Abstract
The amygdala is a fascinating, complex structure that lies at the center of much of our current thinking about emotion. Here, I will review data that suggest that the amygdala is involved in several processes linked to determining what a stimulus is and what the organism should therefore do – the two questions that are part of the title. This piece will focus on three main aspects of amygdala function, namely attention, value representation, and decision making, by reviewing both non-human and human data. Two mechanisms of affective attention will be described. The first involves projections from the central nucleus of the amygdala to the basal forebrain, which has extensive and diffuse projections throughout the cortical mantle. The second involves projections from the basal amygdala to multiple levels across the visual cortex. I will also describe how the basolateral amygdala is important for the representation of value and in decision making. Overall, it will be argued that the amygdala plays a key role in solving the following problem: How can a limited-capacity information processing system that receives a constant stream of diverse inputs be designed to selectively process those inputs that are most significant to the objectives of the system? “What is it?” and “What’s to be done?” processes can then be viewed as important building blocks in the construction of emotion, a process that is intertwined with cognition. Furthermore, answering the two questions directs how resources should be mobilized as the organism seeks out additional information from the environment.
Keywords: Amygdala, Emotion, Affect, Attention, Decision making, Value
1. Introduction
The amygdala is a fascinating, complex structure that lies at the center of much of our current thinking about emotion. It is well known for its involvement in fear conditioning, but it also has been documented to be involved in a surprisingly broad array of functions, spanning both negative and positive dimensions.
As generally is the case when considering a brain structure, a natural inclination is to attempt to discover the function that the structure implements. In the case of the amygdala, historically, this function has been proposed to be linked to fear and, in fact, the amygdala has been suggested to be a fear module (Ohman & Mineka, 2001). An important component of this proposal is the purported existence of a subcortical thalamo-cortical pathway that conveys information to the amygdala in an expedient manner, a notion that we have critically evaluated elsewhere (Padmala, Lim, & Pessoa, 2010). More generally, the limitations of the fear-module idea have been addressed by Sander and colleagues, too, who rejected the idea that amygdala function is centered on “negative arousing emotions” (Sander, Grafman, & Zalla, 2003).
In the present paper, I will review data that suggest that the amygdala is involved in several processes linked to determining what a stimulus is and what the organism should therefore do – the two questions that are part of the title. The goal of this contribution is not, however, to provide an in-depth review of amygdala function (which is available elsewhere; see (Aggleton, 1992; Aggleton, 2000; Whalen & Phelps, 2009)), but to highlight important ways in which the function of the amygdala goes beyond emotion as traditionally conceived; for recent reviews, see also (Morrison & Salzman, 2010; Salzman & Fusi, 2010). This approach reflects a trend that may be discerned in the field of moving away from viewing the amygdala in terms of its fear-related functions and instead conceptualizing this structure in terms of a broader array of processes.
2. Amygdala: definition and anatomy
Anatomically, the amygdala is a complex structure containing more than a dozen nuclei that are richly interconnected (Fig. 1). Based on multiple types of information, including connectivity and distribution of neurotransmitters, given the amygdala’s complexity, some have even questioned whether it is meaningful to consider this collection of nuclei as a functional-anatomical unit – for instance, “What is the amygdala?” was the provocative title used by Swanson and Petrovich (1998) in a well known case (see also Heimer, Van Hoesen, Trimble, & Zahm, 2008; Holland & Gallagher, 1999). Although this debate is far from settled (see p. 3 of Aggleton & Saunders, 2000), it is useful to consider at least two subdivisions of the amygdala, one involving the basolateral amygdala (including the lateral, basal, and accessory basal nuclei) and the central amygdala (involving the central nucleus) (Cardinal, Parkinson, Hall, & Everitt, 2002; Davis & Whalen, 2001; Heimer et al., 2008) (Fig. 2). For instance, the basolateral amygdala appears to be responsible for Pavlovian learning and the representation of value. As discussed below, the central nucleus is involved in several attentional functions. The two subdivisions also exhibit connectivity patterns that are quite distinct from each other. For instance, the basolateral amygdala receives substantial sensory information from the cortex, and is richly connected with parietal, cingulate, insular, and prefrontal cortices. The central amygdala is at times viewed as a “controller of the brainstem” (Cardinal et al., 2002), and uses its widespread projections to the hypothalamus and other brainstem nuclei, including the midbrain reticular formation, to coordinate behavioral, autonomic, and neuroendochrine responses. The basolateral amygdala is also structurally more similar to the isocortical arrangement of cortex, whereas the central amygdala exhibits a more simplified cytoarchitercture with incipient lamination.
Fig. 1.

Amygdala. Anatomically, the amygdala is a complex of more than a dozen nuclei. Here, we will focus on functions of the basolateral complex (including the lateral, basal, and accessory basal nuclei) and the central nucleus). The central nucleus (CE) and the basolateral complex (B: basal nucleus; L: lateral nucleus; AB: accessory basal nucleus), are shown among other amygdala nuclei (for the remaining abbreviations, see (Aggleton & Saunders, 2000)). Reproduced from (Aggleton & Saunders, 2000) with permission.
Fig. 2.
Amygdala and medial temporal lobe regions in the human brain shown in a coronal slice. Orange: centromedial amygdala (central and medial nuclei); red: basolateral amygdala (lateral, basolateral, basomedial, and paralaminar nuclei); magenta: superficial amygdala. Other colors indicate other medial temporal lobe regions. Reproduced from Amunts et al. (2005) with permission.
The value of some functional-anatomical subdivisions notwithstanding, such as the one in terms of the basolateral and central amygdala (see also Swanson & Petrovich, 1998; Heimer et al., 2008), it must be emphasized that the bulk of the research on the human amygdala using neuroimaging employs methods that do not allow a separation in terms of anatomical nuclei (but for an interesting diffusion tensor imaging study, see (Solano-Castiella et al., 2010); for a high-resolution functional study, see (Gamer, Zurowski, & Buchel, 2010)). In addition, many lesion studies are too coarse to offer information in terms of the multiple nuclei that have been identified anatomically. This is certainly the case in human studies, as well as many nonhuman animal studies. As discussed further below, the division of labor between the basolateral and central amygdala is not entirely clear cut, however, as both of these subdivisions may be involved in attentional processes, for instance.
3. Vigilance, arousal, and ambiguity
Early stimulation studies of the amygdala in non-human animals revealed a consistent “attention response” (Ursin & Kaada, 1960); see also (Kaada, 1951), where this behavior was termed the “arrest response”), consisting of a rapid arrest of all activities in progress (e.g., in the cat, licking and walking), followed by movements of an orienting character. For instance, Ursin and Kaada (1960) noted that the animal usually raises its head and looks in an inquisitive manner (Fig. 3). The same amygdala stimulation produced EEG “desynchronization”, as revealed by both scalp electrodes and electrodes implanted into the cortex of the frontal, temporal, and parieto-occipital brain (Ursin & Kaada, 1960). Evidence of cortical desynchronization is particularly interesting as such pattern has long been considered to be a signature of cortical activation or arousal (Moruzzi & Magoun, 1949) – to be contrasted to regular, synchronous activity typically observed in less alert states. These and many other findings led to proposals in which the amygdala played a key role in arousal and/or attention (Kaada, 1972; Pribram & McGuinness, 1975). Indeed, Pribram and McGuinness (1975) suggested that the amygdala is a core structure in a system that is involved in a “What is it?” form of processing in contrast, for instance, with a “What’s to be done?” system (Germana, 1969). Contrary to Pribram and McGuinnes, as discussed below, considerable evidence exists linking the amygdala to a “What’s to be done?” function, too – thus motivating the title of the present piece.
Fig. 3.

Attention response. Stimulation of the amygdala with mild electrical currents elicits an “attention response”. (A) Before stimulation. (B, C) During stimulation. Adapted from Ursin and Kaada (1960) with permission. Illustration by Gatis Cirulis.
Modern studies have picked up on some of these early themes. For instance, Kapp and colleagues reported that, in rabbits, electrical stimulation of the central amygdala suppresses low-frequency activity, namely it produces desynchronization (Kapp, Supple, & Whalen, 1994); see also (Stock, Rupprecht, Stumpf, & Schlor, 1981). Because of the well established role of the cholinergic system in cortical activation and arousal (Sarter & Bruno, 2000), Kapp and colleagues investigated the potential effects of a cholinergic antagonist during amygdala stimulation. Their results revealed that desynchronization was markedly attenuated by such antagonists, consistent with a role for acetylcholine in cortical activation (an effect that is likely dependent on the basal forebrain system, as discussed below).
The involvement of the central amygdala in cortical arousal is also suggested by preliminary findings that spontaneous activity of central nucleus neurons was correlated with spontaneous fluctuations in the excitability of cortical neurons as measured by the electrocorticogram (Kapp, Silvestri, & Guarraci, 1996; Kapp, Silvestri, Guarraci, Moynihan, & Cain, 1997). Given that central nucleus neurons also exhibited increased firing to cues that were predictive of an aversive shock, as suggested by Whalen (1998), it is possible that the engagement of the central nucleus by fear-related cues is part of a more widespread role of this region in modulating the vigilance level of the organism (see also below for discussion of mechanisms). Indeed, based on these findings as well as those from human neuroimaging, Whalen suggested that the amygdala is a “continuous vigilance system, one that is preferentially invoked in ambiguous learning situations of biological relevance” (Whalen, 1998).
The notion that the amygdala is attuned to stimuli of biological significance is consistent with a host of neuroimaging findings, including results of amygdala engagement during biological motion (Bonda, Petrides, Ostry, & Evans, 1996) and stimuli that are of social relevance (Adolphs, 2010). Furthermore, in neuroimaging, the amygdala is robustly engaged by neutral faces (Fig. 4) (see also Pessoa, McKenna, Gutierrez, & Ungerleider, 2002) – which arguably constitute an important class of stimuli for humans – consistent with unit recordings in both monkeys (Gothard, Battaglia, Erickson, Spitler, & Amaral, 2007; Rolls, 2005) and humans (Kreiman, Koch, & Fried, 2000; Mormann et al., 2008).
Fig. 4.
Amygdala activation to neutral faces. Contrast of viewing neutral faces vs. viewing buildings during a one-back working memory task (N=30). Robust differential responses were observed in the amygdala (approximately indicated by the blue circles), and extended dorsally into the basal forebrain, and even into the globus pallidus. Data from “localizer runs” re-analyzed from Lim et al. (2009).
It has also been suggested that the amygdala is more readily engaged when stimuli have more than one interpretation, that is, in situations in which there is ambiguity (Whalen, 1998). In such situations, the gathering of additional environmental information is beneficial and possibly critical. Thus, the amygdala would be a key component of an “information gathering system” (Whalen, 1998), in line with findings that evoked responses in the amygdala to fearful faces are stronger than those evoked by angry faces (Whalen et al., 2001). Here, the reasoning is that although both fearful and angry faces are significant to an individual, the former provide less information than the latter, as the source of threat is unknown. Accordingly, fearful faces would be expected to recruit the amygdala more strongly.
Further findings of how temporal unpredictability affects amygdala processing (Herry et al., 2007) refine our understanding of amygdala function. In an interesting cross-species study, both mouse and human participants heard a simple repeating tone, which in one condition was part of a predictable sequence (e.g., every 200 ms), and in another was part of an unpredictable sequence (e.g., a variable interval with 200ms mean). In humans, fMRI results revealed increased responses in the unpredictable vs. predictable condition in the amygdala, bilaterally. In addition, evoked responses were observed throughout the entire stimulation period – i.e., they did not habituate, as commonly observed (Buchel, Morris, Dolan, & Friston, 1998; LaBar, Gatenby, Gore, LeDoux, & Phelps, 1998). In mice, enhanced amygdala engagement was revealed by increased expression of c-fos (a so-called immediate-early gene) in the basolateral amygdala. In addition, the unpredictable sound sequence prevented the rapid habituation of neuronal activity in the same region. Interestingly, the unpredictable auditory stimulation had a clear behavioral impact. In humans, spatial attention appeared to be enhanced towards emotional faces, as revealed by the dot-probe task, when participants performed the task while the unpredictable auditory sequence was simultaneously played. In mice, the unpredictable sequence led to both avoidance and anxiety-related behavior, as revealed by testing the mice on a stress-inducing maze test.
Taken together, the above findings document an important role for the amygdala in vigilance and arousal, as well as ambiguity processing. A related proposal was offered by Sander and colleagues, who proposed that the amygdala may constitute an evolved system for relevance detection (Sander et al., 2003). Based on a careful review of the available evidence, they rejected the notion that responses in the amygdala are strictly tied to fear-related processing or, more generally, to the processing of unpleasant stimuli (as it responds to many positive stimuli). Instead, they suggested that a “common computational profile” can be inferred and that it involves the detection of relevance: “an event is relevant for an organism if it can significantly influence (positively or negatively) the attainment of his or her goals, the satisfaction of his or her needs, the maintenance of his or her own well-being, and the well-being of his or species” (Sander et al., 2003) (p. 311).
Another important conclusion that can be garnered by this brief review is that the amygdala is engaged by stimuli of biological relevance, even though they do not necessarily influence emotional state (Whalen, 1998). This is the case, for instance, for pictures of affectively neutral faces, and even pictures conveying emotional expressions, which are unlikely to elicit emotions per se. It is also noteworthy that in early stimulation studies, whereas mild amygdala stimulation produced alerting-type responses, stronger stimulation generated reactions more closely related to emotion. Thus, it is useful to distinguish between “affective information processing” and affect (Whalen, 1998), especially given that the former shares many properties with cognitive processes, such as attention.
4. Attention
Although attention is closely related to concepts such as vigilance and arousal, it is often thought to assume a more specific role. Generally speaking, we can think of a central function of attention as highlighting some stimuli (or more generally, task components) so as to receive additional processing, while at the same time other stimuli (or task components) are deemphasized or discarded in terms of further processing. Whereas attention is a heterogeneous concept linked to diverse mechanisms (e.g., “bottom-up competition”, “top-down selection”, etc.), when viewed in this general manner, many amygdala functions can be profitably viewed as attentional.
4.1. Non-human animals
Findings by Holland, Gallagher, and colleagues indicate that the central nucleus is selectively involved in learning to orient to a stimulus that reliably predicts a biologically important event, such as food. As the same lesions do not impair the acquisition of other relevant conditioned responses, explanations of the role of the central nucleus in orienting processes solely based on broad impairments of learning or motivation are unsatisfactory (Holland & Gallagher, 1999).
Conditioning, while naturally involving learning, is subject to several attentional modulations and, more generally, can be viewed as a simplified system for studying selective information processing. The following problem is worth considering in this context: “How can a limited-capacity information processing system that receives a constant stream of diverse inputs be designed to selectively process those inputs that are most significant to the objectives of the system?” (Grossberg & Levine, 1987). Selection of information for further analysis is a key problem that needs to be solved for effective learning – and, arguably, many other processes.
One way in which selective aspects of learning are investigated in several behavioral paradigms is via the study of the rate of learning of a conditioned stimulus (CS), also known as associability. Associability determines how much processing is devoted to a CS, and in this manner determines the extent to which new information can be learned about it. Several theoretical frameworks of Pavlovian conditioning (e.g., Pearce & Hall, 1980) propose that in a situation in which a CS is completely predictive of an unconditioned stimulus (US), the CS is actually not worth learning about. In other words, attention to learning about a stimulus should be reserved for stimuli whose consequences are less well established. In this sense, associability can be greatly increased by surprising events. Notably, increased learning when a predictive relation is altered to a less predictive one depends on the integrity of the central nucleus, including its projections to cholinergic neurons in the basal forebrain (Cardinal et al., 2002). For instance, control rats learned the relationship between a light CS and a tone CS faster when this relationship was less predictive than when it was completely consistent. In contrast, rats with central nucleus legions showed the opposite pattern (Holland & Gallagher, 1993).
The importance of the central nucleus for selective attentional processing is further highlighted by a study by Holland and colleagues (Holland, Han, & Gallagher, 2000). In this experiment, on each trial, after a ready signal, rats were required to poke their noses into one of three ports, guided by the brief illumination of one of them. Rats with central nucleus lesions were slower to learn the task than control animals. Additional attentional challenges, including reducing the duration of the illumination and varying the duration of the ready signal, had greater impact on the performance of the lesioned than control rats. Interestingly, the results were analogous to those observed after damage to the basal forebrain system, consistent with the idea that the observed effects of central nucleus lesion are mediated via this system, as discussed below.
The role of expectations (hence surprise) in modulating amygdala responses has also been documented in the basolateral region, in this case in an experiment involving monkeys (Belova, Paton, Morrison, & Salzman, 2007). During trace conditioning, the initial CS cue is separated from the US by a temporal gap (unlike the situation in delay conditioning in which the two stimuli co-terminate). During the task, neural responses were larger when monkeys incorrectly predicted reward or punishment. For instance, neurons driven by reward (water) responded more strongly to the reward when it occurred unexpectedly (i.e., punishment was expected). Likewise, many neurons driven by punishment (air puff) responded more strongly to unexpected as compared to expected air puff (but some cells that responded to air puff did not exhibit this effect). Cells showing a valence non-specific effect of expectation also exhibited a selective response to rewards and air puffs, while still showing expectation-related modulation of those responses. Interestingly, this expectation-dependent modulation of responses bears resemblance to “prediction error signals”, which are suggested to indicate the difference between expected and received reinforcement in theoretical models of learning (Pearce & Hall, 1980; Rescorla & Wagner, 1972; Sutton & Barto, 1988). Notably, a recent link between “prediction errors” and attention for learning has been reported for the basolateral amygdala, both in terms of neural activity and lesions (Roesch, Calu, Esber, & Schoenbaum, 2010a; Roesch, Calu, Esber, & Schoenbaum, 2010b), suggesting that not only the central nucleus is involved in attentional functions (Holland & Gallagher, 1999), but also the basolateral amygdala.
In summary, the amygdala plays an important role in several selective information processing functions that are encountered during the learning process – one of the multiple forms of affective attention discussed in this paper. In particular, “surprise”, which is directly linked to prediction errors, is an important variable in determining the engagement of amygdala nuclei.
4.2. Mechanisms of vigilance and attention
What are the mechanisms by which the amygdala is involved in the regulation of the attentional functions discussed above? The central nucleus has significant projections to several basal forebrain structures, including the magnocellular basal forebrain, which contains the basal nucleus of Meynert (whose synonym “substantia innominata” is falling into disuse as more of the “unnamed” areas are being characterized), but also cell groups within the septum and the horizontal limb of the diagonal band. The magnocellular basal forebrain system originates an “ascending” (i.e., corticopetal) cholinergic (and GABAergic) projection system that innervates extensively throughout the cortical mantle. This system is thus in a favorable position to influence cortical sites across the brain, including sensory cortex, which plays a central role in responding to environmental stimuli. Consequently, the basal forebrain affects the current mode of information processing as a function of the content of the available information. Such topographically widespread effects can thus result in increased vigilance, alertness, and/or attention (Sarter & Bruno, 1999; Sarter, Bruno, & Turchi, 1999; Sarter & Bruno, 2000).
Acetylcholine released onto cortical neurons is known to facilitate their response (Everitt & Robbins, 1997). Recent evidence suggests that basal forebrain cholinergic effects operate at a time scale that is consistent with fast attentional processes (Parikh, Kozak, Martinez, & Sarter, 2007; Parikh & Sarter, 2008) – and not simply at a slower time scale that would be more consistent with “cortical arousal states”, as often assumed. Furthermore, direct stimulation of the basal forebrain was recently shown to enhance the cortical coding of natural scenes in visual cortex (Goard & Dan, 2009) by improving the reliability of visual responses (i.e., stimulation reduced the trial-by-trial variability of responses when a natural movie stimulus was repeated).
In all, one mechanism by which activity in the central nucleus influences cortical processing is by engaging basal forebrain neurons, whose terminals release acetylcholine onto cortical sensory neurons (Fig. 5) – although cholinergic mechanisms are more commonly emphasized, other non-cholinergic systems may be involved, too (Lin & Nicolelis, 2008). Consistent with the notion that some of the attentional functions of the amygdala depend on its close link with the basal forebrain, lesions of the latter have been shown to impair a host of attentional tasks. These involve not only tasks requiring sustained attention, but also those involving selective processing components (e.g., filtering of irrelevant information) (Sarter & Bruno, 1999; Sarter et al., 1999; Sarter & Bruno,2000).
Fig. 5.
Basal forebrain modulation of sensory processing. The basal forebrain (blue ellipses) has widespread connections throughout the cortical mantle, including to sensory cortex. These latter projections are suggested to have an important role in influencing how sensory information is registered and processed. Here, the basal forebrain is represented only schematically and situated atop the amygdala. For the precise localization of the magnocellular cell groups in humans, see Fig. 8 of (Zaborszky et al., 2008).
It is opportune to contrast the “ascending” projection of the central nucleus to the basal forebrain, and its effects on cortical arousal and attention, and projections more traditionally emphasized, namely “descending” ones. As briefly mentioned earlier, these projections from the central nucleus to the hypothalamus and other brainstem sites affect several structures implicated in autonomic control and neuroendochrine responses, including the periaqueductal gray, the reticular formation, and the pituitary gland. Given the effects of these structures on bodily states and the regulation of the internal milieu, a more direct link with emotional states, such as fear, may be established – in contrast to the alerting and attentional effects of the “ascending” projections of the central nucleus (Fig. 6).
Fig. 6.
Ascending and descending projections of the central nucleus. The central nucleus influences information processing throughout cortex, an effect that is mediated via the basal forebrain. At the same time, descending projections via the hypothalamus and other brainstem sites leads to the mobilization of bodily resources. Both ascending and descending systems are suggested to contribute to affective attention. BF: basal forebrain; HYP: hypothalamus.
4.3. Humans
One of the key functions of attention is to help select specific items that will further shape information processing. One way in which emotional content guides information processing is linked to the prioritization of this class of stimuli relative to neutral items. A host of experimental paradigms have documented the many ways in which the processing of emotion-laden stimuli is privileged, including detection, search, interference, masking, and the attentional blink. A particularly rich paradigm to study capacity limitations is the attentional blink, in which subjects are asked to report the occurrence of two targets (T1 and T2) among a rapid stream of visual stimuli. When T2 follows T1 by a brief delay, participants are more likely to miss it, as if they had blinked (hence the name). The attentional blink is believed to reflect a capacity-limited processing stage linked to the processing of the first target (possibly connected to short-term memory processes). Notably, the strength of the attentional blink is influenced by the emotional content of the stimuli involved, such that participants are better at detecting the second target when it is emotionally laden. For instance, in a task involving the detection of words written in a green font (among distractors written in white font), subjects were better at detecting T2 words such as “rape” compared to words such as “house” (Anderson & Phelps, 2001), when these followed a neutral first target. Importantly, this counteracting of the attentional blink by words with emotional content was not observed in patients with lesions of the amygdala, revealing a critical role for this structure in modulating perceptual experience (but see below for further discussion).
In a recent study (Lim, Padmala, & Pessoa, 2009), we reasoned that if the amygdala is involved in shaping perceptual experience when affectively significant visual items are encountered, as indicated by the above study, responses in this structure should be correlated with both visual cortex responses and behavioral reports. We investigated how affective significance shapes visual perception during an attentional blink paradigm combined with aversive conditioning (Fig. 7A). Behaviorally, following aversive learning, affectively significant scenes (CS+) were better detected than neutral (CS−) ones (72% vs. 62%, respectively). In terms of mean brain responses, both amygdala and visual cortical responses were stronger during CS+ relative to CS− trials. Increased responses in these regions were associated with improved behavioral performance across participants and followed a mediation-like pattern (Fig. 7B). Specifically, although amygdala responses were predictive of behavioral performance, once responses in visual cortex were taken into account, the initial relationship was no longer statistically significant, consistent with the idea that the influence of the amygdala was mediated via visual cortex – it is also possible that the link between amygdala and visual cortex was itself mediated via the basal forebrain (Pessoa, 2010), as discussed above.
Fig. 7.
Attentional blink paradigm. (A) Participants were asked to report on the face stimulus (T1) and on whether the stream contained a house, a building, or no scene (T2). Houses or buildings were paired with mild electrical stimulation during an initial learning phase. During the main experimental phase, only trials in which no stimulation was administered were analyzed. (B) It was hypothesized that the link between responses evoked in the amygdala and behavior (i.e., detection of T2) was mediated via specific regions of visual cortex – in this case, the parahippocampal gyrus given its involvement in the processing of scenes and spatial layouts. It was further anticipated that this relationship would be observed in terms of mean responses (across participants; shown schematically in red and blue), but also in terms of moment-to-moment fluctuations in evoked brain responses and behavior (shown in purple). Reproduced from Lim et al. (2009) with permission.
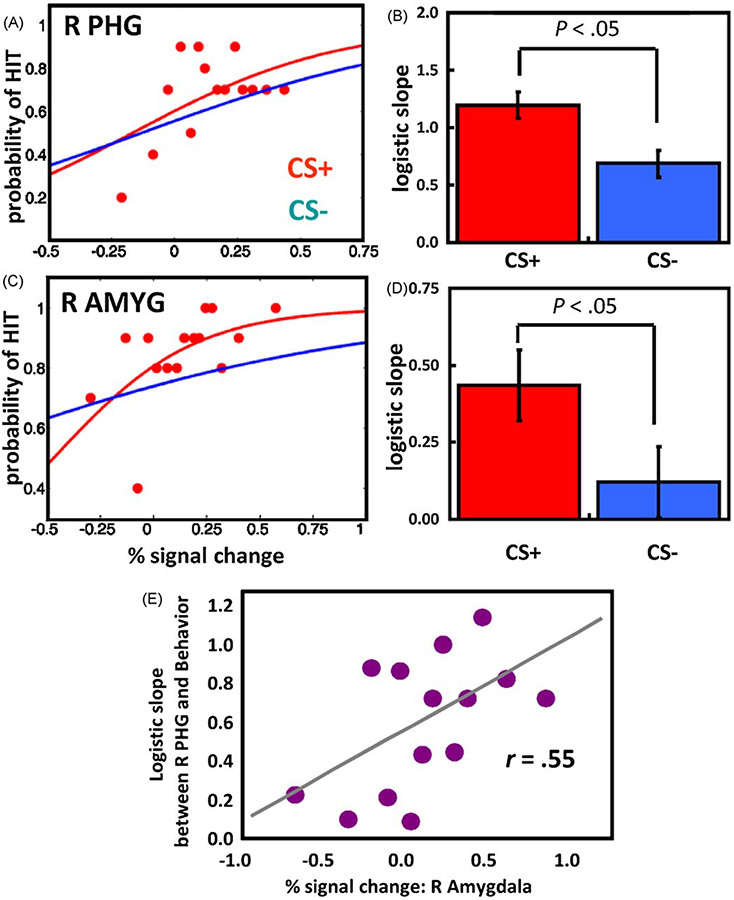
We further hypothesized that if fluctuations in evoked responses in the brain determine the accuracy of the detection of the second target, trial-by-trial variability in response amplitude should predict behavioral reports. To evaluate this prediction, we performed logistic regression analysis and modeled the probability of a hit trial (i.e., correctly reporting “house” or “building”) as a function of single-trial amplitude. In visual cortex, the mean logistic regression slopes, which represent the strength of the predictive effect, were significant for both CS+ and CS− trials, indicating that trial-by-trial fluctuations in fMRI signals reliably predicted perceptual T2 decisions (Fig. 8A and B). Importantly, a direct comparison of the CS+ and CS− conditions revealed that the predictive power of the logistic regression fit was stronger during CS+ relative to CS− scenes. An analogous trial-by-trial analysis was performed for the amygdala (Fig. 8C and D). The mean logistic regression slope was significant for CS+ trials, but not for CS− trials, indicating that variability in fMRI signals in the amygdala contributed to perceptual T2 decisions more robustly when these stimuli were affectively significant (a direct paired comparison was also significant).
Fig. 8.
Trial-by-trial analysis. (A) Logistic regression analysis of evoked responses in the right parahippocampal gyrus (PHG) as a function of affective significance (CS+ and CS−) for one individual (dichotomous variable: hits vs. misses). The slope of the logistic fit indicates the strength of the predictive effect. For clarity, only binned data for the CS+ condition are shown (red dots). (B) Mean logistic slopes across individuals for the parahippocampal gyrus. (C) The same analysis as in (A) but for the right amygdala (AMYG). (D) Mean logistic slopes across individuals for the amygdala. (E) The strength of the visual cortex-to-behavior relationship (as indexed via the slope of the logistic fit) was correlated with the magnitude of evoked responses in the amygdala: The stronger the response in the amygdala, the tighter the relationship between visual responses and behavior. Reproduced from Lim et al. (2009) with permission.
If the region interactions summarized in Fig. 7B subserve behavioral performance, they should be observed in a moment-to-moment basis, too. Importantly, the mediation would be expected to be predictive of the behavioral outcome on individual trials, namely, whether or not a subject correctly detected a target scene (hit vs. miss). Indeed, the mediation pattern was observed in a trial-by-trial analysis, revealing that the specific pattern of trial-based variability in brain responses was closely related to fluctuations in behavioral performance.
Finally, we anticipated that if the amygdala shapes perception, the strength of the predictive effect between visual cortex and behavior should depend on the strength of amygdala signals. In other words, because we could not directly influence amygdala or visual cortical responses (for instance, with microstimulation), we investigated how the relationship between evoked responses in visual cortex and behavior itself varied as a function of response strength in the amygdala. We found that when responses in the amygdala were relatively weak, the relationship between visual cortex and behavior was also relatively weak; when responses in the amygdala were strong, the relationship between visual cortex and behavior was stronger (Fig. 8E). Thus, the relationship between visual cortex and behavior varied as a function of the response strength of the amygdala. These results are consistent with the notion that trial-by-trial fluctuations in visual cortex, which are strongly tied to behavior, depend on amygdala responses.
Taken together with other findings (e.g., Anderson & Phelps, 2001), our results suggest that affective significance potentially determines the fate of a visual item during competitive interactions by enhancing sensory processing. By helping establish affective significance, the amygdala helps separate the significant from the mundane. One way to interpret the above results is in terms of an attentional function of the amygdala. For example, in studies of attention and visual cortical function, fluctuation of responses in visual cortex is often conceptualized as dependent on “source” regions in parietal and frontal cortices (Corbetta & Shulman, 2002; Kastner & Ungerleider, 2000), and these mechanisms are typically viewed as linked to how the processing of attended objects is prioritized. In our study of the attentional blink, something quite similar was observed insofar as fluctuations in the amygdala were predictive of the strength of the link between visual cortex and behavior. In this case, the amygdala was found to behave much like an “attentional device” would be expected to – namely, as a device that helps to prioritize the processing of certain stimuli over others.
4.4. Additional attentional mechanisms
One of the goals of reviewing the study above in some detail was to highlight another attentional mechanism that is carried out by the amygdala, one that further indicates the potential of the amygdala to directly shape how we see the world and even what is seen.
Just as attention in general (i.e., involving neutral information) can favor the processing of attended items, so too does emotional valence. Attended items are associated with faster reaction times and with increased neural activity. In a similar fashion, the processing of emotion-laden stimuli is prioritized and leads to stronger activation in visual processing regions – for reviews, see (Pessoa, Kastner, & Ungerleider, 2002; Vuilleumier, 2005). I suggest that the increased activation produced by emotional stimuli reflects processes of emotional modulation by which the processing of this stimulus category is favored as compared to the processing of neutral stimuli. What are the mechanisms by which this form of affective attention is carried out?
The pattern of connectivity between the amygdala and visual cortex is well characterized in monkeys (Freese & Amaral, 2005). The amygdala receives highly processed inputs from anterior portions of inferior temporal cortex but, remarkably, efferent projections from the amygdala reach nearly all levels of the ventral stream, including the primary visual cortex (V1). Notably, these connections originate in the basolateral amygdala, and are a distinct system from those discussed previously in the context of the central nucleus. This connectivity pattern has led a number of researchers to propose that these “feedback” connections exert a modulatory influence on visual responses according to the affective significance of the item being processed (which may be ascertained in the amygdala per se). As alluded to above, emotion-laden stimuli evoke stronger responses in visual cortex relative to neutral ones. For instance, stronger responses to fearful faces relative to neutral ones are observed in the fusiform gyrus (Pessoa, McKenna, Gutierrez, & Ungerleider, 2002; Vuilleumier, Armony, Driver, & Dolan, 2001), and stronger responses to a fear-conditioned grating stimulus were observed in early, retinotopically organized visual cortex, including areas V1 and V2 (Padmala & Pessoa, 2008). Projections from the amygdala to visual cortex may thus provide the substrate for these effects.
An important piece of evidence supporting this notion comes from a study that compared visual responses to faces in patients with a compromised amygdala and controls. Whereas in control participants fearful faces evoked increased responses relative to neutral ones, no significant differences were detected in the patient group (Vuilleumier, Richardson, Armony, Driver, & Dolan, 2004). It thus appears that efferent projections from the amygdala constitute an important mechanism by which cortical visual responses are modulated according to affective significance. Furthermore, increased responses may influence competition taking place in visual cortex (Pessoa, Kastner, & Ungerleider, 2002). An interesting example comes from a study by Vuilleumier and colleagues in which subjects fixated a central cue and matched either two faces or two houses presented eccentrically (Vuilleumier et al., 2001). When faces were presented to the left and right of fixation, the houses were presented above and below fixation (and vice-versa). When subjects performed the house-matching task (i.e., the faces were unattended), reaction times were slower if the (ignored) faces were fearful than if they were neutral. These results indicate that emotion-laden faces compete more strongly for processing resources in visual cortex, as suggested in Fig. 9.
Fig. 9.
Competition in visual cortex. Competition is influenced by both bottom-up stimulus dimensions (e.g., stimulus contrast) and by top-down mechanisms that are dependent on task goals (e.g., “search for faces”). Importantly, competition is also influenced by the affective significance and value of items. The latter is suggested to be supported by several potentially parallel mechanisms that rely on the amygdala, orbitofrontal cortex, and pulvinar nucleus of the thalamus, among others. Overall, the processing of affectively significant items receives prioritized processing. Note that the contribution of the pulvinar is suggested to be indirect (green arrow), given that the “visual” pulvinar is separate from the more “associational” pulvinar, which is suggested to be more directly involved in determining value (see Pessoa & Adolphs, submitted for publication).
Affective attention also influences stimulus visibility, as supported by studies of the attentional blink paradigm (see above) and detection studies (Padmala & Pessoa, 2008). Complementary evidence comes from variants of the attentional blink task in which a critical distractor stimulus precedes a target stimulus (e.g., a rotated image), and both are presented within a rapid stream of visual stimuli (Most, Chun, Widders, & Zald, 2005), as in the attentional blink. When the critical distractor is emotional, detection of the target stimulus is impaired relative to that observed when the distractor is neutral. These findings from rapid visual stream paradigms (see also Most, Chun, Johnson, & Kiehl, 2006; Most, Smith, Cooter, Levy, & Zald, 2007) suggest that, relative to neutral items, emotion-laden stimuli compete more effectively in the temporal domain, possibly by more forcefully withholding processing resources that are needed to detect the target stimulus.
Taken together, affective attention has several effects. Behaviorally, there is evidence that competition is influenced, such that affectively significant information competes more vigorously for processing resources. The studies outlined above suggest that both spatial and temporal competition processes are influenced. In terms of neural processes, it is likely that emotional modulation plays an important role in both types of behavioral effect. Indeed, the studies with amygdala patients, as well as the investigation of trial-by-trial fluctuations in both the amygdala and visual cortex, suggest that the amygdala plays an important role in these effects.
Several aspects of the contributions of the amygdala to affective attention should be noted, however. In a recent study, it was reported that a patient with bilateral amygdala lesions still exhibited a behavioral advantage for the processing of fearful faces, even though the tasks investigated required rapid detection or involved manipulations of awareness (Tsuchiya, Moradi, Felsen, Yamazaki, & Adolphs, 2009). These findings raise the possibility that the amygdala is not necessary for the advantage of emotion-laden information in these paradigms, as often assumed – although it may play a role when intact. In another recent study, Bach and colleagues reported an advantage for emotion-laden stimuli in the attentional blink in two patients with bilateral amygdala damage (Bach, Talmi, Hurlemann, Patin, & Dolan, submitted for publication) – results that directly counter the earlier findings by Anderson and Phelps (2001). In other words, a reduction of the attentional blink when T2 was an emotional word (comparable to that observed with control participants) was observed even though the patients did not have a functioning amygdala. An additional recent study revealed that enhanced search for fearful faces persisted after amygdala lesions (Piech et al., 2010). The authors proposed that the amygdala is not necessary for emotion-guided visual search and suggested that other mechanisms beyond the amygdala help guide attention toward threatening stimuli.
In this context, I suggest that another structure that is important in determining the significance of incoming stimuli is the pulvinar nucleus of the thalamus. Indeed, in a recent review (Pessoa & Adolphs, submitted for publication), based on both anatomical and physiological data, it was proposed that the pulvinar is a significant hub region (i.e., a region with a high degree of connectivity; see also below for the discussion of the amygdala as a hub region) with key contributions to the processing of biologically significant stimuli (see Ward, Calder, Parker, & Arend, 2007; Ward, Danziger, & Bamford, 2005). Indeed, in a re-analysis of the attentional blink study described in the previous section (Lim et al., 2009), responses in the pulvinar were also closely tied to the behavioral advantage of emotional stimuli (Padmala et al., 2010).
In summary, at least two important systems for affective attention were highlighted here (Fig. 10). The first system involved a circuit that links the central nucleus of the amygdala with the magnocellular basal forebrain. Although this system involves diffuse projections to most of the cortical mantle, its effects are not only global, such as arousal, but also can be quite specific (e.g., such as increasing signal-to-noise during visual processing). A second system is centered on the basolateral amygdala and appears to influence both spatial and temporal competition processes in visual cortex, thereby having an important role in shaping visual perception and awareness. An additional system involves descending projections of the central nucleus (and those via the bed nucleus of the stria terminalis), and are involved in the mobilization of bodily resources (Fig. 10). For further discussion, see (Barrett & Bliss-Moreau, 2009; Pessoa, 2010).
Fig. 10.
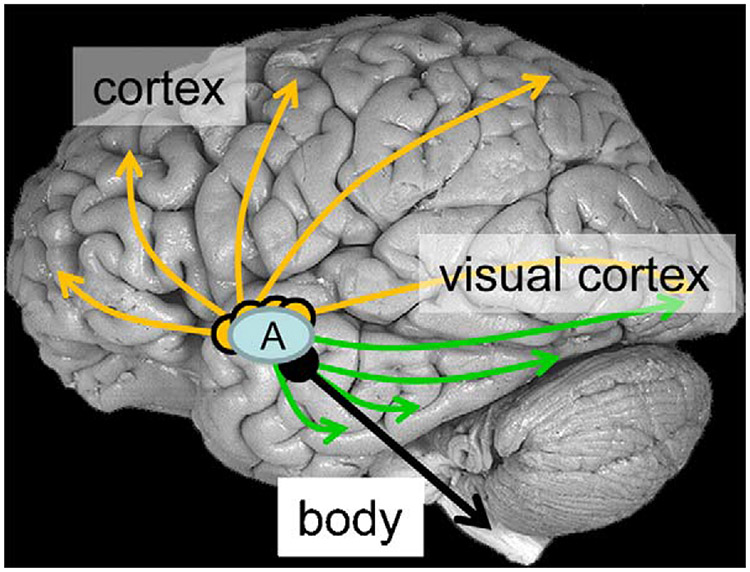
Affective attention. Affective information shapes information processing in the brain via several mechanisms. The mechanisms of affective attention highlighted here depend on the amygdala (blue ellipse). (1) Diffuse projections from the basal forebrain (yellow arrows) modulate responses throughout the cortical mantle, including sensory cortex. (2) Efferent projections from several amygdala nuclei (green arrows) reach multiple stages of the visual cortex, including both “late” (e.g., area TE) and “early” ones (e.g., V1). (3) The central nucleus also originates descending projections (black arrow) via the hypothalamus and other brainstem nuclei that mobilize bodily resources as a function of the affective significance of the item at hand. A: amygdala.
5. Representation of value and decision making
5.1. Value representation
Another important dimension of amygdala function involves its role in linking stimuli (or more generally, object representations) to current estimates of biological value. This role is pertinent when considering studies of appetitive function and reward, as well as negative functions.
In animal learning studies, a whole gamut of tasks involving reinforcer revaluation demonstrates the role of the basolateral complex in the representation of value. For example, both rats and monkeys with basolateral lesions fail to adjust their responding (e.g., orienting and food cup approach in the rat) to a CS after a food US is devalued (e.g., via satiety). In one study (Malkova, Gaffan, & Murray, 1997), monkeys first learned to discriminate pairs of objects, which were either “positive” (i.e., paired with food) or “negative” (i.e., unpaired). Two different food rewards were employed (peanuts or fruit snacks), one of which was presented on a given trial. Visual discrimination learning proceeded until monkeys performed this task rather well – that is, they could discriminate between objects paired with food and objects that were not paired with food. Prior to the subsequent critical sessions, the animals were then sated in one of the foods by having ample access to this food type (but not the other). During critical trials, the monkey was asked to choose between two objects, each of which had been paired with one of the food items – unlike in the initial discrimination learning phase, both items were “positive”. After being sated with one of the foods, intact monkeys avoided displacing objects that covered this food type in favor of objects that covered the food that had not been recently consumed. Monkeys with amygdala lesions did not exhibit this behavior, however, and instead displaced a similar number of objects that had been associated with the two different foods. These results are thus consistent with the notion that the amygdala is necessary to learning the association between stimuli and the value of particular rewards. See also (Cardinal et al., 2002; Holland & Gallagher, 1999; Murray, 2007).
Another paradigm that illustrates this issue is second-order conditioning. In one experiment (Hatfield, Han, Conley, Gallagher, & Holland, 1996), rats first experienced light → food pairings, which were readily learned as evidenced by appropriate food cup responding. Both animals with intact amygdala and those with basolateral lesions learned this task equally well. In a second phase, the rats experienced tone → light pairings. In this case, whereas intact rats acquired conditioned food cup responses to the tone, rats with lesions did not. Thus, although the lesions had no effect on the acquisition of first-order conditioned responses to the light, rats with lesions failed to acquire second-order conditioning to the tone. These findings suggest that, in rats with basolateral lesions, the light failed to acquire reinforcing value as a consequence of its first-order pairings with food (see also Everitt & Robbins, 1992).
A recent paper also suggests that amygdala neurons may help track value on a moment-to-moment basis (Belova, Paton, & Salzman, 2008). During a trace-conditioning task, monkeys learned the association between visual stimuli (CS) and positive (water delivery) and negative (airpuff to the face) outcomes (US). During a trial, presentation of different stimuli induced “state transitions”, operationally defined simply as eliciting approach or defensive behaviors. Stimuli included unconditioned stimuli, learned reinforcement-predictive visual stimuli, and familiar stimuli long associated with reinforcement (fixation point, which had a positive value to monkeys, because they chose to foveate it to initiate trials). Different populations of neurons in both the basolateral amygdala and central nucleus were observed that tracked the positive or negative value of the current state. Positive value-coding neurons increased their firing during the fixation interval and fired more strongly after positive CSs and rewards than after negative CSs and punishments. Negative value-coding neurons did the opposite, decreasing their firing during the fixation interval and firing more strongly after negative CSs and punishments than after positive CSs and rewards. Thus, the overall pattern of responses in the amygdala was consistent with the notion that neurons tracked the moment-to-moment value of the state.
Furthermore, responses in the amygdala appeared to provide a graded representation of value that spanned positive and negative valences. For instance, in a positive value-coding cell, responses elicited by the cue were high on a large-reward trial, low on a punished trial, and intermediate on small-reward trials. Analogously, in a negative value-coding cell, responses to the cue were strong on punished trials, weak on large-reward trials, and again intermediate on small-reward trials (Fig. 11). This pattern of response is important because it indicates that amygdala neurons did not simply represent the association of the CS with the sensory properties of a preferred US (water or airpuff). Instead, the responses to the CSs reflected an integration of information about multiple reinforcers with different sensory properties – involving somatosensory, gustatory, and auditory pathways. These response properties suggest, therefore, that amygdala neurons build associations between learned cues and the value of the corresponding US, which can differ in sensory modality, valence, and magnitude.
Fig. 11.
Graded representation of value. (A) Cell responses during presentation of the visual CS stimulus from a cell that encoded negative value. (B) Cell responses during the “trace interval” (i.e., interval between the visual stimulus offset and outcome delivery [US]) from a cell that encoded positive value. Reproduced from Belova et al. (2008) with permission.
Consistent with the idea that the basolateral amygdala is involved in value representation, this nucleus also exhibits signals linked to reward expectancy. For instance, Richmond and colleagues investigated a task in which specific cues indicated if reward could be obtained on that same trial after a correct red-green color discrimination task, or whether one or two additional color discrimination phases were needed for obtaining a reward (in which case, more effort was necessary for obtaining the reward). In other words, trials involved one or multiple phases. Notably, amygdala neurons signaled whether trials would involve one, two, or three phases (Sugase-Miyamoto & Richmond, 2005). Presumably, these signals could then be used to adjust the motivational level of the animal while attempting to obtain the reward.
Taken together, the above findings suggest that the amygdala plays an important role in encoding value. Notably, this encoding is observed not only at the level of the stimulus, but also at the level of reinforcer representations. For instance, conditioned responses to a tone that originally was paired with food are reduced if the food is devalued via pairing with a toxin, even though this occurs in the absence of the tone itself (Cardinal et al., 2002; Holland & Gallagher, 1999). Evidence for a role in value representations is particularly well established for the basolateral amygdala, as revealed by manipulations of a rich set of learning paradigms, but some forms of value encoding are observed in the central nucleus, too (Belova et al., 2008).
5.2. Decision making: non-human animal data
A central component of decision making involves the evaluation of the costs associated with different candidate actions relative to their potential rewards. In experimental studies, animals are required to choose between options that yield smaller, easily obtainable rewards and larger, more costly rewards. Imposing these costs, including the delay of the delivery of the reward or the requirement of increased physical effort to obtain it, leads to a “discounting” (i.e., a devaluation) of larger rewards, thus increasing preference for smaller, low-cost rewards (Floresco, St Onge, Ghods-Sharifi, & Winstanley, 2008). A growing literature indicates that the basolateral amygdala plays a critical role in these forms of decision making. Lesions or inactivations of the basolateral complex reduce preference for larger, delayed rewards (Winstanley, Theobald, Cardinal, & Robbins, 2004), larger rewards that require rats to exert increased effort (Floresco & Ghods-Sharifi, 2007; Ghods-Sharifi, St Onge, & Floresco, 2009), in addition to reducing tolerance for uncertainty (Ghods-Sharifi et al., 2009).
During a delay-based decision making task, an animal is typically confronted with a simple choice: an immediate delivery of a small reward versus the delayed delivery of a larger reward. Within certain parameters, animals, like adult humans, will choose the latter over the former. In one type of paradigm, a rat chooses between two levers, one of which provides a small reward, the other a large reward. Because the trial length is kept constant across conditions, the optimal strategy is to choose the lever linked to the larger reward (as it maximizes the total reward provided). Lesions of the basolateral amygdala increase impulsive choices (i.e., no-delay choices), indicating that the intact amygdala reduces the discounting of the delay (Winstanley et al., 2004) – that is, the intact amygdala allows the animal to wait for longer. Thus, maintaining a representation of the reward value on-line is compromised by basolateral lesions.
Another way to impose a choice between two options is to contrast options that are linked to different amounts of reward and effort. For instance, the animal may be asked to choose a food well located to the left or right of a decision location. However, to acquire the high vs. low reward, the animal is required to exert greater effort in the former vs. the latter case (e.g., climb over a scalable barrier). Intact rats typically choose the high- over the low-reward option (given that they are typically hungry in these experimental manipulations). In contrast, rats with inactivations/lesions of the basolateral amygdala choose the low-reward option (Floresco & Ghods-Sharifi, 2007; Ghods-Sharifi et al., 2009). In other words, a compromised basolateral complex reduces an animal’s preference to work harder to obtain rewards of larger magnitudes. These findings are consistent with the notion that basolateral neurons encode the expected magnitude of rewards associated with different choices.
Yet another way to evaluate decision making (and one that is quite appealing to humans) is to manipulate the probability of reward delivery. For instance, an animal may be faced with a certain, though smaller reward, and an uncertain reward of larger magnitude. In one study (Ghods-Sharifi et al., 2009), rats chose between a certain-reward lever and a “risky” lever that was linked to 50, 25, or 12.5% probability of receiving a larger reward (where the probability was fixed during a block of trials). Rats with lesions of the basolateral amygdala exhibited less tolerance for the risky choice, revealing a form of risk aversion.
Taken together, these studies reveal an important contribution of the basolateral amygdala in decision making (Seymour & Dolan, 2008). Notably, this role necessitates the integration of multiple types of information, including response cost, incentive magnitude, incentive valence, motivational state, and prior learning history. In this manner, the potential rewards that may be accrued from distinct courses of actions can be gauged in the determination of unfolding actions. On the whole, the basolateral amygdala can then lead to choices that generate greater long-term payoffs. Conversely, lesions of this region will reveal behaviors that are at times impulsive, lazy, or risk-averse, for instance, depending on the task at hand (Floresco et al., 2008). Important findings that we were not able to cover here include those by Schoenbaum and colleagues (e.g., Pickens et al., 2003; Stalnaker et al., 2007).
Naturally, the basolateral amygdala does not operate in a vacuum in shaping decision making. The value representations in the basolateral complex and how they guide choice behaviors, in all likelihood, engage multiple systems that involve the frontal lobe, the ventral striatum, and the mesolimbic dopamine system (Cardinal et al., 2002; Floresco et al., 2008; Haber & Knutson, 2010; Robbins & Everitt, 1996).
5.3. Decision making: human data
What is known about the involvement of the amygdala in decision making in humans? Impairments in decision making as experienced during the Iowa Gambling Task are well known for patients with ventromedial prefrontal cortex (VMPFC) lesions, as studied by Bechara, Damasio, and colleagues. Interestingly, a similar deficit is observed in patients with bilateral amygdala lesions, too (Bechara, Damasio, Damasio, & Lee, 1999). Similar to VMPFC patients, amygdala patients fail to choose consistently from the advantageous decks ofcards relative to the disadvantageous decks. The impairments in decision making during this experimental setting are also consistent with observations that amygdala patients exhibit poor judgment and decision making in real-life situations (Tranel & Hyman, 1990). For instance, one patient was described as exhibiting “mild improprieties and irrationalities in her social behavior” (Tranel & Hyman, 1990). More recent investigations of patients with amygdala lesion have refined our understanding of potential impairments in decision making. In addition to tasks involving uncertainty, such as the Iowa Gambling Task, tasks involving risk (i.e., probabilistic gains and losses) have also been investigated. For instance, in one study (Weller, Levin, Shiv, & Bechara, 2007), amygdala patients were impaired during scenarios involving potential gains, but not when potential losses were involved (VMPFC patients, on the other hand, were compromised in both situations). Specifically, they showed elevated risk taking and insensitivity to differences in expected value between choice options in the gain domain, but a pattern of decision making that was very similar to that of healthy individuals in the loss domain – namely, taking more risks when it was advantageous to do so and fewer risks when it was disadvantageous to do so. A second study involving patients with amygdala lesions also documented impairments in risky decision making (Brand, Grabenhorst, Starcke, Vandekerckhove, & Markowitsch, 2007). Interestingly, the deficit appeared to be more intense in those patients who also demonstrated deficits in executive function (e.g., a modified card sorting task).
Neuroimaging studies also support the idea that the amygdala is involved in encoding value, in particular, and decision making, more generally. In an appetitive conditioning task, responses evoked by a predictive stimulus in the amygdala (and OFC) were decreased after devaluation, whereas responses to the non-devalued stimulus were maintained, suggesting that the amygdala (and OFC) encodes the current value of reward representations (Gottfried, O’Doherty, & Dolan, 2003), much like that suggested in animal learning paradigms. Specifically, when a food changed from appetitive (given hunger) to disagreeable (given satiety), the responses evoked by the predictive cue were attenuated in areas that continued to respond to predictors of other palatable stimuli.
Humans are quite sensitive to the way choices are framed (Kahneman & Tversky, 1979). Interestingly, amygdala responses are sensitive to this framing effect, suggestive of a role in biasing decision making. Specifically, in one study, amygdala responses were driven by a combination of a subject’s decision and the frame (gain vs. loss frame) in which it took place, rather than the valence of the frame per se (De Martino, Kumaran, Seymour, & Dolan, 2006). These findings accord with the notion that frame-related valence information, as provided by amygdala responses, is incorporated into the relative assessment of options, thus affecting individual decisions. Amygdala responses are also sensitive to the experience of regret. In one experiment, across the experiment, participants became increasingly regret-aversive, and such cumulative process was reflected by changes in responses in the amygdala (and OFC) (Coricelli et al., 2005). In this manner, it may be possible that the amygdala has a role in biasing future decisions, possibly to minimize regret.
Consistent with a role in representing value, a recent study investigated the contributions of the amygdala to reward expectancy (Hampton, Adolphs, Tyszka, & O’Doherty, 2007). To investigate the contribution of the amygdala to reward-related signals in the PFC, two patients with bilateral amygdala lesions were scanned with fMRI during a reversal learning task. In the task, subjects learned which of two choices was the more rewarding and then flexibly switched their choice when contingencies changed. Behaviorally, the two patients with bilateral amygdala lesions exhibited markedly different performance relative to controls. For instance, one patient was significantly more likely to switch stimulus choice than controls; another patient was significantly more likely to switch choices following receipt of reward than controls. In terms of brain responses, choice-related signals (switch vs. stay) in anterior cingulate cortex and sites in the anterior insula abutting the posterior lateral OFC, bilaterally, were significantly attenuated in the amygdala patients. Likewise, expected reward signals during the choice period (based on a computational model taking into account the reward and punishment history) in orbital and medial PFC were also attenuated in the patients. Taken together, these results reveal that signals in orbital, medial, and lateral PFC that are linked to the computation of expected reward and the determination of behavioral choice based on such reward depend on input from the amygdala for their integrity. They also demonstrate the importance of amygdala-PFC interactions to the computation of expected reward value in humans, supporting a model of decision making whereby these expected reward signals are used to guide behavioral decisions.
During decision making, some situations are naturally ambiguous – namely, when information is missing – while others involve risk – that is, probabilistic outcomes – but where the judged probabilities are available. Interestingly, both types of decisions engage the amygdala, although uncertainty engages it more strongly than risk (Hsu, Bhatt, Adolphs, Tranel, & Camerer, 2005); but see (Bach, Seymour, & Dolan, 2009).
The current section on humans, together with the previous one on animals, documents a role for the amygdala in decision making and value representation. However, the reader may have detected the following apparent contradiction: whereas amygdala lesions in humans appear to, for instance, lead to risky decisions, lesions in the rat rendered the animal risk averse (as well as less willing to exert effort or tolerate uncertainty in order to gain larger rewards). A possible way to reconcile these findings is by noting that in both cases, animal and human, amygdala lesions rendered the behavioral choices sub-optimal (Stan Floresco, personal communication). For instance, in one case, rats chose the certain, low-reward outcome instead of the larger though riskier outcome, a decision that was actually disadvantageous – for instance, the certain reward condition yielded fewer pellets (on average) relative to the riskier conditions (Ghods-Sharifi et al., 2009). Humans in the Iowa Gambling Task choose from the disadvantageous decks of cards. Removal of the amygdala may thus deprive the organism from circuits that establish the relative values of different rewards, rendering the choices sub-optimal. However, it is also possible that true species differences are partly responsible for the discrepant behavioral outcomes. In addition, it is conceivable that, in the animal studies reviewed here, the involvement of the amygdala is more closely linked to other computations than those suggested here, as multiple processes may have contributed to the underlying behavioral changes.
The data reviewed here indicates that the basolateral amygdala makes important contributions to decision making and the representation of value. Nevertheless, more data are clearly needed in both humans and animals, as many issues remain unresolved. For instance, as described earlier, the study by Weller and colleagues (2007) indicates that humans do not exhibit a general tendency to behave in a riskier manner when amygdala lesions are present. Instead, impaired judgment was observed in cases involving potential gains, but not when losses were at stake.
6. Discussion
In the past two decades, our understanding of amygdala function has greatly expanded. At the same time that great advances have been made concerning the role of the amygdala in fear-related processes, concurrently, a great deal has been learned about many other functions of this structure. Instead of exhaustively trying to cover every aspect of amygdala function, here we chose to focus on examples of functions that connect more directly with the notion that its role is indeed quite broad and connected with cognitive functions, including attention (Holland & Gallagher, 1999) and decision making (Seymour & Dolan, 2008).
It could be said, then, that the field is now moving away from conceptualizing the amygdala as a fear structure and is, in a way, experiencing a reconnection with some of the historically earlier notions of amygdala function tied to vigilance and attention, in particular. Insofar as there will always be a desired to attribute a central, specific function to a brain structure, new proposals are likely to be put forward, as has been the case in the past. It appears, however, that a fundamental property of the amygdala is related to a “What is it?” function. This is the case for the basolateral amygdala, which is involved in determining the value of stimuli in the world. This is also the case for the central nucleus, which is involved in alerting and attention functions, allowing the significance of an item to be ascertained. But studies of the role of the amygdala in decision making, both in animals and humans, indicate that the amygdala is also involved in guiding “What’s to be done?”. As reviewed, both rats and humans behave sub-optimally when the amygdala is compromised.
In several ways, these two functions are aligned with “affective information processing”, as distinct from affect (see Whalen, 1998). Yet, “What is it?” and “What’s to be done?” can also be viewed as important basic building blocks that are fundamental in the construction of emotion (Kober et al., 2008). Answering “What is it?” and “What’s to be done” requires that resources be mobilized as the organism seeks out additional information from the environment (Fig. 10). And, when answering these questions redirects mental and bodily resources more forcefully, especially by engaging the hypothalamus and other brain stem structures (via the central nucleus), an emotion per se will typically result (Fig. 10, black arrow).
The two functions that we emphasized here bring us back to the problem outlined previously: “How can a limited-capacity information processing system that receives a constant stream of diverse inputs be designed to selectively process those inputs that are most significant to the objectives of the system?” (Grossberg & Levine, 1987). As reviewed here, the amygdala is intimately involved in solving this problem. Put another way, the amygdala is crucially involved in selective information processing. According to this broader perspective, the amygdala is not an “emotional structure”. Furthermore, although some of its functions can be adequately described in terms of processing ambiguity and/or detecting relevance (Sander et al., 2003; Whalen, 1998), the roles of the amygdala extend far beyond these two. This is true, for instance, when considering the attentional functions of the amygdala and even more so when considering its complex roles in the representation of value and decision making.
In this contribution, most of the discussion on the amygdala neglected to describe how this structure works in close fashion with several other brain regions while carrying out the functions outlined here. Given that the amygdala is richly interconnected with both cortical and subcortical structures (Petrovich, Canteras, & Swanson, 2001; Young, Scannell, Burns, & Blakemore, 1994), this structure has been proposed to be part of many intersecting networks of regions that are formed while behaviors unfold (Pessoa, 2008). More generally, network analyses of structural and functional data have refined our understanding of the critical role of hub regions – namely, those that exhibit a very high degree of connectivity. I propose that the amygdala complex contains at least two hubs, one involving basolateral nuclei and another involving the central nucleus. That the amygdala is increasingly recognized as playing important roles in cognitive, emotional, and social processes is suggested to be a reflection of the crucial role of these two hubs in integrating and coordinating information flow that is associated with these domains.
Readers skeptical of the central role of the amygdala in the broader array of functions described here may object that the amygdala is too “primitive”, and that it may be better viewed as tied to fear-related functions and to functioning as an effective “alarm system” – one that has been evolutionarily conserved for good reasons. Yet, even the brief exposition here that focused on rodents revealed important roles for the amygdala in “cognitive” operations, such as attention and decision making. And in primates, an order of mammals in which the cortex is richly expressed, the potential for such roles is likely to be even greater. As pointed out by Sander and colleagues, the primate amygdala may have evolved into a less specialized system in order to cope with new environmental problems (Sander et al., 2003). One way in which this may have occurred may be related to a potential expansion of the connectivity of the amygdala with a wider range of cortical territories. This may involve new direct connections, such as the connectivity documented between the amygdala and lateral PFC (Ghashghaei, Hilgetag, & Barbas, 2007) and, more extensively, in an indirect fashion via other important cortical hubs, such as those in the anterior cingulate and orbitofrontal cortices. Altered and enhanced connectivity may be one way in which a system expands the repertoire of functions it is involved in. Although the evolution of the brain is highly constrained, dramatic changes in the pattern of connectivity have been carefully documented – such as those involving the somatosensory cortex and thalamus in several groups of mammals (Kaas, 2004; Krubitzer, 2009). Whereas mice have about 10 cortical fields, and macaque monkeys have more than 50 fields, humans may have more than a hundred fields (Krubitzer, 2009). The combinatorial nature of connectivity is such that, in humans, the amygdala, which is extremely highly interconnected, may indeed be in a position to be an important player in an impressive array of cognitive-emotional behaviors.
Acknowledgements
Support for this work was provided in part by the National Institute of Mental Health (R01 MH071589). I would like to thank Stan Floresco for valuable discussions, Melissa Roemmele for assistance with references, Dan Salzman for assistance with Fig. 11, and Andrew Bauer and Jena Wierwille for help with figures.
Footnotes
A publishers’ error resulted in this article appearing in the wrong issue. The article is reprinted here for the reader’s convenience and for the continuity of the special issue. For citation purposes, please use the original publication details; Neuropsychologia, 48(12), 2010, pp. 3416–3429.
References
- Adolphs R (2010). What does the amygdala contribute to social cognition? Annals of the New York Academy of Sciences, 1191, 42–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton J (Ed.). (2000). The amygdala: A functional analysis. Oxford: Oxford University Press. [Google Scholar]
- Aggleton JP (1992). The amygdala: Neurobiological aspects of emotion, memory and mental dysfunction. New York: John Wiley & Sons. [Google Scholar]
- Aggleton JP, & Saunders RC (2000). In Aggleton JP (Ed.), The amygdala – what’s happened in the last decade. The amygdala: A functional analysis (pp. 1–30). Oxford: Oxford University Press. [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, & Zilles K (2005). Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl), 210, 343–352. [DOI] [PubMed] [Google Scholar]
- Anderson AK, & Phelps EA (2001). Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature, 411, 305–309. [DOI] [PubMed] [Google Scholar]
- Bach DR, Seymour B, & Dolan RJ (2009). Neural activity associated with the passive prediction of ambiguity and risk for aversive events. Journal of Neuroscience, 29, 1648–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach DR, Talmi D, Hurlemann R, Patin A, Dolan RJ (submitted for publication). Relevance detection in the absence of a functional amygdala. Journal of Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, & Bliss-Moreau E (2009). Affect as a psychological primitive. Advances in Experimental Social Psychology, 41, 167–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, & Lee GP(1999). Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. Journal of Neuroscience, 19, 5473–5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belova MA, Paton JJ, & Salzman CD (2008). Moment-to-moment tracking of state value in the amygdala. Journal of Neuroscience, 28, 10023–10030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belova MA, Paton JJ, Morrison SE, & Salzman CD (2007). Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala. Neuron, 55, 970–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonda E, Petrides M, Ostry D, & Evans A (1996). Specific involvement of human parietal systems and the amygdala in the perception of biological motion. Journal of Neuroscience, 16, 3737–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M, Grabenhorst F, Starcke K, Vandekerckhove MM, & Markowitsch HJ (2007). Role of the amygdala in decisions under ambiguity and decisions under risk: Evidence from patients with Urbach-Wiethe disease. Neuropsychologia, 45, 1305–1317. [DOI] [PubMed] [Google Scholar]
- Buchel C, Morris J, Dolan RJ, & Friston KJ (1998). Brain systems mediating aversive conditioning: An event-related fMRI study. Neuron, 20, 947–957. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, & Everitt BJ (2002). Emotion and motivation: The role of the amygdala, ventral striatum, and prefrontal cortex. Neuroscience & Biobehavioral Reviews, 26, 321–352. [DOI] [PubMed] [Google Scholar]
- Corbetta M, & Shulman GL (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3, 201–215. [DOI] [PubMed] [Google Scholar]
- Coricelli G, Critchley HD, Joffily M, O’Doherty JP, Sirigu A, & Dolan RJ (2005). Regret and its avoidance: A neuroimaging study of choice behavior. Nature Neuroscience, 8, 1255–1262. [DOI] [PubMed] [Google Scholar]
- Davis M, & Whalen PJ (2001). The amygdala: Vigilance and emotion. Molecular Psychology, 6, 13–34. [DOI] [PubMed] [Google Scholar]
- De Martino B, Kumaran D, Seymour B, & Dolan RJ (2006). Frames, biases, and rational decision-making in the human brain. Science, 313, 684–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, & Robbins TW (1992). In Aggleton JP (Ed.), Amygdala-ventral striatal interactions and reward-related processes. The amygdala: Neurological aspects of emotion, memory and mental dysfunction (pp. 401–429). New York: John Wiley & Sons. [Google Scholar]
- Everitt BJ, & Robbins TW (1997). Central cholinergic systems and cognition. Annual Review of Psychology, 48, 649–684. [DOI] [PubMed] [Google Scholar]
- Floresco SB, & Ghods-Sharifi S (2007). Amygdala-prefrontal cortical circuitry regulates effort-based decision making. Cereb Cortex, 17, 251–260. [DOI] [PubMed] [Google Scholar]
- Floresco SB, St Onge JR, Ghods-Sharifi S, & Winstanley CA (2008). Cortico-limbic-striatal circuits subserving different forms of cost-benefit decision making. Cognitive, Affective, & Behavioral Neuroscience, 8, 375–389. [DOI] [PubMed] [Google Scholar]
- Freese JL, & Amaral DG (2005). The organization of projections from the amygdala to visual cortical areas TE and V1 in the macaque monkey. Journal of Comparative Neurology, 486, 295–317. [DOI] [PubMed] [Google Scholar]
- Gamer M, Zurowski B, & Buchel C (2010). Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proceedings of the National Academy of Sciences of United States of America, 107, 9400–9405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germana J (1969). Central efferent processes and autonomic-behavioral integration. Psychophysiology, 6, 78–90. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, & Barbas H (2007). Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage, 34, 905–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghods-Sharifi S, St Onge JR, & Floresco SB (2009). Fundamental contribution by the basolateral amygdala to different forms of decision making. Journal of Neuroscience, 29, 5251–5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goard M, & Dan Y (2009). Basal forebrain activation enhances cortical coding of natural scenes. Nature Neuroscience, 12, 1444–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothard KM, Battaglia FP, Erickson CA, Spitler KM, & Amaral DG (2007). Neural responses to facial expression and face identity in the monkey amygdala. Journal of Neurophysiology, 97, 1671–1683. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, & Dolan RJ (2003). Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science, 301, 1104–1107. [DOI] [PubMed] [Google Scholar]
- Grossberg S, & Levine DS (1987). Neural dynamics of attentionally modulated Pavlovian conditioning: Blocking, interstimulus interval, and secondary reinforcement. Applied Optics, 26, 5015–5030. [DOI] [PubMed] [Google Scholar]
- Haber SN, & Knutson B (2010). The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology, 35, 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton AN, Adolphs R, Tyszka MJ, & O’Doherty JP (2007). Contributions of the amygdala to reward expectancy and choice signals in human prefrontal cortex. Neuron, 55, 545–555. [DOI] [PubMed] [Google Scholar]
- Hatfield T, Han JS, Conley M, Gallagher M, & Holland P (1996). Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer devaluation effects. Journal of Neuroscience, 16, 5256–5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L, Van Hoesen GW, Trimble M, & Zahm DS (2008). Anatomy of neuropsychiatry: The new anatomy of the basal forebrain and its implications for neuropsychiatric illness. Burlington, MA: Academic Press. [Google Scholar]
- Herry C, Bach DR, Esposito F, Di Salle F, Perrig WJ, Scheffler K, et al. (2007). Processing of temporal unpredictability in human and animal amygdala. Journal of Neuroscience, 27, 5958–5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, & Gallagher M (1993). Effects of amygdala central nucleus lesions on blocking and unblocking. Behavioral Neuroscience, 107, 235–245. [DOI] [PubMed] [Google Scholar]
- Holland PC, & Gallagher M (1999). Amygdala circuitry in attentional and representational processes. Trends in Cognitive Sciences, 3, 65–73. [DOI] [PubMed] [Google Scholar]
- Holland PC, Han JS, & Gallagher M (2000). Lesions of the amygdala central nucleus alter performance on a selective attention task. Journal of Neuroscience, 20, 6701–6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M, Bhatt M, Adolphs R, Tranel D, & Camerer CF (2005). Neural systems responding to degrees of uncertainty in human decision-making. Science, 310, 1680–1683. [DOI] [PubMed] [Google Scholar]
- Kaada BR (1951). Somato-motor, autonomic and electrocorticographic responses to electrical stimulation of rhinencephalic and other structures in primates, cat, and dog; A study of responses from the limbic, subcallosal, orbito-insular, piriform and temporal cortex, hippocampus-fornix and amygdala. Acta Physiologica Scandinavica Supplement, 24, 1–262. [PubMed] [Google Scholar]
- Kaada BR (1972). In Eleftheriou BE (Ed.), Stimulation and regional ablation of the amygdaloid complex with reference to functional representations. The neurobiology of the amygdala (pp. 205–281). New York: Plenum. [Google Scholar]
- Kaas JH (2004). Evolution of somatosensory and motor cortex in primates. Anatomical Record A Discoveries in Molecular, Cellular and Evolutionary Biology, 281, 1148–1156. [DOI] [PubMed] [Google Scholar]
- Kahneman D, & Tversky A (1979). Prospect theory: An analysis of decision under risk. Econometrica, 47, 263–291. [Google Scholar]
- Kapp BS,Supple WF Jr., & Whalen PJ (1994). Effects of electrical stimulation of the amygdaloid central nucleus on neocortical arousal in the rabbit. Behavioral Neuroscience, 108, 81–93. [DOI] [PubMed] [Google Scholar]
- Kapp BS, Silvestri AJ, & Guarraci FA (1996). Amygdaloid central nucleus neuronal activity: Correlations with EEG arousal. Society for Neuroscience, 2049. [Google Scholar]
- Kapp BS, Silvestri AJ, Guarraci FA, Moynihan JE, & Cain ME (1997). Associative and EEG arousal-related characteristics of amygdaloid central nucleus neurons in rabbit. Society for Neuroscience, 787. [Google Scholar]
- Kastner S, & Ungerleider LG (2000). Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience, 23, 315–341. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF,Joseph J, Bliss-Moreau E, Lindquist K, & Wager TD (2008). Functional grouping and cortical-subcortical interactions in emotion: A metaanalysis of neuroimaging studies. Neuroimage, 42, 998–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiman G, Koch C, & Fried I (2000). Imagery neurons in the human brain. Nature, 408, 357–361. [DOI] [PubMed] [Google Scholar]
- Krubitzer L (2009). In search of a unifying theory of complex brain evolution. Annals of the New York Academy of Sciences, 1156, 44–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, & Phelps EA (1998). Human amygdala activation during conditioned fear acquisition and extinction: A mixed-trial fMRI study. Neuron, 20, 937–945. [DOI] [PubMed] [Google Scholar]
- Lim SL, Padmala S, & Pessoa L (2009). Segregating the significant from the mundane on a moment-to-moment basis via direct and indirect amygdala contributions. Proceedings of the National Academy of Sciences of United States of America, 106, 16841–16846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SC, & Nicolelis MA (2008). Neuronal ensemble bursting in the basal forebrain encodes salience irrespective of valence. Neuron, 59, 138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova L, Gaffan D, & Murray EA (1997). Excitotoxic lesions of the amygdala fail to produce impairment in visual learning for auditory secondary reinforcement but interfere with reinforcer devaluation effects in rhesus monkeys. Journal of Neuroscience, 17, 6011–6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormann F, Kornblith S, Quiroga RQ, Kraskov A, Cerf M, Fried I, et al. (2008). Latency and selectivity of single neurons indicate hierarchical processing in the human medial temporal lobe. Journal of Neuroscience, 28, 8865–8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SE, & Salzman CD (2010). Re-valuing the amygdala. Current Opinion in Neurobiology, 20, 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moruzzi G, & Magoun HW (1949). Brain stem reticular formation and activation of the EEG. Electroencephalography and Clinical Neurophysiology, 1, 455–473. [PubMed] [Google Scholar]
- Most SB, Chun MM, Widders DM, & Zald DH (2005). Attentional rubbernecking: Cognitive control and personality in emotion-induced blindness. Psychonomic Bulletin & Review, 12, 654–661. [DOI] [PubMed] [Google Scholar]
- Most SB,Chun MM,Johnson MR, & Kiehl KA (2006). Attentional modulation of the amygdala varies with personality. Neuroimage, 31, 934–944. [DOI] [PubMed] [Google Scholar]
- Most SB, Smith SD, Cooter AB, Levy BN, & Zald DH (2007). The naked truth: Positive, arousing distractors impair rapid target perception. Cognition and Emotion, 21, 964–981. [Google Scholar]
- Murray EA (2007). The amygdala, reward and emotion. Trends in Cognitive Sciences, 11, 489–497. [DOI] [PubMed] [Google Scholar]
- Ohman A, & Mineka S (2001). Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychology Review, 108, 483–522. [DOI] [PubMed] [Google Scholar]
- Padmala S, & Pessoa L (2008). Affective learning enhances visual detection and responses in primary visual cortex. Journal of Neuroscience, 28, 6202–6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmala S, Lim S, & Pessoa L (2010). Pulvinar and affective significance: responses track moment-to-moment stimulus visibility. Frontiers in Human Neuroscience, 4, 64. doi: 10.3389/fnhum.2010.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, & Sarter M (2008). Cholinergic mediation of attention: Contributions of phasic and tonic increases in prefrontal cholinergic activity. Annals of the New York Academy of Sciences, 1129, 225–235. [DOI] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, & Sarter M (2007). Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron, 56, 141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce JM, & Hall G (1980). A model for Pavlovian learning: Variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychology Review, 87, 532–552. [PubMed] [Google Scholar]
- Pessoa L (2008). On the relationship between emotion and cognition. Nature Review Neuroscience, 9, 148–158. [DOI] [PubMed] [Google Scholar]
- Pessoa L (2010). Emergent processes in emotion and cognition. Dialogues in Clinical Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Adolphs R (submitted). Affective processing: From a“low road” to “many roads” of evaluating biological significance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, & Ungerleider LG (2002). Attentional control of the processing of neutral and emotional stimuli. Cognitive Brain Research, 15, 31–45. [DOI] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, & Ungerleider LG (2002). Neural processing of emotional faces requires attention. Proceedings of the National Academy of Sciences of United States of America, 99, 11458–11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, & Swanson LW (2001). Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Research Brain Research Reviews, 38, 247–289. [DOI] [PubMed] [Google Scholar]
- Pickens CL, Saddoris MP, Setlow B, Gallagher M, Holland PC, & Schoenbaum G (2003). Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. Journal of Neuroscience, 23, 11078–11084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piech RM, McHugo M, Smith SD, Dukic S, Van Der Meer J, Abou-Khali B, et al. (2010). Fear-enhanced visual search persists after amygdala lesions. Neuropsychologia, 48, 3430–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribram KH, & McGuinness D (1975). Arousal, activation, and effort in the control of attention. Psychology Review, 82, 116–149. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, & Wagner AR (1972). In Black AH, & Prokasy WF (Eds.), A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and non-reinforcement Classical conditioning II: Current research and theory (pp. 64–99). New York: Appleton Century Crofts. [Google Scholar]
- Robbins TW, & Everitt BJ (1996). Neurobehavioural mechanisms of reward and motivation. Current Opinions in Neurobiology, 6, 228–236. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Calu DJ, Esber GR, & Schoenbaum G (2010a). All that glitters dissociating attention and outcome-expectancy from prediction errors signals. Journal of Neurophysiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Calu DJ, Esber GR, & Schoenbaum G (2010b). Neural correlates of variations in event processing during learning in basolateral amygdala. Journal of Neuroscience, 30, 2464–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET (2005). Emotion explained. Oxford: Oxford University Press. [Google Scholar]
- Salzman CD, & Fusi S (2010). Emotion, cognition, and mental state representation in amygdala and prefrontal cortex. Annual Reviews of Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander D, Grafman J, & Zalla T (2003). The human amygdala: An evolved system for relevance detection. Review Neuroscience, 14, 303–316. [DOI] [PubMed] [Google Scholar]
- Sarter M, & Bruno JP (1999). Abnormal regulation of corticopetal cholinergic neurons and impaired information processing in neuropsychiatric disorders. Trends Neuroscience, 22, 67–74. [DOI] [PubMed] [Google Scholar]
- Sarter M, & Bruno JP (2000). Cortical cholinergic inputs mediating arousal, attentional processing and dreaming: Differential afferent regulation of the basal forebrain by telencephalic and brainstem afferents. Neuroscience, 95, 933–952. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP, & Turchi J (1999). Basal forebrain afferent projections modulating cortical acetylcholine, attention, and implications for neuropsychiatric disorders. Annals of the New York Academy of Sciences, 877, 368–382. [DOI] [PubMed] [Google Scholar]
- Seymour B, & Dolan R (2008). Emotion, decision making, and the amygdala. Neuron, 58, 662–671. [DOI] [PubMed] [Google Scholar]
- Solano-Castiella E, Anwander A, Lohmann G, Weiss M, Docherty C, Geyer S, et al. (2010). Diffusion tensor imaging segments the human amygdala in vivo. Neuroimage, 49, 2958–2965. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Roesch MR, Franz TM, Calu DJ, Singh T, & Schoenbaum G (2007). Cocaine-induced decision-making deficits are mediated by miscoding in basolateral amygdala. Nature Neuroscience, 10, 949–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock G, Rupprecht U, Stumpf H, & Schlor KH (1981). Cardiovascular changes during arousal elicited by stimulation of amygdala, hypothalamus and locus coeruleus. Journal of the Autonomic Nervous System, 3, 503–510. [DOI] [PubMed] [Google Scholar]
- Sugase-Miyamoto Y, & Richmond BJ (2005). Neuronal signals in the monkey basolateral amygdala during reward schedules. Journal of Neuroscience, 25, 11071–11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton R, & Barto A (1988). Reinforcement learning. Cambridge, MA: MIT Press. [Google Scholar]
- Swanson LW, & Petrovich GD (1998). What is the amygdala? Trends Neuroscience, 21, 323–331. [DOI] [PubMed] [Google Scholar]
- Tranel D, & Hyman BT (1990). Neuropsychological correlates of bilateral amygdala damage. Archives of Neurology, 47, 349–355. [DOI] [PubMed] [Google Scholar]
- Tsuchiya N, Moradi F, Felsen C, Yamazaki M, & Adolphs R (2009). Intact rapid detection of fearful faces in the absence of the amygdala. Nature Neuroscience, 12, 1224–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursin H, & Kaada BR (1960). Functional localization within the amygdaloid complex in the cat. Electroencephalography and Clinical Neurophysiology, 12, 1–20. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P (2005). How brains beware: Neural mechanisms of emotional attention. Trends in Cognitive Sciences, 9, 585–594. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P,Armony JL, Driver J, & Dolan RJ (2001). Effects of attention and emotion on face processing in the human brain: An event-related fMRI study. Neuron, 30, 829–841. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP,Armony JL, Driver J, & Dolan RJ (2004). Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature Neuroscience, 7, 1271–1278. [DOI] [PubMed] [Google Scholar]
- Ward R, Danziger S, & Bamford S (2005). Response to visual threat following damage to the pulvinar. Current Biology, 15, 571–573. [DOI] [PubMed] [Google Scholar]
- Ward R, Calder AJ, Parker M, & Arend I (2007). Emotion recognition following human pulvinar damage. Neuropsychologia, 45, 1973–1978. [DOI] [PubMed] [Google Scholar]
- Weller JA, Levin IP, Shiv B, & Bechara A (2007). Neural correlates of adaptive decision making for risky gains and losses. Psychology Science, 18, 958–964. [DOI] [PubMed] [Google Scholar]
- Whalen PJ (1998). Fear, vigilance, and ambiguity: Initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science, 7, 177–188. [Google Scholar]
- Whalen PJ, & Phelps EA (Eds.). (2009). The human amygdala. New York, NY: Guilford Press. [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H,Wright CI, & Rauch SL(2001). A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion, 1, 70–83. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Cardinal RN, & Robbins TW (2004). Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. Journal of Neuroscience, 24, 4718–4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MP, Scannell JW, Burns GAPC, & Blakemore C (1994). Analysis of connectivity: Neural systems in the cerebral cortex. Reviews in the Neurosciences, 5, 227–249. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Hoemke L, Mohlberg H, Schleicher A, Amunts K, & Zilles K (2008). Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. Neuroimage, 42, 1127–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]