the synthesis of one (or at most several) protein is regulated. In three of the best-studied examples, (i) translation of mammalian ribosomal protein mRNA is selectively repressed during growth arrest when the requirement for protein synthesis is minimal (4, 34), (ii) translation of ferritin mRNA is inhibited during cellular iron deprivation to maintain an adequate pool of intracellular free iron (30), and (iii) 15-lipoxygenase mRNA is silenced in early stages of erythropoiesis to protect the mitochondrial membranes (43). In most cases of translational control, a cellular RNA-binding protein binds to a cis-acting element of the targeted message which inhibits the efficiency of coupling of the transcript with the ribosome. In eukaryotic cells, interaction of a protein with a specific mRNA usually represses rather than activates translation (50).
In early studies of cis-acting sequences that regulate translation, most attention has been given to the 5′ untranslated region (5′-UTR). In the case of ferritin, the best-understood example of translational control, iron regulatory proteins 1 and 2 bind to their cognate iron-responsive element in the 5′-UTR of ferritin mRNA and block initiation by preventing the association of 43S preinitiation complex with the cap structure (24). More recently, many investigators have recognized the importance of the 3′-UTR in regulating multiple facets of mRNA metabolism, including mRNA translation initiation (6, 28, 42), stability (47), localization (57), and poly(A) chain length (48). In one example of translational control, 15-lipoxygenase mRNA translation is repressed before erythroid differentiation by binding of the heterogeneous nuclear ribonucleoproteins (hnRNP) K and E1 to pyrimidine-rich repeated regions in the 3′-UTR of the transcript (42). Other vertebrate examples of mRNAs that are translationally regulated by interaction of RNA-binding proteins with the 3′-UTR, include myocyte enhancer factor 2 (6) and β-F1-ATPase (28).
Ceruloplasmin (Cp) is a plasma glycoprotein containing seven Cu atoms per molecule and 95% of the total plasma Cu (see references 21 and 46 for review). It is a monomer of 132 kDa comprised almost entirely of three major domains that have 40% sequence homology to each other (40) and to homologous domains in factors Va and VIIIa in the coagulation cascade (9). Cp has multiple enzymatic activities including ferroxidase (41), amine oxidase (12), and pro-oxidant (39) and antioxidant (1) activities. It is an acute-phase reactant protein of uncertain physiological function; roles in Cu transport, inflammation, coagulation, angiogenesis, and defense against bacterial infection have been proposed (21, 31, 46). An important role of Cp in iron metabolism is suggested by its ferroxidase activity, by its homology to yeast copper proteins involved in iron transport (2), by hemochromatosis in human “aceruloplasminemia” patients with Cp gene defects (26), and by our own studies showing that Cp stimulates cellular iron uptake (3, 37).
The primary site of synthesis of Cp in adult humans is the liver, but cells of myeloid lineage also synthesize and secrete Cp (14, 20, 35, 56). A role for Cp in monocyte/macrophage host defense mechanisms is suggested by the stimulation of Cp synthesis by yeast cell wall fragments (14) and by gamma interferon (IFN-γ) (35). Although bactericidal activity of Cp has been reported (31), the specific function or functions of Cp in host defense are not known. Cp ferroxidase activity may drive cellular iron homeostasis in a direction unfavorable to the invasive organism, a mechanism consistent with a report that IFN-γ-activated human monocytes limit the growth of Legionella pneumophila by limiting intracellular iron (7). Alternatively, the pro-oxidant activity of Cp (39) may cause oxidative damage to invasive organisms.
We here investigate the regulation of Cp production by IFN-γ in monocytic cells. We show that IFN-γ induces Cp gene expression and protein synthesis, but these processes become uncoupled after about 8 h when the rate of Cp synthesis is low even in the presence of abundant Cp mRNA. Our experiments indicate the presence of an IFN-γ-inducible RNA-binding protein (or complex) that binds to the 3′-UTR of Cp mRNA and specifically blocks the coupling of the Cp transcript to ribosomes, thereby silencing its translation.
MATERIALS AND METHODS
Reagents.
Rabbit reticulocyte lysate, methionine-minus amino acid mixture, and RNasin were purchased from Promega (Madison, Wis.). Puromycin, cycloheximide, and other assay reagents were obtained from Sigma (St. Louis, Mo.). Human IFN-γ was from Life Technologies (Gaithersburg, Md.). [35S]methionine was purchased from NEN-DuPont (Boston, Mass.) for in vitro translation (translation grade) and from ICN (Costa Mesa, Calif.) for metabolic labeling (Trans-label). Purified human Cp was obtained from Calbiochem (La Jolla, Calif.).
Cultured cells.
U937 cells (American Type Culture Collection, Rockville, MD; CRL 1593.2) were preincubated for 3 h in serum-free RPMI 1640 medium (108 cells per 50 ml) before addition of IFN-γ (500 U/ml). HT1080, HeLa, and HepG2 cells were cultured in Dulbecco’s modified Eagle’s medium containing 5% fetal bovine serum, and human umbilical vein endothelial cells were cultured in MCDB 107 medium containing 15% fetal bovine serum. After overnight incubation of the cells, the medium was replaced with serum-free medium and incubated for 3 h before IFN-γ treatment.
Immunoblot analysis.
Conditioned medium from 8 × 106 U937 cells was centrifuged at 14,000 × g, and the supernatant was concentrated by ultrafiltration with Centricon-30 filters (Amicon, Beverly, Mass.). The concentrate was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (7% polyacrylamide) (SDS-PAGE) with Protogel (National Diagnostics, Atlanta, Ga.) and transferred by a semidry method to an Immobilon-P membrane (Millipore, Bedford, Mass.). The membrane was incubated with polyclonal rabbit anti-human Cp immunoglobulin G (IgG) (1:20,000; Accurate Chemical, Westbury, N.Y.) as a primary antibody and then with peroxidase-conjugated secondary antibody (1:10,000; Boehringer Mannheim, Indianapolis, Ind.). The blot was developed by chemiluminescence by using the ECL (Amersham, Arlington Heights, Ill.) enhanced chemiluminescence system and XAR-5 film (Kodak, Rochester, N.Y.). Immunoblots were quantitated densitometrically with a Microtek III flatbed scanner and the N.I.H. Image program, provided by Wayne Rasband, National Institutes of Mental Health.
Metabolic labeling.
U937 cells (8 × 106 cells in 4 ml of RPMI 1640 medium) were treated with IFN-γ (500 U/ml) for up to 24 h. The cells were collected by centrifugation at 7,000 × g, resuspended, and metabolically labeled by incubation for 2 h with [35S]methionine (100 μCi/ml; Trans-Label) in methionine-free medium. The cells were pelleted by centrifugation at 7,000 × g, and the conditioned medium was collected. To prepare lysates, the cells were suspended in a mixture of 50 mM Tris (pH 7.6), 50 mM NaCl, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM dithiothreitol (DTT), and 0.5% NP40; subjected to three freeze-thaw cycles; and passed several times through a 26-gauge needle. Newly synthesized, 35S-labeled Cp was immunoprecipitated from conditioned medium and lysates by using rabbit anti-human Cp IgG and protein A-Sepharose in buffer containing 50 mM Tris (pH 7.6), 150 mM NaCl, 0.5% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, and 1 mM PMSF. Proteins were resolved by SDS-PAGE (7% polyacrylamide), and the gel was fixed and treated with Amplify (Amersham, Arlington Heights, Ill.) and then dried and allowed to expose MR film (Kodak, Rochester, N.Y.).
RNA blot analysis.
Treated U937 cells (108 cells) were collected by centrifugation, and total RNA was extracted with Trizol reagent (Life Technologies) according to the manufacturer’s instructions and subjected to poly(A) selection with an OligoTex mRNA kit (Qiagen, Stanford, Calif.). The mRNA isolated from 100 μg of total RNA was fractionated on a 1% agarose–formaldehyde gel and transferred to Nytran membranes (Schleicher and Schuell, Keene, N.H.). The blot was hybridized with a random primer-labeled 646-bp human Cp cDNA probe (nucleotides [nt] 984 to 1629 in the open reading frame). The blot was stripped and rehybridized with full-length glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or human γ-actin cDNA probes.
Cloning of the 3′-UTR of human Cp.
For studies of the human Cp 5′-flanking region not described here, a human genomic library in the bacterial artificial chromosome vector pBeloBAC11 (Research Genetics, Huntsville, Ala.) was examined by PCR screening with primers corresponding to the extremes of Cp exon 1 (bp 1 to 25 and 117 to 140) (13). A pool of putative positive clones was rescreened by PCR with anchor primers in exon 1, and a single positive clone was isolated. DNA sequencing with an exon 1-specific primer 40 bp downstream of the start codon gave a sequence identical to the known Cp exon 1 sequence, demonstrating its authenticity. The same clone was sequenced with a Cp exon 19-specific primer (13) and gave a sequence which was identical to the published 247-bp Cp 3′-UTR (56), except for substitution of an A for a G in position 13 and TTG in place of GCC in positions 164 to 166; identical results were obtained from multiple sequencing reactions of three individual clones. The entire 247-bp Cp 3′-UTR was subcloned into pcDNA3 downstream of the T7 promoter (pcDNA3/Cp 3′-UTR).
Synthesis of deletion fragments of Cp 3′-UTR.
The 247-bp 3′-UTR was excised from pcDNA3/Cp 3′-UTR by digestion with BamHI and XhoI. The insert was purified by gel electrophoresis and amplified by PCR with Pfu polymerase and primers appropriate for desired segment; the 5′ primer also contained the T7 promoter sequence upstream of the Cp-specific sequence. The PCR products were gel purified, sequenced, and used as a template to generate the corresponding RNA segments by in vitro transcription with T7 RNA polymerase by using Megascript (Ambion, Austin, Tex.). Finally, the transcripts were purified on a 5% acrylamide–8 M urea gel. The synthetic Cp 3′-UTR segments are designated 1–247, 51–247, 101–247, 1–200, 1–150, and 1–100, where 1 is the first nucleotide after the stop codon and 247 is the last nucleotide before the poly(A) tail.
Isolation of polysomal mRNA.
Polysomal mRNA was isolated from U937 cells as described previously (54). In brief, U937 cells (5 × 108 cells) were treated with IFN-γ for up to 24 h and homogenized in 5 ml of polysome buffer consisting of 20 mM Tris (pH 7.4), 10 mM MgCl2, 300 mM KCl, 10 mM DTT, 100 U of RNasin per ml, and 100 μg of cycloheximide per ml, followed by centrifugation at 10,000 × g for 15 min. The postmitochondrial supernatant was layered over a sucrose (20% [wt/vol]) cushion containing cycloheximide and centrifuged at 149,000 × g for 2 h. The polysome-containing pellet was collected, and the nonpolysomal fraction was obtained by ethanol precipitation of the supernatant. Both fractions were subjected to SDS-proteinase K digestion.
Preparation of cell extracts.
U937 cells (108 cells), or in some experiments, HeLa, HT1080, HepG2, and human umbilical vein endothelial cells, were treated with IFN-γ, harvested by scraping, and suspended in a mixture of 50 mM Tris (pH 7.6), 50 mM NaCl, 1 mM PMSF, and 1 mM DTT. The suspension was subjected to three freeze-thaw cycles, was passed several times through a 26-gauge needle, and was ultracentrifuged at 100,000 × g for 30 min. The protein concentration of the supernatant was adjusted to 1 mg/ml, and 4 μg was used in the in vitro translation reaction.
In vitro translation of Cp mRNA by reticulocyte lysate.
Total RNA from U937 cells (108 cells) was isolated by two rounds of Trizol extraction. An aliquot (100 μg) was subjected to in vitro translation by addition of rabbit reticulocyte lysate, 20 μM a methionine-free amino acid mixture, 40 U of RNasin, and 20 μCi of translation-grade [35S]methionine in 50 μl for 1 h at 30°C. A 45-μl aliquot was subjected to immunoprecipitation with rabbit anti-human Cp IgG and protein-A Sepharose in buffer containing 50 mM Tris, 150 mM NaCl, 0.5% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, and 1 mM PMSF (pH 7.6). Immunoprecipitated protein was resolved by SDS-PAGE (7% polyacrylamide). The gel was fixed, soaked in Amplify, dried, and allowed to expose Kodak MR film. To evaluate the total pool of newly synthesized proteins, a 5-μl aliquot that was not subjected to immunoprecipitation was similarly resolved by SDS-PAGE (7% polyacrylamide) and fluorography. In decoy experiments, 500 ng of synthetic unlabeled transcript of Cp 3′-UTR, 15-lipoxygenase 3′-UTR, Cp exon 5, and deletion fragments of the Cp 3′-UTR were preincubated for 10 min with the cell extract before being added to the in vitro translation reaction.
In vitro transcription of Cp 3′-UTR.
A synthetic transcript of the 247-nt Cp 3′-UTR was prepared by in vitro transcription of XbaI-linearized pcDNA3/Cp 3′-UTR using T7 polymerase in the presence of [α-32P]UTP (MaxiScript kit; Ambion). The full-length transcript was purified by electrophoresis on a 5% acrylamide gel containing 8 M urea. Unlabeled synthetic transcripts of the Cp 3′-UTR, the 241-nt 15-lipoxygenase 3′-UTR, and the 255-nt Cp exon 5 were prepared by in vitro transcription of pcDNA3/Cp 3′-UTR, pBS[SK(10R)] (42, 43), and pcDNA3/Cp exon 5, respectively, and were gel purified.
RNA gel shift assay.
[α-32P]UTP-labeled Cp 3′-UTR (10 fmol) was incubated for 30 min at room temperature with U937 cell extract (10 μg of protein) in 20 μl of reaction buffer containing 12 mM HEPES (pH 8.0), 15 mM KCl, 0.25 mM DTT, 5 mM MgCl2, 0.1 mM PMSF, 200 μg of yeast tRNA per ml, 40 U of RNasin, and 10% glycerol. In competition experiments, a 25-fold molar excess of unlabeled Cp 3′-UTR and 25- or 100-fold molar excess of Cp 3′-UTR segments were added to the extract 10 min before the addition of radiolabeled probe. RNA-protein complexes were resolved by native gel electrophoresis (5% polyacrylamide in 0.5× Tris-buffered EDTA). The gel was dried, and the retarded probe was visualized by autoradiography.
RESULTS
Termination of Cp synthesis in the presence of abundant Cp mRNA.
The temporal relationship between Cp synthesis and Cp mRNA levels in IFN-γ-treated U937 cells was examined. The steady-state level of Cp mRNA was measured by Northern blot analysis and was normalized by comparison to GAPDH mRNA (Fig. 1A and B). In agreement with previous results (35), maximum Cp mRNA was reached after about 8 h and remained high for at least 24 h (Fig. 1D). Cp synthesis and secretion during 4- or 8-h intervals were measured by Western blot analysis (Fig. 1C). During the first 8 h, Cp synthesis was nearly proportional to Cp mRNA (Fig. 1D). At later times, Cp synthesis decreased disproportionately compared to Cp mRNA; between 16 and 24 h, there was essentially no Cp synthesis despite the presence of abundant Cp mRNA. To confirm the low rate of synthesis at late times after IFN-γ treatment, de novo Cp synthesis was measured by pulse-labeling of cells with [35S]methionine. Labeled Cp in the conditioned medium and lysate was determined by immunoprecipitation with rabbit anti-human Cp IgG, SDS-PAGE, and autoradiography. The lysate and conditioned medium of cells treated with IFN-γ for 8 h contained abundant newly synthesized Cp, but essentially no new Cp was detected in either pool after treatment of cells for 24 h (Fig. 1E).
FIG. 1.
Cessation of Cp synthesis in the presence of abundant Cp mRNA. (A) A steady-state amount of Cp mRNA was measured by mRNA blot analysis. U937 cells (108 cells in 50 ml) were treated with IFN-γ (500 U/ml) for up to 24 h. Poly(A)-selected mRNA was fractionated on 1% agarose–formaldehyde, transferred to Nytran membranes, and hybridized with a random primer-labeled 646-bp human Cp probe. The 18S and 28S rRNA bands are indicated by arrows. (B) The mRNA blot was stripped and rehybridized with a GAPDH cDNA probe. (C) The release of Cp into conditioned medium was measured by immunoblot analysis. U937 cells (2 × 106 cells/ml) were treated with IFN-γ (500 U/ml) for up to 24 h. The conditioned medium was collected at the time indicated and replaced with fresh medium for the next collection. The conditioned medium was concentrated and subjected to SDS-PAGE and immunoblot analysis with rabbit anti-human Cp IgG. A purified human Cp standard (Std., 25 ng) is in the leftmost lane; the arrow indicates intact 132-kDa Cp. (D) Quantitation of Cp mRNA and protein synthesis. Cp protein made during each collection period in panel C was quantitated by densitometry, normalized by comparison to the Cp standard, and expressed as nanograms per hour (gray bars). Cp mRNA in panel A and GAPDH mRNA in panel B were quantitated by densitometry, and Cp mRNA was expressed as relative densitometric units after normalization with GAPDH mRNA (o). (E) The rate of Cp synthesis was measured by metabolic labeling. U937 cells (8 × 106 cells in 4 ml) were treated with IFN-γ (500 U/ml) for 0, 8, or 24 h. At the end of each interval, cells were metabolically labeled by incubation with [35S]methionine in methionine-free medium for 2 h. The conditioned medium (CM) and lysates (Lys.) were immunoprecipitated (IP) with rabbit anti-human Cp IgG and resolved by SDS-PAGE, and radiolabeled bands were detected by fluorography. The arrow indicates the position of intact 132-kDa Cp.
Mechanism of translational silencing of Cp.
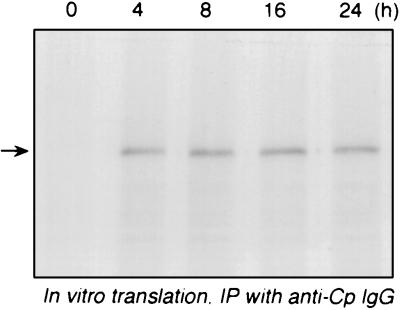
The low translation rate of Cp mRNA was consistent with either a modified and untranslatable transcript or translational silencing of an intact transcript. To test transcript integrity, U937 cells were treated with IFN-γ for up to 24 h, and total RNA was isolated and subjected to cell-free translation by a rabbit reticulocyte lysate in the presence of [35S]methionine. Newly translated, radiolabeled Cp was detected by immunoprecipitation with anti-Cp IgG and fluorography. The in vitro translational efficiencies of Cp mRNA derived from cells treated with IFN-γ for from 4 to 24 h were nearly identical (Fig. 2). The translated Cp product comigrated with an authentic human Cp standard, as shown by Coomassie blue staining (not shown). In control experiments, the Cp band was not seen with rabbit IgG in the immunoprecipitation reaction, and background translation in the absence of added RNA was negligible (not shown). The amount of RNA used gave a rate of Cp translation within the linear range of the reticulocyte lysate system (not shown). These results suggest that the low Cp translation rate in U937 cells treated with IFN-γ for 24 h is not due to a defective Cp transcript, but rather is due to a cellular defect in Cp translation.
FIG. 2.
In vitro translation of Cp mRNA from IFN-γ-treated U937 cells. U937 cells (108 cells) were incubated with IFN-γ (500 U/ml) for up to 24 h. Total RNA was isolated at the time shown, and an aliquot (100 μg) was subjected to in vitro translation for 1 h at 30°C with a rabbit reticulocyte lysate system in the presence of [35S]methionine. Translated Cp was immunoprecipitated (IP) with rabbit antihuman Cp IgG, resolved by SDS-PAGE (7% polyacrylamide), and detected by fluorography.
Transcript-specific translational control often involves translocation of regulated transcripts between two defined pools: an inactive, nonpolysomal pool containing cytoplasmic messenger ribonucleoprotein particles and an active polyribosomal pool of mRNA-ribosome complexes. We thus investigated whether inefficient Cp translation was due to a defect in the association of Cp mRNA with polyribosomes. U937 cells were treated with IFN-γ for 8 or 24 h, cell homogenates were separated into polysomal and nonpolysomal fractions by centrifugation through a sucrose cushion, and Cp mRNA was detected by Northern analysis. After IFN-γ treatment for 8 h (when the rate of Cp protein synthesis was high) Cp mRNA was primarily associated with the polysomal fraction (Fig. 3A). In contrast, after IFN-γ treatment for 24 h (when Cp synthesis was low), Cp mRNA was found almost exclusively in the inactive nonpolysomal fraction. In a control experiment, puromycin was added to the 8-h fractions to release ribosomes from mRNA (54); puromycin shifted the Cp transcript into the nonpolysomal fraction, showing that the presence of Cp in the polysomal fraction was not due to nonspecific interactions or aggregate formation (Fig. 3A). Reprobing the same blot with human γ-actin cDNA showed that this transcript was not shifted from the polysomal to nonpolysomal fraction during prolonged treatment with IFN-γ, indicating at least partial transcript specificity of the translocation process (Fig. 3B).
FIG. 3.
Release of Cp mRNA from polysomes by IFN-γ. U937 cells (5 × 108 cells) were incubated with IFN-γ (500 U/ml) for 0, 8, and 24 h. The cells were homogenized in buffer containing cycloheximide (100 μg/ml) to prevent further elongation and centrifuged at low speed. The postmitochondrial supernatant was separated into polysomal (P) and nonpolysomal (NP) fractions by centrifugation through a sucrose (20% wt/vol) cushion. In one tube, cycloheximide was replaced by puromycin (100 μg/ml) to release mRNA from ribosomes. Total mRNA from both fractions was isolated by SDS-proteinase K digestion, Trizol reagent extraction, and poly(A) selection. (A) The blot was subjected to RNA blot analysis with a human Cp cDNA probe. The 18S and 28S rRNA bands are indicated by arrows. (B) The blot was stripped and rehybridized with a human γ-actin cDNA probe.
Translational silencing of Cp transcript by a cellular factor.
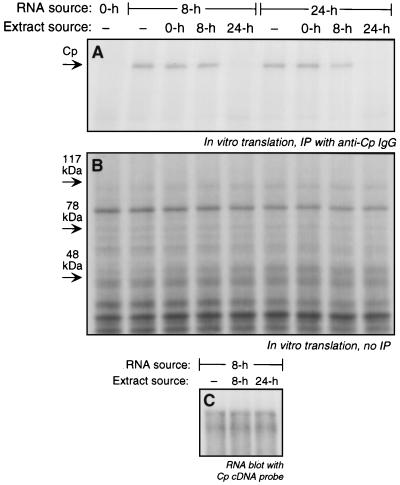
Transcript-specific translational control in eukaryotes generally involves binding of inhibitory trans-acting factors to specific mRNA sequences. To investigate the presence of inhibitory factors, extracts were made from IFN-γ-treated U937 cells and tested for their ability to block Cp mRNA translation in cell-free reticulocyte lysates. Extracts made from cells treated with IFN-γ for 24 h completely inhibited in vitro translation of Cp mRNA derived from cells treated with IFN-γ for 8 h (Fig. 4A). In contrast, extracts made from untreated cells or from cells treated with IFN-γ for 8 h were not inhibitory, showing specificity with respect to the extract source. Similar results were obtained with RNA isolated from cells treated with IFN-γ for 24 h, verifying that this transcript is regulatable as well as translatable (Fig. 4A). The transcript specificity of the inhibitor in the 24-h extract was investigated by analysis of the translated products before immunoprecipitation with anti-Cp IgG. The lack of inhibition of translation of other major U937 cellular proteins indicates a high degree of transcript specificity (Fig. 4B). In a control experiment, we tested the possibility that the 24-h extracts did not inhibit translation of Cp mRNA, but instead increased its rate of degradation. Total RNA from cells treated with IFN-γ for 8 h was incubated with rabbit reticulocyte lysate in the presence of extracts from cells treated with IFN-γ for 8 and 24 h. The RNA was reisolated and subjected to poly(A) selection and Northern blot hybridization with a Cp cDNA probe. Neither extract caused measurable Cp mRNA degradation (Fig. 4C), consistent with a factor in the 24-h extract that represses translation.
FIG. 4.
Inhibition of Cp translation by extracts from IFN-γ-treated U937 cells. U937 cells (5 × 108 cells) were treated with IFN-γ (500 U/ml) for 0, 8, and 24 h. Total RNA (100 μg) was subjected to in vitro translation with the rabbit reticulocyte lysate system. Extracts (4 μg of protein) made from cells treated with IFN-γ for the same times were added to the translation reaction. (A) Translated, 35S-labeled Cp was immunoprecipitated (IP) with rabbit anti-human Cp IgG and resolved by SDS-PAGE, and radiolabeled bands were detected by fluorography. (B) Total in vitro protein synthesis was determined with an aliquot of the translated material described in panel A that was not subjected to immunoprecipitation. Translated, 35S-labeled protein was resolved by SDS-PAGE and detected by fluorography. (C) Analysis of Cp mRNA stability. U937 cells (5 × 108 cells) were treated with IFN-γ (500 U/ml) for 8 and 24 h. Total RNA was isolated from the 8-h-treated cells, and 100 μg was incubated for 1 h at 30°C in a translation reaction mixture (without [35S]methionine) containing cell extract (4 μg of protein) from 8- and 24-h-treated cells. The RNA was reisolated with Trizol reagent and subjected to poly(A) selection and RNA blot analysis with a human Cp cDNA probe.
To investigate cell-type specificity, the presence of translational inhibitory activity in extracts of several cell lines was examined. HT1080, HeLa, HepG2, and human umbilical vein endothelial cells were treated with IFN-γ for 24 h, and extracts were prepared. All of these cells contain IFN-γ receptors, and HT1080 and HeLa cell lines are commonly used in studies of IFN-γ signaling. HepG2 cells were chosen since IFN-γ stimulates (albeit modestly) Cp synthesis by these cells (35). None of these extracts inhibited the cell-free translation of Cp mRNA isolated from U937 cells after 8 h of IFN-γ treatment (Fig. 5A). Since U937 cells are a human promonocytic cell line, we examined the activity of extracts from freshly isolated human peripheral blood monocytes. These extracts inhibited Cp translation, suggesting a specificity for cells of myeloid origin (Fig. 5B).
FIG. 5.
Cell specificity of the inhibitory activity. (A) The presence of translation inhibitory activity in HT1080, HeLa, HepG2, and human umbilical vein endothelial cells (HUVEC) was tested after incubating cells with IFN-γ for 24 h. Extracts (4 μg of protein) made from 108 cells were added to the in vitro translation reaction mixture in the presence of RNA prepared from U937 cells treated with IFN-γ for 8 h. Translated 35S-labeled Cp was immunoprecipitated (IP) with rabbit antihuman Cp IgG, resolved by SDS-PAGE (7% polyacrylamide), and detected by fluorography. (B) The presence of translation inhibitory activity in peripheral blood monocytes (PBM) was determined as in panel A.
Role of Cp 3′-UTR in translational Control by IFN-γ.
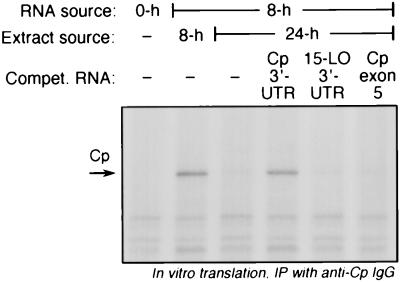
Assuming that the human Cp 5′-UTR has a length comparable to the 30-nt 5′-UTR of rat Cp (19), this transcript region is unlikely to provide secondary structure necessary for translational control. To test the role of the Cp 3′-UTR in translational inhibition, we tested whether a synthetic Cp 3′-UTR, added in excess as a decoy, could overcome the translational silencing activity of putative RNA-binding proteins. The synthetic Cp 3′-UTR almost completely restored translation in the presence of inhibitory extracts from U937 cells treated with IFN-γ for 24 h (Fig. 6). As a control to show specificity of the Cp 3′-UTR, a synthetic transcript of Cp exon 5 was found to have no effect on the translational inhibition by the cell extract. In the same experiment, we showed that the 15-lipoxygenase 3′-UTR, known to contain a regulatory region responsible for translational silencing of that transcript (42, 43), also was ineffective (Fig. 6). To determine the region of the Cp 3′-UTR responsible for translational control, we measured the ability of deletion fragments of the UTR (Fig. 7, top [schematic]) to overcome the silencing activity of the 24-h extract. Cp 3′-UTR segments 51–247, 1–200, and 1–150 were as effective as the full-length UTR, but fragment 1–100 had only marginal activity, and 101–247 was completely inactive (Fig. 7, bottom). These results suggest that nt 50 to 150 are critical for Cp translational control by IFN-γ.
FIG. 6.
RNA decoy experiment to evaluate the role of 3′-UTR in translational silencing of Cp mRNA. U937 cells (5 × 108 cells) were incubated with IFN-γ (500 U/ml) for 0, 8, and 24 h. Total RNA was isolated from these cells, and 100 μg was subjected to in vitro translation using rabbit reticulocyte lysate in the presence of cytosolic extracts (4 μg of protein from 100,000 × g supernatant) made from IFN-γ-treated cells. Synthetic unlabeled transcripts of Cp 3′-UTR (247 nt), 15-lipoxygenase (LO) 3′-UTR (241 nt), and Cp exon 5 (255 nt) were tested for their ability to restore translation in the presence of the inhibitory extract. The unlabeled transcripts (500 ng) were preincubated for 10 min with extracts made from U937 cells treated with IFN-γ for 24 h and then added to the translation reaction mixture. Newly translated, 35S-labeled Cp was detected as in Fig. 2. Compet., competitor.
FIG. 7.
RNA decoy experiment using Cp 3′-UTR deletion fragments. (Top) Schematic representation of the Cp 3′-UTR deletion fragments and results from using these fragments. (Bottom) In vitro translation of U937 total RNA was done as described in the legend to Fig. 6, except that the cell extract was preincubated with unlabeled transcripts (500 ng) of each of the deletion fragments of the Cp 3′-UTR before addition to the translation reaction mixture. Compet., competitor.
Translational control generally results from an interaction between cellular RNA-binding proteins and specific cis-acting sites in transcript UTRs. We therefore investigated the presence of a factor(s) in extracts of IFN-γ-treated U937 cells that bind to the Cp 3′-UTR. Interacting proteins were determined by an RNA electrophoretic mobility shift assay by using [α-32P]UTP-labeled Cp 3′-UTR as probe. The probe was retarded by extracts from U937 cells treated with IFN-γ for 24 h (Fig. 8). Probe-binding complexes were not detected in extracts from untreated cells or from cells treated with IFN-γ for 8 h. The specificity of the RNA-protein interaction was shown by efficient competition by an excess of unlabeled Cp 3′-UTR and also by the lack of competition by the synthetic 15-lipoxygenase 3′-UTR or by RNA corresponding to Cp exon 5.
FIG. 8.
RNA gel shift assay to detect protein or proteins binding to Cp 3′-UTR. α-32P-labeled Cp 3′-UTR transcript (10 fmol) was incubated for 30 min at room temperature with cytosolic extract (10 μg of protein) prepared from U937 cells treated with IFN-γ for 0, 8, and 24 h. RNA-protein complexes were resolved by electrophoresis on a nondenaturing 5% polyacrylamide gel and detected by autoradiography. In the lanes showing competition, a 25-fold molar excess of unlabeled Cp 3′-UTR transcript, 15-lipoxygenase 3′-UTR, and RNA corresponding to Cp exon 5 were preincubated for 10 min with the extract before addition of labeled probe.
To investigate whether the complex observed in the RNA gel shift assay may be responsible for the translational silencing activity in the 24-h extract, the complex-forming activity of the Cp 3′-UTR was mapped and compared to the map of the silencing activity. Formation of the complex with the deletion fragments of the Cp 3′-UTR was measured as competition by unlabeled fragments for binding of the complex to radiolabeled full-length 3′-UTR. UTR segments 51–247, 1–200, and 1–150 were as effective competitors as unlabeled, full-length UTR, but segments 1–100 and 101–247 did not compete for binding even at a 25-fold molar excess (Fig. 9). The segments gave essentially the same results in the two assay systems; namely, only those segments that competed for binding to complexes when incubated with extracts also overcame the translational silencing activity when added as decoys (Fig. 7, top). The minor difference between the relative activities of segments 1–100 and 101–247 in the two assays may be due to the dissimilar experimental conditions or the nonquantitative nature of the assays. Overall, these results are consistent with the presence of a single factor (or complex) that binds to the Cp 3′-UTR and translationally silences the transcript.
FIG. 9.
RNA gel shift assay with Cp 3′-UTR deletion fragments. The RNA gel shift assay was done as in Fig. 8, except that binding of the extract to α-32P-labeled, full-length Cp 3′-UTR transcript (10 fmol) was competed for by a 25-fold molar excess of the full-length transcript or by a 25- or 100-fold molar excess of the deletion fragments illustrated in Fig. 7 (top).
DISCUSSION
Our results show that IFN-γ inhibits translation of Cp mRNA by activating or inducing a translational repressor that specifically binds to the Cp mRNA 3′-UTR and uncouples it from the polyribosomes. Although IFN-γ is known to cause global inhibition of translation via activation of PKR, to our knowledge, this is the first demonstration of delayed translational silencing of a specific transcript by IFN-γ. In fact, translational control of synthesis of specific proteins by any cytokine is not common. An exception is the translational regulation of tumor necrosis factor-α by lipopolysaccharide in monocytes (16). In contrast to the silencing of Cp translation by IFN-γ, lipopolysaccharide upregulates tumor necrosis factor-α translation by recruitment of its transcript to polyribosomes.
A paradigm that has been helpful in understanding transcript-specific translational control was first introduced for studies of regulation of ferritin mRNA translation, namely, that a cellular RNA-binding protein binds to specific cis-acting elements in target transcripts, uncoupling them from polyribosomes (5, 45). Our finding that a cellular factor suppresses in vitro translation of Cp is consistent with this model. Our results suggest that the activity in the extract that blocks translation and the factor or complex that binds to the Cp 3′-UTR are the same. In support of this conjecture, both activities are present in extracts of cells treated with IFN-γ for 24 h, but absent in 8-h extracts, and both require the same part of the Cp 3′-UTR. The specific factor involved in translational control of Cp has not been identified. The “decoy” experiments suggest that hnRNP proteins that bind to the 3′-UTR of 15-lipoxygenase (42) are ineffective; this result is not surprising in view of the absence of any of the critical pyrimidine-rich motifs that are binding sites in the 15-lipoxygenase 3′-UTR (42). Likewise, the hexanucleotide consensus sequence of the iron-responsive element (30) is not present in the Cp 3′-UTR, suggesting that iron regulatory proteins 1 and 2 are not involved in Cp translational control. There are reports of translational suppression by the translated protein product itself (8, 15, 55). As a secreted protein, it is unlikely that Cp autoregulates translation, and, in fact, addition of Cp to the in vitro translation system did not inhibit Cp translation (not shown). A role of PKR may be considered, because it is activated by IFN-γ and it inhibits protein synthesis (by phosphorylation and inhibition of eIF2α) (10, 18). The long delay and the observed high specificity with respect to target transcripts observed for Cp regulation suggest that PKR is not involved. Addition of the PKR inhibitor adenovirus-associated RNA1 (53) during in vitro translation failed to overcome the inhibitory activity of cell extract, providing further evidence that PKR is not involved (not shown).
The induction of two different Cp transcripts after IFN-γ treatment of U937 cells is consistent with reports of two human Cp transcripts of about 3.7 and 4.2 kb in monocytic or liver cells (23, 33). Inspection of human expressed sequence tag databases reveals a 539-nt Cp 3′-UTR (accession no. AA165482) which contains a proximal sequence essentially identical to the 247-nt Cp 3′-UTR described in the present work [and originally cloned as a full-length transcript with a poly(A) tail (56)]. Thus, at least part of the difference between the sizes of the observed transcripts is accounted for by the different 3′-UTR lengths. Our observation that both transcripts are subject to similar translational control, as evidenced by uncoupling of both transcripts from the polysomes (Fig. 3), is not surprising, given that the critical regulatory region described in our work is present in both 3′-UTRs.
Our findings are consistent with a model in which the prolonged treatment of monocytic cells with IFN-γ activates or induces an RNA-binding protein or proteins which specifically bind to the 3′-UTR of Cp and interfere with ribosome assembly. Recent studies have begun to elucidate the mechanism or mechanisms by which ribosome binding to RNA is inhibited by regulatory proteins binding to transcript UTRs, (e.g., binding of iron regulatory protein 1 to the ferritin 5′-UTR prevents recruitment of the 40S ribosomal subunit despite assembly of the cap binding complex) (36). A key unanswered issue is the mechanism by which a protein that binds to 3′-UTR inhibits ribosome binding to the distant 5′ terminus of the transcript. One possibility is that 3′-UTR binding proteins interfere with translational control mechanisms associated with the adjacent poly(A) tail. There is substantial evidence that the poly(A) tail synergizes with cap-binding proteins to give optimal translation rates (49). Two possible sources of this synergy are the ability of poly(A) tails to increase the efficiency of delivery of ribosomes to the 5′ end of the mRNA (44) and the interaction of poly(A) binding proteins with eIF4G, a member of the cap-binding complex (11, 27, 51). These experiments suggest that 5′- and 3′-UTRs are spatially proximate, and consistent with this concept, circular complexes of capped, polyadenylated mRNA (in the presence of eIF4E, eIF4G, and poly(A) binding protein 1) have been visualized by atomic force microscopy (52). It is therefore possible that proteins binding to the Cp 3′-UTR alter the interaction of poly(A) binding proteins with the 3′-poly(A) chain or the 5′ cap-binding complex, thereby suppressing ribosome assembly.
The physiological function of translational silencing of Cp is unknown. Given the known capacity of Cp to oxidatively damage macromolecules (38, 39), one possibility is that rapid silencing of translation prevents accumulation of Cp in the cell microenvironment before reaching the point at which oxidative damage occurs. This mechanism would parallel the known silencing of mRNAs encoding potentially harmful enzymes such as 15-lipoxygenase (42), and also certain growth factors and oncogenes (32). Alternatively, translational silencing of Cp may be important for iron homeostasis. The role of Cp in iron metabolism is well documented (29), and we have recently reported that Cp increases high-affinity cellular iron uptake (3, 37). The importance of translational regulation in iron homeostasis is well known. Hemoglobin was one of the first mammalian proteins shown to be under translational control (22, 25), and the hemin-inactivated protein kinase inhibits global translation by phosphorylating and inhibiting eIF-2α (17). The specific repression of ferritin mRNA translation is perhaps the best-understood example of eukaryotic translational control (5, 45). Thus, translational silencing of Cp may be a mechanism by which cells prevent excess iron uptake and may represent an additional example of the special role that translational control plays in regulation of eukaryotic cell iron homeostasis.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants HL-29582 and HL-52692 from the National Heart Lung and Blood Institute, National Institutes of Health.
We gratefully acknowledge Paul Copeland, Donna Driscoll, and Nicholas Tripoulas for helpful discussions; Jim Finke and Pat Rayman for human peripheral blood monocytes; and Matthias Hentze for helpful discussions and for the 15-lipoxygenase 3′-UTR construct.
REFERENCES
- 1.Al-Timimi D J, Dormandy T L. The inhibition of lipid autoxidation by human caeruloplasmin. Biochem J. 1977;168:283–288. doi: 10.1042/bj1680283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Askwith C, Eide D, Van Ho A, Bernard P S, Li L, Davis-Kaplan S, Sipe D M, Kaplan J. The FET3 gene of S. cerevisiae encodes a multicopper oxidase required for ferrous iron uptake. Cell. 1994;76:403–410. doi: 10.1016/0092-8674(94)90346-8. [DOI] [PubMed] [Google Scholar]
- 3.Attieh Z K, Mukhopadhyay C K, Seshadri V, Tripoulas N A, Fox P L. Ceruloplasmin ferroxidase activity stimulates cellular iron uptake by a trivalent cation-specific transport mechanism. J Biol Chem. 1999;274:1116–1123. doi: 10.1074/jbc.274.2.1116. [DOI] [PubMed] [Google Scholar]
- 4.Avni D, Shama S, Loreni F, Meyuhas O. Vertebrate mRNAs with a 5′-terminal pyrimidine tract are candidates for translational repression in quiescent cells: characterization of the translational cis-regulatory element. Mol Cell Biol. 1994;14:3822–3833. doi: 10.1128/mcb.14.6.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aziz N, Munro H N. Iron regulates ferritin mRNA translation through a segment of its 5′ untranslated region. Proc Natl Acad Sci USA. 1987;84:8478–8482. doi: 10.1073/pnas.84.23.8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black B L, Lu J, Olson E N. The MEF2A 3′ untranslated region functions as a cis-acting translational repressor. Mol Cell Biol. 1997;17:2756–2763. doi: 10.1128/mcb.17.5.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrd T F, Horwitz M A. Interferon gamma-activated human monocytes downregulate transferrin receptors and inhibit the intracellular multiplication of Legionella pneumophila by limiting the availability of iron. J Clin Investig. 1989;83:1457–1465. doi: 10.1172/JCI114038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu E, Koeller D M, Casey J L, Drake J C, Chabner B A, Elwood P C, Zinn S, Allegra C J. Autoregulation of human thymidylate synthase messenger RNA translation by thymidylate synthase. Proc Natl Acad Sci USA. 1991;88:8977–8981. doi: 10.1073/pnas.88.20.8977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Church W R, Jernigan R L, Toole J, Hewick R M, Knopf J, Knutson G J, Nesheim M E, Mann K G, Fass D N. Coagulation factors V and VIII and ceruloplasmin constitute a family of structurally related proteins. Proc Natl Acad Sci USA. 1984;81:6934–6937. doi: 10.1073/pnas.81.22.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clemens M J. Protein kinases that phosphorylate eIF2 and eIF2B, and their role in eukaryotic cell translational control. In: Hershey J W B, Mathews M B, Sonenberg N, editors. Translational control. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 139–172. [Google Scholar]
- 11.Craig A W, Haghighat A, Yu A T, Sonenberg N. Interaction of polyadenylate-binding protein with the eIF4G homologue PAIP enhances translation. Nature. 1998;392:520–523. doi: 10.1038/33198. [DOI] [PubMed] [Google Scholar]
- 12.Curzon G. Some properties of coupled iron-caeruloplasmin oxidation systems. Biochem J. 1961;79:656–663. doi: 10.1042/bj0790656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daimon M, Yamatani K, Igarashi M, Fukase N, Kawanami T, Kato T, Tominaga M, Sasaki H. Fine structure of the human ceruloplasmin gene. Biochem Biophys Res Commun. 1995;208:1028–1035. doi: 10.1006/bbrc.1995.1437. [DOI] [PubMed] [Google Scholar]
- 14.Ehrenwald E, Fox P L. Role of endogenous ceruloplasmin in LDL oxidation by human U937 monocytic cells. J Clin Investig. 1996;97:884–890. doi: 10.1172/JCI118491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ercikan-Abali E A, Banerjee D, Waltham M C, Skacel N, Scotto K W, Bertino J R. Dihydrofolate reductase protein inhibits its own translation by binding to dihydrofolate reductase mRNA sequences within the coding region. Biochemistry. 1997;36:12317–12322. doi: 10.1021/bi971026e. [DOI] [PubMed] [Google Scholar]
- 16.Espel E, Garcia-Sanz J A, Aubert V, Menoud V, Sperisen P, Fernandez N, Spertini F. Transcriptional and translational control of TNF-α gene expression in human monocytes by major histocompatibility complex class II ligands. Eur J Immunol. 1996;26:2417–2424. doi: 10.1002/eji.1830261023. [DOI] [PubMed] [Google Scholar]
- 17.Fagard R, London I M. Relationship between phosphorylation and activity of heme-regulated eukaryotic initiation factor 2α kinase. Proc Natl Acad Sci USA. 1981;78:866–870. doi: 10.1073/pnas.78.2.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farrell P J, Balkow K, Hunt T, Jackson R J, Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977;11:187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- 19.Fleming R E, Gitlin J D. Structural and functional analysis of the 5′-flanking region of the rat ceruloplasmin gene. J Biol Chem. 1992;267:479–486. [PubMed] [Google Scholar]
- 20.Fleming R E, Whitman I P, Gitlin J D. Induction of ceruloplasmin gene expression in rat lung during inflammation and hyperoxia. Am J Physiol. 1991;260:L68–L74. doi: 10.1152/ajplung.1991.260.2.L68. [DOI] [PubMed] [Google Scholar]
- 21.Fox P L, Mukhopadhyay C, Ehrenwald E. Structure, oxidant activity, and cardiovascular mechanisms of human ceruloplasmin. Life Sci. 1995;56:1749–1758. doi: 10.1016/0024-3205(95)00146-w. [DOI] [PubMed] [Google Scholar]
- 22.Fuhr J E, Natta C. Translational control of globin chain synthesis. Nat New Biol. 1972;240:274–276. doi: 10.1038/newbio240274a0. [DOI] [PubMed] [Google Scholar]
- 23.Gitlin J D. Transcriptional regulation of ceruloplasmin gene expression during inflammation. J Biol Chem. 1988;263:6281–6287. [PubMed] [Google Scholar]
- 24.Gray N K, Hentze M W. Iron regulatory protein prevents binding of the 43S translation pre-initiation complex to ferritin and eALAS mRNAs. EMBO J. 1994;13:3882–3891. doi: 10.1002/j.1460-2075.1994.tb06699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gross M, Rabinovitz M. Control of globin synthesis in cell-free preparations of reticulocytes by formation of a translational repressor that is inactivated by hemin. Proc Natl Acad Sci USA. 1972;69:1565–1568. doi: 10.1073/pnas.69.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris Z L, Klomp L W, Gitlin J D. Aceruloplasminemia: an inherited neurodegenerative disease with impairment of iron homeostasis. Am J Clin Nutr. 1998;67:972S–977S. doi: 10.1093/ajcn/67.5.972S. [DOI] [PubMed] [Google Scholar]
- 27.Imataka H, Gradi A, Sonenberg N. A newly identified N-terminal amino acid sequence of human eIF4G binds poly(A)-binding protein and functions in poly(A)-dependent translation. EMBO J. 1998;17:7480–7489. doi: 10.1093/emboj/17.24.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izquierdo J M, Cuezva J M. Control of the translational efficiency of β-F1-ATPase mRNA depends on the regulation of a protein that binds the 3′ untranslated region of the mRNA. Mol Cell Biol. 1997;17:5255–5268. doi: 10.1128/mcb.17.9.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplan J, O’Halloran T V. Iron metabolism in eukaryotes: Mars and Venus at it again. Science. 1996;271:1510–1512. doi: 10.1126/science.271.5255.1510. [DOI] [PubMed] [Google Scholar]
- 30.Klausner R D, Rouault T A, Harford J B. Regulating the fate of mRNA: the control of cellular iron metabolism. Cell. 1993;72:19–28. doi: 10.1016/0092-8674(93)90046-s. [DOI] [PubMed] [Google Scholar]
- 31.Klebanoff S J. Bactericidal effect of Fe2+, ceruloplasmin, and phosphate. Arch Biochem Biophys. 1992;295:302–308. doi: 10.1016/0003-9861(92)90522-x. [DOI] [PubMed] [Google Scholar]
- 32.Korth M J, Katze M G. mRNA metabolism and cancer. In: Harford J B, Morris D R, editors. mRNA metabolism and posttranscriptional gene regulation. New York, N.Y: Wiley-Liss, Inc.; 1997. pp. 265–280. [Google Scholar]
- 33.Koschinsky M L, Funk W D, van Oost B A, MacGillivray R T. Complete cDNA sequence of human preceruloplasmin. Proc Natl Acad Sci USA. 1986;83:5086–5090. doi: 10.1073/pnas.83.14.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levy S, Avni D, Hariharan N, Perry R P, Meyuhas O. Oligopyrimidine tract at the 5′ end of mammalian ribosomal protein mRNAs is required for their translational control. Proc Natl Acad Sci USA. 1991;88:3319–3323. doi: 10.1073/pnas.88.8.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazumder B, Mukhopadhyay C K, Prok A, Cathcart M K, Fox P L. Induction of ceruloplasmin synthesis by IFN-γ in human monocytic cells. J Immunol. 1997;159:1938–1944. [PubMed] [Google Scholar]
- 36.Muckenthaler M, Gray N K, Hentze M W. IRP-1 binding to ferritin mRNA prevents the recruitment of the small ribosomal subunit by the cap-binding complex eIF4F. Mol Cell. 1998;2:383–388. doi: 10.1016/s1097-2765(00)80282-8. [DOI] [PubMed] [Google Scholar]
- 37.Mukhopadhyay C K, Attieh Z K, Fox P L. Role of ceruloplasmin in cellular iron uptake. Science. 1998;279:714–717. doi: 10.1126/science.279.5351.714. [DOI] [PubMed] [Google Scholar]
- 38.Mukhopadhyay C K, Ehrenwald E, Fox P L. Ceruloplasmin enhances smooth muscle cell- and endothelial cell-mediated low density lipoprotein oxidation by a superoxide-dependent mechanism. J Biol Chem. 1996;271:14773–14778. doi: 10.1074/jbc.271.25.14773. [DOI] [PubMed] [Google Scholar]
- 39.Mukhopadhyay C K, Mazumder B, Lindley P F, Fox P L. Identification of the prooxidant site of human ceruloplasmin: a model for oxidative damage by copper bound to protein surfaces. Proc Natl Acad Sci USA. 1997;94:11546–11551. doi: 10.1073/pnas.94.21.11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ortel T L, Takahashi N, Putnam F W. Structural model of human ceruloplasmin based on internal triplication, hydrophilic/hydrophobic character, and secondary structure of domains. Proc Natl Acad Sci USA. 1984;81:4761–4765. doi: 10.1073/pnas.81.15.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Osaki S, Johnson D A, Frieden E. The possible significance of the ferrous oxidase activity of ceruloplasmin in normal human serum. J Biol Chem. 1966;241:2746–2751. [PubMed] [Google Scholar]
- 42.Ostareck D H, Ostareck-Lederer A, Wilm M, Thiele B J, Mann M, Hentze M W. mRNA silencing in erythroid differentiation: hnRNP K and hnRNP E1 regulate 15-lipoxygenase translation from the 3′ end. Cell. 1997;89:597–606. doi: 10.1016/s0092-8674(00)80241-x. [DOI] [PubMed] [Google Scholar]
- 43.Ostareck-Lederer A, Ostareck D H, Standart N, Thiele B J. Translation of 15-lipoxygenase mRNA is inhibited by a protein that binds to a repeated sequence in the 3′ untranslated region. EMBO J. 1994;13:1476–1481. doi: 10.1002/j.1460-2075.1994.tb06402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Preiss T, Hentze M W. Dual function of the messenger RNA cap structure in poly(A)-tail-promoted translation in yeast. Nature. 1998;392:516–520. doi: 10.1038/33192. [DOI] [PubMed] [Google Scholar]
- 45.Rouault T A, Hentze M W, Caughman S W, Harford J B, Klausner R D. Binding of a cytosolic protein to the iron-responsive element of human ferritin messenger RNA. Science. 1988;241:1207–1210. doi: 10.1126/science.3413484. [DOI] [PubMed] [Google Scholar]
- 46.Rydén L. Ceruloplasmin. In: Lontie R, editor. Copper proteins and copper enzymes. III. Boca Raton, Fla: CRC Press; 1984. pp. 37–100. [Google Scholar]
- 47.Sachs A B. Messenger RNA degradation in eukaryotes. Cell. 1993;74:413–421. doi: 10.1016/0092-8674(93)80043-e. [DOI] [PubMed] [Google Scholar]
- 48.Sachs A B, Deardorff J A. Translation initiation requires the PAB-dependent poly(A) ribonuclease in yeast. Cell. 1992;70:961–973. doi: 10.1016/0092-8674(92)90246-9. [DOI] [PubMed] [Google Scholar]
- 49.Sachs A B, Sarnow P, Hentze M W. Starting at the beginning, middle, and end: translation initiation in eukaryotes. Cell. 1997;89:831–838. doi: 10.1016/s0092-8674(00)80268-8. [DOI] [PubMed] [Google Scholar]
- 50.Standart N, Jackson R J. Regulation of translation by specific protein/mRNA interactions. Biochimie. 1994;76:867–879. doi: 10.1016/0300-9084(94)90189-9. [DOI] [PubMed] [Google Scholar]
- 51.Tarun S Z, Sachs A B. Association of the yeast poly(A) tail binding protein with translation initiation factor eIF-4G. EMBO J. 1996;15:7168–7177. [PMC free article] [PubMed] [Google Scholar]
- 52.Wells S E, Hillner P E, Vale R D, Sachs A B. Circularization of mRNA by eukaryotic translation initiation factors. Mol Cell. 1998;2:135–140. doi: 10.1016/s1097-2765(00)80122-7. [DOI] [PubMed] [Google Scholar]
- 53.Wold W S, Hermiston T W, Tollefson A E. Adenovirus proteins that subvert host defenses. Trends Microbiol. 1994;2:437–443. doi: 10.1016/0966-842x(94)90801-x. [DOI] [PubMed] [Google Scholar]
- 54.Wormington M. Preparation of synthetic mRNAs and analyses of translational efficiency in microinjected Xenopus oocytes. Methods Cell Biol. 1991;36:167–183. doi: 10.1016/s0091-679x(08)60277-0. [DOI] [PubMed] [Google Scholar]
- 55.Wu J, Bag J. Negative control of the poly(A)-binding protein mRNA translation is mediated by the adenine-rich region of its 5′-untranslated region. J Biol Chem. 1998;273:34535–34542. doi: 10.1074/jbc.273.51.34535. [DOI] [PubMed] [Google Scholar]
- 56.Yang F, Naylor S L, Lum J B, Cutshaw S, McCombs J L, Naberhaus K H, McGill J R, Adrian G S, Moore C M, Barnett D R, Bowman B H. Characterization, mapping, and expression of the human ceruloplasmin gene. Proc Natl Acad Sci USA. 1986;83:3257–3261. doi: 10.1073/pnas.83.10.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zoladek T, Vaduva G, Hunter L A, Boguta M, Go B D, Martin N C, Hopper A K. Mutations altering the mitochondrial-cytoplasmic distribution of Mod5p implicate the actin cytoskeleton and mRNA 3′ ends and/or protein synthesis in mitochondrial delivery. Mol Cell Biol. 1995;15:6884–6894. doi: 10.1128/mcb.15.12.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]