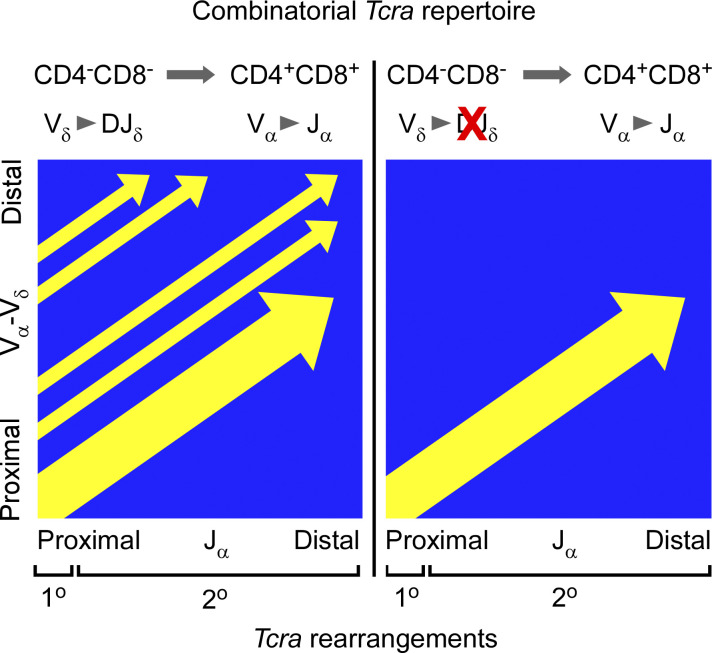
Dauphars et al. show that prior Tcrd rearrangement is essential for a combinatorially diverse Tcra repertoire in mouse thymocytes. Trav15-dv6 family Tcrd rearrangements are critical for Tcra repertoire formation because of their central and distal locations in the Vα-Vδ array.
Abstract
The Tcra repertoire is generated by multiple rounds of Vα-Jα rearrangement. However, Tcrd recombination precedes Tcra recombination within the complex Tcra-Tcrd locus. Here, by ablating Tcrd recombination, we report that Tcrd rearrangement broadens primary Vα use to diversify the Tcra repertoire in mice. We reveal that use of Trav15-dv6 family V gene segments in Tcrd recombination imparts diversity in the Tcra repertoire by instigating use of central and distal Vα segments. Moreover, disruption of the regions containing these genes and their cis-regulatory elements identifies the Trav15-dv6 family as being responsible for driving central and distal Vα recombinations beyond their roles as substrates for Tcrd recombination. Our study demonstrates an indispensable role for Tcrd recombination in general, and the Trav15-dv6 family in particular, in the generation of a combinatorially diverse Tcra repertoire.
Graphical Abstract
Introduction
TCR and BCR repertoires rely on combinatorial diversity imparted by V(D)J recombination of variable (V), diversity (D), and joining (J) gene segments at their respective antigen receptor (AgR) loci (Schatz and Ji, 2011). The lymphocyte-specific recombination-activating gene (RAG) proteins mediate double-stranded DNA breaks at recognition sites, called “recombination signal sequences” (RSSs), flanking the V, D, and J segments of the AgR loci. These breaks are then modified, and the segments are ligated via nonhomologous end joining and spliced to a constant gene segment (C) to make AgR chain transcripts (Schatz and Ji, 2011; Helmink and Sleckman, 2012). RAG binding at AgR loci is primarily restricted to recombination centers (RCs), chromatin regions characterized by highly accessible clusters of J (and sometimes D) segments (Ji et al., 2010; Schatz and Ji, 2011). In order for V-to-(D)J recombination to occur, V segments must be brought into proximity of the RC (Jhunjhunwala et al., 2009; Lin et al., 2018). This process is tightly regulated, and ordered recombinations are integral to successful generation of a diverse TCR repertoire. Tcrd, Tcrg, and Tcrb rearrangements occur at the CD4−CD8− (double-negative [DN]) stage of thymocyte development (Capone et al., 1998). Subsequently, Tcra undergoes Vα-to-Jα recombination at the CD4+CD8+ (double-positive [DP]) stage (Petrie et al., 1995).
Because Tcra recombinations involve only Vα and Jα segments, Tcra is capable of undergoing multiple rounds of rearrangement to generate a functional, in-frame TCRα chain (Petrie et al., 1993; Wang et al., 1998). The first V-to-Jα rearrangement is referred to as the “primary rearrangement,” and all subsequent Tcra recombination events are referred to as “secondary rearrangements” (Carico and Krangel, 2015). Primary Tcra rearrangements typically involve the most Vα-proximal Jα segments (Thompson et al., 1990), which constitute the initial Tcra RC. High-throughput sequencing (HTS) of the Tcra repertoire demonstrates that early Vα rearrangements are broadly distributed, including the proximal half of the Vα array as well as substantial central and distal Vα contributions. Secondary rearrangements proceed to more distal Vα and Jα segments over time, with the Tcra RC continually retargeted in stepwise fashion to more distal Jα segments following each round of Vα-Jα rearrangement (Hawwari and Krangel, 2007; Ji et al., 2010; Schatz and Ji, 2011; Carico et al., 2017). Notably, in a mouse in which all thymocytes have a knock-in of the rearrangement Trav17-Traj57 (Buch et al., 2002), the progression of secondary rearrangements was found to be tightly constrained, with each round of secondary recombinations limited to the most proximal of the remaining Vα and Jα segments (Carico et al., 2017). Because the combinatorial space occupied by secondary rearrangements downstream of a single primary rearrangement is narrow, repertoire diversity must depend on broad use of Vα segments in primary rearrangements.
The Tcra-Tcrd locus displays a unique structure among AgR loci, wherein Dδ and Jδ gene segments are nested between the Vα and Jα gene segments, and Vδ segments are interspersed among the Vα segments in both mouse and man (Fig. 1 A; Glusman et al., 2001; Carico and Krangel, 2015). Intriguingly, many of the central and distal Vα segments used in primary and early secondary rearrangements were found to be immediately upstream of segments that can be used as Tcrd recombination substrates (Carico et al., 2017). Most cells that express αβTCRs first undergo Tcrd rearrangement on at least one allele (Livak et al., 1995; Nakajima et al., 1995; Sleckman et al., 1998; Shih et al., 2012). These truncating recombinations delete Vα segments located between the recombined Vδ and Dδ segments before Tcra rearrangement begins. Therefore, Tcrd rearrangement could bias Tcra recombination toward the use of more distal Vα segments for primary rearrangements than would be observed on alleles that do not recombine Tcrd.
Figure 1.
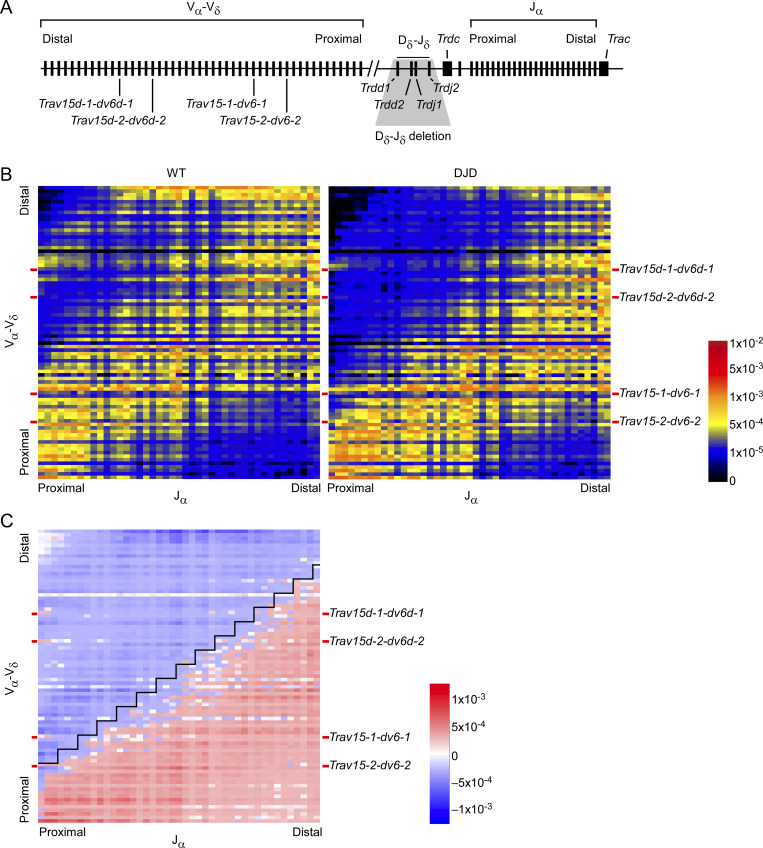
Tcrd recombination diversifies Tcra repertoire. (A) Schematic of the Tcra-Tcrd locus with gene segments depicted. Shaded region corresponds to deletion on DJD alleles. (B) Average frequencies of V-Jα rearrangements in CD4+CD8+CD3εlo thymocytes from WT (left) and DJD (right) mice. Two DJD mice and two WT littermates (mixed 129 and C57BL/6 background) were analyzed in two independent experiments by HTS of 5′ RACE-amplified Tcra transcripts. Red bars on left and right edges of heatmaps indicate locations of Trav15-dv6 family V segments. (C) Map depicting difference between average V-Jα rearrangements in DJD and WT mice. Blue represents rearrangements underrepresented in DJD; red represents rearrangements more common in DJD. Black line indicates step-function diagonal with a slope of 4Vα:3Jα (or 1.33 Vα per Jα, the previously established relationship for the progression of secondary rearrangements across the V and J arrays; Carico et al., 2017). With the origin of the diagonal just distal to Trav15-2-dv6-2, the line separates all primary and secondary Tcra rearrangements hypothesized to arise as a result of Trav15-dv6 and other central and distal Tcrd rearrangements from those expected to occur on alleles that lack such rearrangements. Normalized Shannon’s entropy values were 0.953 and 0.950 for replicate WT samples (541,510 and 396,722 unique sequences per sample) and 0.936 and 0.935 for replicate DJD samples (388,593 and 377,173 unique sequences per sample).
Most deletional V-to-DJδ recombination events in mice involve Dδ-proximal Vδ segments, thereby leaving most of the Vα array intact (Chen et al., 2015; Zhao et al., 2016). One Vδ family, Trav15-dv6, accounts for most of the distal Tcrd rearrangements that do occur. As Trav15-dv6 rearrangements would truncate the Vα array and change the starting point for primary Tcra rearrangements, we hypothesized that Trav15-dv6 rearrangement may be fundamental to combinatorial diversity of the Tcra repertoire.
In a mouse model of altered chromatin looping at the Tcra-Tcrd locus, V-to-DJδ recombination was found to be skewed toward rearrangement of the proximal Trdv2-2 and Trdv3 gene segments, with concomitant reductions in rearrangements to distal Vδ segments (Chen et al., 2015; Zhao et al., 2016). Tcra rearrangements were also found to be less diverse, consistent with a role for Tcrd recombination in Tcra repertoire formation. However, because the primary effect of mutation in this model is on chromatin looping, it remains unclear whether the observed Tcra repertoire phenotype may be a direct consequence of this structural change as opposed to an indirect consequence of altered Tcrd rearrangements.
Here, we employed an HTS approach (Carico et al., 2017) to determine the impact of Tcrd recombination on the Tcra repertoire in DP thymocytes using several novel mouse models. In primary mouse thymocytes incapable of Tcrd rearrangement, we observed a dramatic contraction in Tcra repertoire diversity, directly implicating Tcrd recombination in Tcra repertoire formation. Moreover, deletion of Trav15d-1-dv6d-1 or Trav15-1-dv6-1 revealed the rearrangement of these Vδ segments to be particularly important in targeting primary Tcra rearrangements to the distal and central portions of the Vα array, respectively. Finally, we provide evidence that Trav15-dv6 or flanking elements may also have a direct effect on the Tcra repertoire by facilitating secondary Vα-to-Jα rearrangement events.
Results and discussion
Tcrd recombination diversifies Tcra repertoire
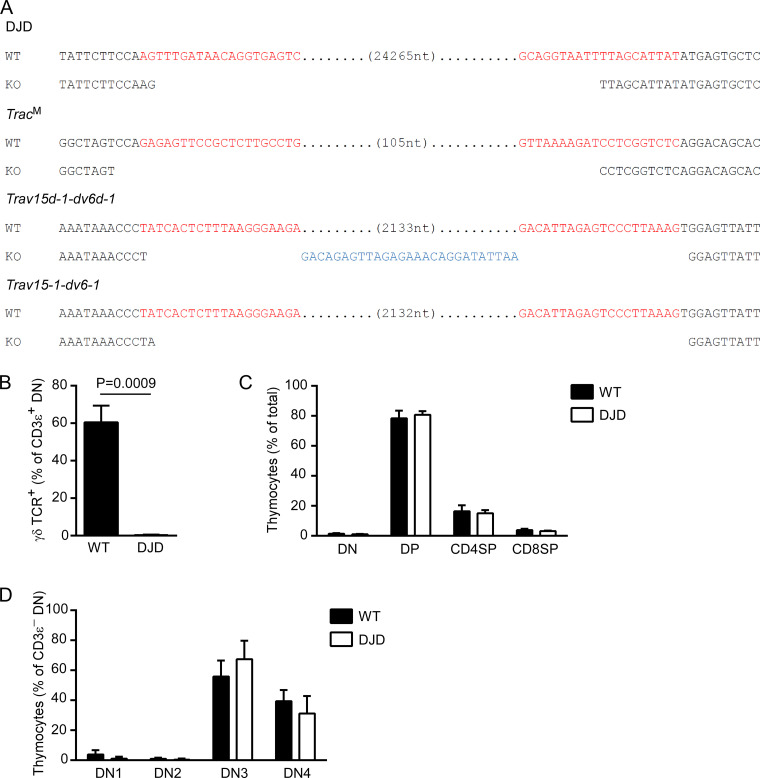
To directly assess the impact of Tcrd recombination on the Tcra repertoire, we sought a mouse strain incapable of Tcrd recombination. In this regard, the Cδ-KO mouse undergoes normal Tcrd rearrangement (Itohara et al., 1993), and the Eδ-KO mouse has only a partial defect (Monroe et al., 1999). Therefore, we generated a novel line of mice lacking a 24-kb region containing the Dδ and Jδ segments (Figs. 1 A and S1 A). The resulting mouse, Dδ and Jδ deficient (DJD), ablates V-to-DJδ rearrangement and γδT cells, with otherwise normal thymocyte development (Fig. S1, B–D).
Figure S1.
Characterization of mutant mouse strains. (A) DNA sequence of mutant alleles. Red, guide sequence for CRISPR/Cas9 targeting. Blue, nucleotides inserted in KO alleles. Gaps, nucleotides deleted in KO alleles. (B–D) Flow cytometric analysis of total thymocytes from WT and DJD littermate mice. Data are presented as mean and SD of four WT and five DJD littermates (mixed 129 and C57BL/6 background) analyzed in two independent experiments. Statistical significance was evaluated with unpaired t test with Welch’s correction. Cells were pregated as 7-AAD−CD11b−CD11c−Ter119−B220−Gr-1−F4/80−.
Preselection DP thymocytes from DJD mice and their WT littermates were subjected to 5′ rapid amplification of cDNA ends (RACE) and Tcra sequencing. Unique clones were analyzed to determine the frequencies of V-to-Jα rearrangements. As previously reported (Carico et al., 2017), in WT thymocytes, we found the Tcra repertoire to be diverse (Fig. 1 B, left panel; and Fig. S2 A). Proximal Jα segments frequently rearranged with proximal, central, and distal Vα segments. Secondary rearrangements occurred mostly along two distinct diagonals (Fig. S2 B). The major diagonal started with proximal and central V-to-proximal Jα primary rearrangements, followed by secondary rearrangements through the central and distal Vα and Jα segments. The minor diagonal arose from distal V-to-proximal Jα primary rearrangements. In DJD thymocytes, absent Tcrd recombination, we observed restricted Vα use; proximal Jα segments recombined almost exclusively with the most proximal Vα segments (Fig. 1 B, right panel). This reduction in diversity of Vα use in early rearrangements with proximal Jα segments depressed the diversity of secondary rearrangements, with a notable loss in the use of distal Vα segments, even in recombinations with central and distal Jα segments. The major diagonal was constrained and shifted toward more proximal Vα segments along its entire length; the minor diagonal was largely depleted of unique clones.
Figure S2.
Tcra repertoire in strain 129 preselection DP thymocytes. (A) Average frequencies of V-Jα rearrangements in two WT strain 129 CD4+CD8+CD3εlo thymocytes (mixed 129 and C57BL/6 background; identical to Fig 1 B, left). Gene segment names are indicated on the right and lower margins. Red and blue lettering identifies Trav15-dv6 family Vδ segments and other Vδ segments, respectively. (B) Diagram indicating locations of major and minor diagonals in yellow, corresponding to the heatmap in A.
Previous work described the progression of secondary rearrangements emanating from a single primary recombination as proceeding ∼1.33 Vα segments per Jα (Carico et al., 2017). According to our hypothesis, DJD thymocytes should be depleted of V-Jα combinations resulting from primary rearrangements distal to the most proximal Trav15-dv6 family member, Trav15-2-dv6-2, as well as any secondary rearrangements that occur as a consequence of those primary rearrangements. To assess this, we overlaid a step-function diagonal (4Vα:3Jα, corresponding to 1.33 Vα per Jα) over the DJD−WT difference map, with its origin just distal to Trav15-2-dv6-2 (Fig. 1 C). The step-function diagonal predicts almost exactly the region of depletion of the combinatorial Tcra repertoire in DJD thymocytes. In WT thymocytes, the region above the diagonal accounts for 43% of the repertoire; in DJD thymocytes, it accounts for 22% (Fig. 1, B and C; P < 0.0001 by χ2 test with Yates’s correction). However, this is a substantial underestimate of the impact on some distal Vα rearrangements. Prior work demonstrated that the SD of the Vα distribution used with any Jα gradually increases from proximal to distal across the Jα array (Carico et al., 2017). Because this increase is not accounted for by the step-function diagonal, we would expect representation of the most distal Vα-distal Jα combinations to persist, although at reduced frequencies, in the region above the diagonal in DJD thymocytes (compare Fig. 1 B, right panel, with Fig. 1 C). This effect should not impact quantification along the minor diagonal; accordingly, rearrangements in this region account for 7.7% of the repertoire in WT but only 2% in DJD thymocytes, a reduction of 74%. We conclude that Tcrd rearrangement expands the combinatorial diversity of the Tcra repertoire by diversifying Vα use.
Primary Tcra rearrangements are poorly represented in the steady-state Tcra repertoire. To more robustly investigate the impact of Tcrd rearrangement on primary Tcra rearrangement, we directly visualized Tcra rearrangements in the earliest DP thymocytes. We previously analyzed these cells in mice carrying a tamoxifen-inducible TcrdCreER allele together with a Rosa26fl-STOP-fl-ZsGreen (hereafter Rosa26ZsG) reporter allele (Carico et al., 2017). In these mice, tamoxifen injection causes ZsGreen expression in cells with transcriptionally active Trdc, including DN thymocytes, most of which, failing to diverge to the γδT cell fate, progress to rearrange Tcra at the DP stage (Madisen et al., 2010; Zhang et al., 2015). To analyze rearrangement on the DJD allele, we mutated Trac on the TcrdCreER allele to prevent annealing of Trac 5′ RACE primers (Fig. S1 A). In mice containing Rosa26ZsG and a Trac-mutated TcrdCreER (TcrdCreER TracM) allele, in combination with either a WT or DJD allele, we analyzed Tcra recombination in ZsGreen+ DP thymocytes at 12 h after tamoxifen injection. These primary and early secondary recombinations, in cells that compose <20% of the DP population (Carico et al., 2017), are typically overshadowed by secondary recombinations in the steady-state repertoire. As previously reported (Carico et al., 2017), on WT alleles, we found these recombinations to be restricted to the first half of the Jα array (Fig. 2 A, left panel), with the majority of rearrangements focused on the most proximal Jα segments, Traj58-Traj48. Vα use involved the first half of the Vα array, along with a cluster of relatively distal Vα segments. Save for an unexpected reduction in use of Traj58, DJD alleles displayed minimal change in overall Jα use (Fig. 2 A, right panel; and Fig. 2 B). However, Vα use on DJD alleles was restricted compared with WT thymocytes, with overall use of segments distal to Trav15-2-dv6-2, the most proximal Trav15-dv6 segment, reduced from 59.4% to 32.2% on DJD alleles (P < 0.0001 by χ2 test with Yates’s correction). We conclude that Tcrd recombination diversifies primary Vα use, leading to a more robust repertoire of secondary rearrangements and ultimately imparting combinatorial diversity upon the Tcra repertoire.
Figure 2.
Early Tcra rearrangements are diversified by Tcrd recombination. (A) Average frequencies of V-Jα rearrangements on WT or DJD alleles in CD4+CD8+CD3εloZsGreen+ thymocytes of mice containing Rosa26ZsG and a TcrdCreER TracM allele paired with either a WT or a DJD Tcra-Tcrd allele at 12 h after tamoxifen injection. Two DJD and two WT littermates (mixed 129 and C57BL/6 background) were analyzed in two independent experiments. (B) Map depicting difference between average V-Jα rearrangements in DJD and WT mice. Normalized Shannon’s entropy values were 0.863 and 0.864 for replicate WT samples (81,039 and 116,598 unique sequences per sample) and 0.835 and 0.838 for replicate DJD samples (83,778 and 106,918 unique sequences per sample).
Trav15d-1-dv6d-1 and Trav15-1-dv6-1 rearrangements facilitate distal and central Vα use, respectively, in primary Tcra recombinations
Trav15-dv6 segments comprise the most frequent of the distal and central Vδ contributions to the Tcrd repertoire; the only other similarly located Vδ gene segments, Trav14d-3-dv8 and Trav16d-dv11 (Fig. S2), are rarely used (Chen et al., 2015; Zhao et al., 2016). We observed that in primary recombinations of the WT Tcra locus, most central and distal Vα use occurs immediately upstream of Trav15-dv6 family members, particularly Trav15-1-dv6-1 and Trav15d-1-dv6d-1 (left panels of Figs. 1 B and 2 A). These early central and distal recombinations are dramatically reduced in DJD mice (Fig. 1 B, right; Fig. 1 C; Fig. 2 A, right; and Fig. 2 B), suggesting that Trav15-dv6 recombinations in DN cells may contribute to diversifying the Tcra repertoire. To analyze the impact of Trav15-dv6 rearrangements on Tcra recombination, we analyzed two lines of mice, one with a deletion of ∼2 kb spanning Trav15d-1-dv6d-1 and a second with a similar deletion of Trav15-1-dv6-1 (Fig. S1 A) for their Tcra clonal repertoires. We hypothesized that the loss of either would result in a substantial reduction in recombination events emanating in a diagonal pattern from the most proximal upstream Vα and moving distally through the remaining Vα segments.
Primary and secondary Tcra recombinations in the minor diagonal, predicted to occur as a consequence of Tcrd rearrangements involving Trav15d-1-dv6d-1, represented 3.1% of the Tcra repertoire in WT DP thymocytes but only 0.8% of the repertoire in cells lacking Trav15d-1-dv6d-1 (Fig. 3, A and B, region 1; P < 0.0001 by χ2 test with Yates’s correction). Similarly, major diagonal primary and secondary Tcra recombinations, predicted to occur as a consequence of Tcrd rearrangements involving Trav15-1-dv6-1, accounted for 21.2% of the repertoire in WT thymocytes but only 16.6% of the repertoire in cells lacking Trav15-1-dv6-1 (Fig. 4, A and B, region 2; P < 0.0001 by χ2 test with Yates’s correction). In contrast, the representation of minor diagonal Tcra recombinations predicted to occur as a consequence of rearrangements involving more distal Trav15-dv6 family members increased slightly in Trav15-1-dv6-1–deleted DP cells (Fig. 4, A and B, region 1). In both lines of mice, rearrangements involving Vα segments downstream of the deleted Trav15-dv6 family member increased proportionally (Fig. 3 B, region 3; and Fig. 4 B, region 4). Because reduced minor diagonal Tcra rearrangements were observed in both DJD mice and Trav15d-1-dv6d-1–deleted mice and reduced major diagonal Tcra rearrangements were observed in both DJD mice and Trav15-1-dv6-1–deleted mice, we conclude that central and distal Tcrd rearrangements involving Trav15-dv6 family members diversify the Tcra repertoire.
Figure 3.
Reduced distal Vα use in Trav15d-1-dv6d-1 KO mice. (A) Average frequencies of V-Jα rearrangements in CD4+CD8+CD3εlo thymocytes of mice carrying WT (left) or Trav15d-1-dv6d-1 KO (right) Tcra-Tcrd alleles. Although irrelevant for this analysis, WT and KO Tcra-Tcrd alleles also carry TcrdCreER (see Materials and methods). Two littermates per genotype (C57BL/6 background) were analyzed in two independent experiments. (B) Map depicting difference between average V-Jα rearrangements on Trav15d-1-dv6d-1 KO and WT alleles. Region 1, primary and secondary Tcra rearrangements predicted to depend on use of Trav15d-1-dv6d-1 in Tcrd recombination. Region 2, secondary rearrangements upstream of Trav15d-1-dv6d-1 expected to arise from primary and secondary recombinations downstream of Trav15d-1-dv6d-1. Region 3, primary and secondary rearrangements downstream of Trav15d-1-dv6d-1. Normalized Shannon’s entropy values were 0.944 and 0.947 for replicate WT samples (171,982 and 318,404 unique sequences per sample) and 0.938 and 0.942 for replicate Trav15d-1-dv6d-1 samples (219,968 and 304,900 unique sequences per sample).
Figure 4.
Reduced central Vα use in Trav15-1-dv6-1 KO mice. (A) Average frequencies of V-Jα rearrangements in CD4+CD8+CD3εlo thymocytes of mice carrying WT (left) or Trav15-1-dv6-1 KO (right) Tcra-Tcrd alleles. Although irrelevant for this analysis, WT and KO Tcra-Tcrd alleles also carry TcrdCreER (see Materials and methods). Two littermates per genotype (C57BL/6 background) were analyzed in two independent experiments. (B) Map depicting difference between average V-Jα rearrangements on Trav15-1-dv6-1 KO and WT alleles. Region 1, primary and secondary Tcra rearrangements predicted to depend on Trav15d-1-dv6d-1 and Trav15d-2-dv6d-2 Tcrd rearrangements. Region 2, primary and secondary Tcra rearrangements predicted to depend on Trav15-1-dv6-1 Tcrd rearrangements. Region 3, secondary Tcra rearrangements upstream of Trav15-1-dv6-1 expected to arise from primary and secondary Tcra recombinations downstream of Trav15-1-dv6-1. Region 4, primary and secondary Tcra rearrangements downstream of Trav15-1-dv6-1. Normalized Shannon’s entropy values were 0.950 and 0.947 for replicate WT samples (262,146 and 324,511 unique sequences per sample) and 0.947 and 0.939 for replicate Trav15-1-dv6-1 samples (223,783 and 264,507 unique sequences per sample).
In thymocytes lacking either Trav15d-1-dv6d-1 or Trav15-1-dv6-1, we observed changes in the Tcra repertoire that were not predicted to arise due to loss of Tcrd recombination to either segment. In the absence of Trav15d-1-dv6d-1, rearrangements between distal Vα and distal Jα segments outside the minor diagonal were reduced to 13.1% of the repertoire from 19.1% in WT; these secondary rearrangements lie on the major diagonal and are predicted to arise from primary rearrangements involving central Vα segments (Fig. 3, A and B, region 2; P < 0.0001 by χ2 test with Yates’s correction). Similarly, in Trav15-1-dv6-1–deleted mice, major diagonal secondary rearrangements involving central and distal Vα segments were unexpectedly reduced to 37.4% of the repertoire from 43.1% in WT (Fig. 4, A and B, region 3; P < 0.0001 by χ2 test with Yates’s correction); these secondary rearrangements are predicted to arise from primary rearrangements involving proximal Vα segments. Moreover, particularly in Trav15-1-dv6-1–deleted mice, reduced secondary rearrangements were associated with increased use of Vα segments immediately Jα-proximal to the deleted region (Fig. 4). None of these changes were observed in the DJD repertoire (Fig. 2). This implies that Trav15-dv6 family members impact Tcra repertoire diversification by an additional mechanism that is separate from their roles as Tcrd recombination substrates. We propose that the Trav15-1-dv6-1 and Trav15d-1-dv6d-1 deletions have these additional effects on Tcra repertoire diversity because they impair the propagation of secondary Tcra rearrangements to Vα segments beyond the deleted region.
The Trav15d-1-dv6d-1 and Trav15-1-dv6-1 deletions encompass regions spanning ∼500 bp upstream of the transcription start site to ∼1 kb downstream of the gene body. These deleted regions are highly conserved between Trav15-1-dv6 family members and include the promoter, the RSS, and downstream E-box sites that are highly accessible and, by chromatin immunoprecipitation followed by sequencing (ChIP-seq), are occupied by E2A at levels surpassed within the Tcra-Tcrd locus only by Tcra enhancer E-box sites (Fig. S3; Heng et al., 2008; Roy et al., 2018; Yoshida et al., 2019). Loss of the Trav15-dv6 RSS could inhibit more distal secondary rearrangements if the propagation of sequential rearrangements requires closely spaced RSSs. However, Trav15-dv6 family members were not found to be frequently recombined in the WT Tcra repertoire, suggesting that Trav15-dv6 RSSs are often skipped during secondary rearrangements (Fig. 1 B). Moreover, the distance between the nearest functional Vα segments flanking Trav15-dv6 is reduced from ∼37 kb to ∼35 kb on the deleted alleles. We considered that Trav15-dv6 deletion might influence secondary rearrangements by disrupting the CCCTC binding factor (CTCF)–mediated chromatin loop organization. However, binding sites for CTCF were not disrupted on the Trav15d-1-dv6d-1– or Trav15-1-dv6-1–deleted alleles (Shih et al., 2012), and the deletions do not obviously create de novo CTCF binding sites (Martin et al., 2011).
Figure S3.
E2A binding at the C57BL/6 Tcra-Tcrd locus. Reanalysis of published E2A ChIP-seq data obtained from Id2- and Id3-deficient DP thymocytes (Roy et al., 2018). (A) E2A binding across the Tcra-Tcrd locus. Vα and Vδ (red bars), Dδ (green bars), Jα and Jδ (blue bars), and Trdc and Trac (orange bars) segments are indicated. Black bars represent pseudogenes. Compared with strain 129, the C57BL/6 Tcra-Tcrd locus contains an extra copy of several Vα segments, including Trav15n-1. (B) E2A binding across 155 kb surrounding Trav15-1-dv6-1 (blue shaded region of A). (C) E2A binding across 155 kb encompassing Trac with major E2A peak at Eα (purple shaded region of A). (D) E2A binding at Trav15-1-dv6-1, with shaded region indicating the region of deletion on the TcrdCreER allele (strain 129). (E) E2A binding at Trav15d-1-dv6d-1, with shaded region as in D.
It remains possible that Trav15-dv6 segments influence secondary Tcra recombinations via effects on chromatin accessibility mediated by flanking cis-acting elements. The Trav15-1-dv6-1 promoter drives accessibility, transcription, and recombination of Trav15-1-dv6-1 in DN thymocytes (Naik et al., 2015) but displays only modest accessibility and is not expected to influence neighboring V segments (Heng et al., 2008; Yoshida et al., 2019). Perhaps a better candidate is the highly accessible downstream E2A-bound element (Fig. S3). E2A is a known regulator of Tcrd recombination and γδT cell fate (Bain et al., 1999) and may influence Trav15-dv6 rearrangement in DN thymocytes. As the E2A-bound region remains highly accessible in DP thymocytes, it could have extended effects on accessibility and RAG binding at RCs that form downstream of Trav15-dv6 family members during secondary rearrangement or on Vα substrates upstream of Trav15-dv6 family members. The cis region may alternatively serve as a mediator of locus structure to facilitate recombination; such features have been observed at other AgR loci (Barajas-Mora et al., 2019). Further exploration of this phenomenon is warranted to better understand these perturbations of secondary Tcra rearrangements on Trav15d-1-dv6d-1– and Trav15-1-dv6-1–deleted alleles.
Our repertoire studies emphasize that capture of V gene segments within the Tcra-Tcrd locus occurs via distinct mechanisms during Tcrd and Tcra rearrangement. Capture of Vδ gene segments by the Tcrd RC in DN thymocytes can occur over very long distances. By contrast, capture of Vα gene segments by Tcra RCs appears to occur predominantly as a result of short-range interactions once those segments are brought into proximity of the RC by prior rounds of rearrangement. One exception is the residual rearrangement of central and distal Vα segments in early rearrangements on DJD alleles (Fig. 2 A, right panel); the cluster of rearrangements between V segments upstream of Trav15d-2-dv6d-2 and J segments Traj58-Traj48 is not ablated, but rather reduced from 11.4% to 3.5% (P < 0.0001 by χ2 test with Yates’s correction). We think that these rearrangements, which include Trav15d-1-dv6d-1, may result from residual chromatin marks carried over from DN thymocytes in the absence of Tcrd recombination, facilitating DN-like V capture in early DP thymocytes.
The change in the mode of V segment capture between the DN and DP stages of thymocyte development correlates with a conformational change in the Tcra-Tcrd locus, with the V array being contracted in DN and extended in DP (Shih and Krangel, 2010). Prior work has shown that long-range capture of VH segments by the Igh RC in pro-B cells occurs via long-range RAG scanning and cohesin-mediated loop extrusion, facilitated by reduced expression of the cohesin unloader Wapl (Hu et al., 2015; Lin et al., 2018; Ba et al., 2020; Hill et al., 2020; Dai et al., 2021). However, RAG scanning from the Tcrd RC appears to be effectively contained within an 80-kb chromatin loop domain formed by the INT1-2 and T early α CTCF binding elements in DN thymocytes (Chen et al., 2015; Zhao et al., 2016). This suggests that long-range capture of Vδ gene segments may occur via diffusion. In contrast, short-range Vα capture by the Tcra RC in DP thymocytes can be envisioned to occur either by relatively short-range RAG scanning or by diffusive interactions. Regardless, our data suggest that Tcra repertoire diversity in mice results from synergy between two different modes of V segment capture. We expect that our conclusions on Tcra repertoire diversity in mice likely extend to humans, since the human Tcra-Tcrd locus is similarly organized with Vδ gene segments distributed among central and distal Vα gene segments.
Materials and methods
Mice
To generate DJD mice, female C57BL/6J mice were mated to male strain 129 mice. The resulting F1 embryos were subjected to pro-nuclear injection of reagents for two guide–mediated CRISPR/Cas9 (Cong et al., 2013; Singh et al., 2015) to delete an ∼24.3-kb region spanning Trdd1-Trdj2 at the Tcra-Tcrd locus. The upstream guide sequence was 5′-GACTCACCTGTTATCAAACT-3′, and the downstream guide sequence was 5′-ATAATGCTAAAATTACCTGC-3′. Offspring were screened for deletion on the 129 Tcra-Tcrd allele using PCR and Sanger sequencing (Duke University DNA Analysis Facility). Appropriately targeted mice of mixed C57BL/6J and 129 genetic background were crossed once to strain 129, and DJD heterozygotes were then intercrossed to obtain homozygous WT and DJD littermates for analysis.
TcrdCreER/CreER Rosa26ZsG/ZsG mice, with modified strain 129 Tcra-Tcrd alleles on a C57BL/6 genetic background (backcrossed >10 generations), were described previously (Zhang et al., 2015). These mice were further modified by deletion of 138 bp spanning the 5′ portion of Trac exon 1 to generate TcrdCreER/CreER TracM/+ Rosa26ZsG/ZsG mice. Two guide–mediated CRISPR/Cas9 targeting was accomplished by electroporation using upstream guide 5′-CAGGCAAGAGCGGAACTCTC-3′ and downstream guide 5′-GAGACCGAGGATCTTTTAAC-3′. The founder mouse was backcrossed once to TcrdCreER/CreER Rosa26ZsG/ZsG, and mice heterozygous for the Trac mutation were then intercrossed to obtain TcrdCreER/CreER TracM/M Rosa26ZsG/ZsG mice. These were then crossed to DJD heterozygotes to obtain littermates containing a Rosa26ZsG allele and TcrdCreER TracM allele paired with either a WT 129 or DJD 129 Tcra-Tcrd allele on a mixed C57BL/6 and 129 genetic background.
Trav15-1-dv6-1 and Trav15d-1-dv6d-1 deletions were generated in TcrdCreER/CreER Rosa26ZsG/ZsG Id3f/f mice (Madisen et al., 2010; Guo et al., 2011; Zhang et al., 2015) containing modified strain 129 Tcra-Tcrd alleles on a C57BL/6 genetic background (backcrossed >10 generations). Two-guide mediated CRISPR/Cas9 targeting was accomplished by electroporation using upstream guide 5′-TCTTCCCTTAAAGAGTGATA-3′, and downstream guide 5′-GACATTAGAGTCCCTTAAAG-3′. Offspring were screened by PCR and Sanger sequencing. Deletions of Trav15-1-dv6-1 and Trav15d-1-dv6d-1 were detected in different founders and maintained separately. Appropriately targeted mice were crossed to TcrdCreER/CreER Rosa26ZsG/ZsG Id3f/f mice to obtain Rosa26ZsG/ZsG Id3f/f mice containing a Trav15-1-dv6-1 KO TcrdCreER or a Trav15d-1-dv6d-1 KO TcrdCreER allele paired with a TcrdCreER allele on a C57BL/6 genetic background. Intercrossing of Trav15-1-dv6-1 KO heterozygous mice generated littermates containing either homozygous Trav15-1-dv6-1 KO TcrdCreER or TcrdCreER alleles for analysis. Intercrossing of Trav15d-1-dv6d-1 KO heterozygous mice generated littermates containing either homozygous Trav15d-1-dv6d-1 KO TcrdCreER or TcrdCreER alleles for analysis.
All CRISPR/Cas9-mediated deletions were performed by the Duke University Transgenic and Knockout Mouse Shared Resource. Mice were sacrificed at 4–5 wk of age. Both male and female mice were used; no differences were observed on the basis of sex. All mice were handled under protocols approved by the Duke University Institutional Animal Care and Use Committee and maintained in specific pathogen–free conditions.
Cell collection and flow cytometry
For analysis of thymocyte subpopulations, thymi were collected from mice at 3–4 wk of age. To sort DP thymocytes for repertoire analysis, thymi were collected from mice at 4–5 wk of age. To label developing thymocytes with ZsGreen, mice were injected i.p. with a single 100-µl dose of 10 mg/ml tamoxifen (Sigma-Aldrich) in corn oil (Sigma-Aldrich) 12 h before sacrifice.
To obtain preselection DP thymocytes (defined as CD4+CD8+Lin−7AAD−CD3εlo), total thymocytes were stained with anti-CD4 (GK1.5), anti-CD8α (53-6.7), anti-CD3ε (145-2C11), 7AAD, and PE-Cy5–conjugated lineage (Lin) markers anti-B220 (RA3-6B2), anti-CD11b (M1/70), anti-CD11c (N418), anti-F4/80 (BM8), anti–Gr-1 (RB6-8C5; Invitrogen), and anti–Ter-119 (TER-119). Preselection DP thymocytes from tamoxifen-injected mice were additionally sorted for ZsGreen+. For analysis of thymocyte subpopulations, total thymocytes were additionally stained with Pacific Blue anti-CD25 (PC61), allophycocyanin-Cy7 anti-cKit (2B8), and allophycocyanin anti-γδTCR (GL3). All antibodies were purchased from BioLegend, unless otherwise specified.
Tcra repertoire library preparation
Tcra sequencing libraries were prepared as previously described (Carico et al., 2017). Briefly, RNA was extracted using TRIzol (Life Technologies) from sorted preselection DP thymocytes. 5′ RACE was performed on total RNA as previously described (Pinto and Lindblad, 2010; Quigley et al., 2011; Carico et al., 2017), with minor modifications. 700 ng RNA was used as input for template-switch 5′ RACE and cDNA synthesis using SuperScript II (Thermo Fisher Scientific). Kapa HiFi polymerase in 1× Kapa HiFi buffer (Kapa Biosystems) was used for all PCRs in 50 µl total volume per reaction; eight reactions were performed per sample for each round of PCR. PCR products were pooled and purified using the QIAquick PCR purification kit (Qiagen) per the manufacturer’s specifications. Purified products of the first PCR were subjected to a second round of PCR amplification as described (Kozich et al., 2013; Carico et al., 2017) to ligate barcodes and Illumina adapter sequences. Libraries were then pooled and purified using the QIAquick PCR purification kit and resuspended in nuclease-free water.
Tcra repertoire sequencing and analysis
Sequencing and analysis were performed as previously described (Carico et al., 2017), with minor modifications. Briefly, barcoded libraries were pooled and sequenced by the Duke University Sequencing and Genomic Technologies Shared Resource using 300-nt paired-end reads on the Illumina MiSeq platform (version 3 chemistry). Agilent Bioanalyzer analysis was used to determine library molarity and quality, and size selection was performed by the Duke University Sequencing and Genomic Technologies Shared Resource for further purification. A PhiX control library and custom primers were added to the standard Illumina primer mix, as previously described (Carico et al., 2017). Libraries were demultiplexed and assessed for quality and yield using Illumina MiSeq Reporter software.
Analysis was performed using MiXCR (version 3.0.7; Bolotin et al., 2015). The reference library was edited to permit alignments only to 129 sequences (Bosc and Lefranc, 2003). The “align” command was used to align sequencing reads to this reference library. The “assemble” command was then used to identify clones with sequences spanning CDR2 through CDR3. Each clone was assigned to the corresponding V and J segments. The “exportClones” command produced a human-readable form of these data; alignment was manually reviewed. Sequences aligning to pseudogenes and other very infrequently uses genes were manually removed, including Traj61, Traj41, Traj25, Trav5d-2, Trav7d-6, Trav7-6, and Trav18. Trav11 and Trav11d are not distinguishable; both were maintained in this analysis, but the computed distribution of reads between the segments should be ignored. The VDJtools (Shugay et al., 2015) command PlotFancyVJUsage was used to calculate clonal frequencies of V-J recombinations. In R (version 3.3.3; R Core Team, 2020), and heatmaps were generated using the gplots (Warnes et al., 2009) and RColorBrewer (Neuwirth, 2014) packages. For difference maps, the WT repertoire was subtracted from the mutant repertoire. Repertoire differences were reported for regions demarcated by step-function diagonals with a slope of 4Vα:3Jα (or 1.33 Vα per Jα), the previously established relationship for the progression of secondary rearrangements across the V and J arrays (Carico et al., 2017). Total numbers of unique sequences in each region were combined from two replicates per genotype, and differences between genotypes were evaluated by two-tailed χ2 test with Yates’s correction for continuity, using GraphPad Prism 6 software. When determining the regional changes to the repertoire in Trav15d-1-dv6d-1– and Trav15-1-dv6-1–deleted models, the deleted segment in each case was excluded from analysis.
Tcra repertoire sequencing data are deposited in the Gene Expression Omnibus under accession no. GSE186044.
Global Shannon’s entropy calculation
Global V-J pair diversity was measured by Shannon entropy index (H). Shannon entropy quantifies both abundance and degree of unevenness of all distinct V-J pairs in a sample. A higher H value indicates more even distribution for distinct V-J pairs, while a lower value suggests that a dominant V-J type occupies overall pairs. The Shannon diversity (H) was calculated using the following equation:
where is the fraction of i-th V-J pairs in a sample, and is the total combinations of V-J pairs. In this study, S = 3,569 (83 V × 43 J). We then normalized to the maximum using the following equation:
The normalized Shannon index is bounded from 0 to 1, and a value of 1 means all V-J pairs have the same frequency.
E2A ChIP-seq analysis
E2A ChIP-seq from sorted DP thymocytes (CD1dTet−CD4+CD8+) isolated from Id2f/f Id3f/f Lck-Cre C57BL/6 mice was previously reported (Roy et al., 2018). Here, alignment was performed to mm10 using Bowtie2 (version 2.3.4.1), allowing multiple read alignment (k = 3) to account for sequence similarity within the Tcra-Tcrd locus; all other parameters were set to default. Peak calling was performed using MACS2 (version 2.1.1.20160309); default options were used. Data visualization was performed using the Integrative Genomics Viewer (version 2.8.2).
Online supplemental material
Fig. S1 shows characterization of mouse strain mutations and thymocyte cell populations in DJD mice. Fig. S2 shows the WT strain 129 Tcra combinatorial repertoire with gene segments and secondary Tcra recombination diagonals identified. Fig. S3 shows Tcra-Tcrd locus E2A ChIP tracks.
Acknowledgments
We thank Lunden Simpson and Abani Naik for comments on the manuscript and Qi-Jing Li for helpful discussions.
This research was supported in part by National Institutes of Health grants R01 GM41052 and R35 GM136284 (to M.S. Krangel) and P01 AI102853 (to Y. Zhuang).
Author contributions: D.J. Dauphars, A. Mihai, Y. Zhuang, and M.S. Krangel designed the study; D.J. Dauphars and A. Mihai performed the experiments; D.J. Dauphars, A. Mihai, and L. Wang analyzed the data; and D.J. Dauphars, A. Mihai, L. Wang, and M.S. Krangel wrote the manuscript.
References
- Ba, Z., Lou J., Ye A.Y., Dai H.-Q., Dring E.W., Lin S.G., Jain S., Kyritsis N., Kieffer-Kwon K.-R., Casellas R., and Alt F.W.. 2020. CTCF orchestrates long-range cohesin-driven V(D)J recombinational scanning. Nature. 586:305–310. 10.1038/s41586-020-2578-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain, G., Romanow W.J., Albers K., Havran W.L., and Murre C.. 1999. Positive and negative regulation of V(D)J recombination by the E2A proteins. J. Exp. Med. 189:289–300. 10.1084/jem.189.2.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barajas-Mora, E.M., Kleiman E., Xu J., Carrico N.C., Lu H., Oltz E.M., Murre C., and Feeney A.J.. 2019. A B-cell-specific enhancer orchestrates nuclear architecture to generate a diverse antigen receptor repertoire. Mol. Cell. 73:48–60.e5. 10.1016/j.molcel.2018.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolotin, D.A., Poslavsky S., Mitrophanov I., Shugay M., Mamedov I.Z., Putintseva E.V., and Chudakov D.M.. 2015. MiXCR: software for comprehensive adaptive immunity profiling. Nat. Methods. 12:380–381. 10.1038/nmeth.3364 [DOI] [PubMed] [Google Scholar]
- Bosc, N., and Lefranc M.P.. 2003. The mouse (Mus musculus) T cell receptor alpha (TRA) and delta (TRD) variable genes. Dev. Comp. Immunol. 27:465–497. 10.1016/S0145-305X(03)00027-2 [DOI] [PubMed] [Google Scholar]
- Buch, T., Rieux-Laucat F., Förster I., and Rajewsky K.. 2002. Failure of HY-specific thymocytes to escape negative selection by receptor editing. Immunity. 16:707–718. 10.1016/S1074-7613(02)00312-6 [DOI] [PubMed] [Google Scholar]
- Capone, M., Hockett R.D. Jr., and Zlotnik A.. 1998. Kinetics of T cell receptor β, γ, and δ rearrangements during adult thymic development: T cell receptor rearrangements are present in CD44(+)CD25(+) Pro-T thymocytes. Proc. Natl. Acad. Sci. USA. 95:12522–12527. 10.1073/pnas.95.21.12522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carico, Z., and Krangel M.S.. 2015. Chromatin dynamics and the development of the TCRα and TCRδ repertoires. Adv. Immunol. 128:307–361. 10.1016/bs.ai.2015.07.005 [DOI] [PubMed] [Google Scholar]
- Carico, Z.M., Roy Choudhury K., Zhang B., Zhuang Y., and Krangel M.S.. 2017. Tcrd rearrangement redirects a processive Tcra recombination program to expand the Tcra repertoire. Cell Rep. 19:2157–2173. 10.1016/j.celrep.2017.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L., Carico Z., Shih H.-Y., and Krangel M.S.. 2015. A discrete chromatin loop in the mouse Tcra-Tcrd locus shapes the TCRδ and TCRα repertoires. Nat. Immunol. 16:1085–1093. 10.1038/ni.3232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong, L., Ran F.A., Cox D., Lin S., Barretto R., Habib N., Hsu P.D., Wu X., Jiang W., Marraffini L.A., and Zhang F.. 2013. Multiplex genome engineering using CRISPR/Cas systems. Science. 339:819–823. 10.1126/science.1231143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, H.-Q., Hu H., Lou J., Ye A.Y., Ba Z., Zhang X., Zhang Y., Zhao L., Yoon H.S., Chapdelaine-Williams A.M., et al. 2021. Loop extrusion mediates physiological Igh locus contraction for RAG scanning. Nature. 590:338–343. 10.1038/s41586-020-03121-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glusman, G., Rowen L., Lee I., Boysen C., Roach J.C., Smit A.F.A., Wang K., Koop B.F., and Hood L.. 2001. Comparative genomics of the human and mouse T cell receptor loci. Immunity. 15:337–349. 10.1016/S1074-7613(01)00200-X [DOI] [PubMed] [Google Scholar]
- Guo, Z., Li H., Han M., Xu T., Wu X., and Zhuang Y.. 2011. Modeling Sjögren’s syndrome with Id3 conditional knockout mice. Immunol. Lett. 135:34–42. 10.1016/j.imlet.2010.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawwari, A., and Krangel M.S.. 2007. Role for rearranged variable gene segments in directing secondary T cell receptor α recombination. Proc. Natl. Acad. Sci. USA. 104:903–907. 10.1073/pnas.0608248104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmink, B.A., and Sleckman B.P.. 2012. The response to and repair of RAG-mediated DNA double-strand breaks. Annu. Rev. Immunol. 30:175–202. 10.1146/annurev-immunol-030409-101320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng, T.S.P., Painter M.W., Elpek K., Lukacs-Kornek V., Mauermann N., Turley S.J., Koller D., Kim F.S., Wagers A.J., Asinovski N., et al. Immunological Genome Project Consortium . 2008. The Immunological Genome Project: networks of gene expression in immune cells. Nat. Immunol. 9:1091–1094. 10.1038/ni1008-1091 [DOI] [PubMed] [Google Scholar]
- Hill, L., Ebert A., Jaritz M., Wutz G., Nagasaka K., Tagoh H., Kostanova-Poliakova D., Schindler K., Sun Q., Bönelt P., et al. 2020. Wapl repression by Pax5 promotes V gene recombination by Igh loop extrusion. Nature. 584:142–147. 10.1038/s41586-020-2454-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, J., Zhang Y., Zhao L., Frock R.L., Du Z., Meyers R.M., Meng F.L., Schatz D.G., and Alt F.W.. 2015. Chromosomal loop domains direct the recombination of antigen receptor genes. Cell. 163:947–959. 10.1016/j.cell.2015.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itohara, S., Mombaerts P., Lafaille J., Iacomini J., Nelson A., Clarke A.R., Hooper M.L., Farr A., and Tonegawa S.. 1993. T cell receptor δ gene mutant mice: independent generation of α β T cells and programmed rearrangements of γ δ TCR genes. Cell. 72:337–348. 10.1016/0092-8674(93)90112-4 [DOI] [PubMed] [Google Scholar]
- Jhunjhunwala, S., van Zelm M.C., Peak M.M., and Murre C.. 2009. Chromatin architecture and the generation of antigen receptor diversity. Cell. 138:435–448. 10.1016/j.cell.2009.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji, Y., Resch W., Corbett E., Yamane A., Casellas R., and Schatz D.G.. 2010. The in vivo pattern of binding of RAG1 and RAG2 to antigen receptor loci. Cell. 141:419–431. 10.1016/j.cell.2010.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozich, J.J., Westcott S.L., Baxter N.T., Highlander S.K., and Schloss P.D.. 2013. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 79:5112–5120. 10.1128/AEM.01043-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, S.G., Ba Z., Alt F.W., and Zhang Y.. 2018. RAG chromatin scanning during V(D)J recombination and chromatin loop extrusion are related processes. Adv. Immunol. 139:93–135. 10.1016/bs.ai.2018.07.001 [DOI] [PubMed] [Google Scholar]
- Livak, F., Petrie H.T., Crispe I.N., and Schatz D.G.. 1995. In-frame TCR δ gene rearrangements play a critical role in the α β/γ δ T cell lineage decision. Immunity. 2:617–627. 10.1016/1074-7613(95)90006-3 [DOI] [PubMed] [Google Scholar]
- Madisen, L., Zwingman T.A., Sunkin S.M., Oh S.W., Zariwala H.A., Gu H., Ng L.L., Palmiter R.D., Hawrylycz M.J., Jones A.R., et al. 2010. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13:133–140. 10.1038/nn.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, D., Pantoja C., Fernández Miñán A., Valdes-Quezada C., Moltó E., Matesanz F., Bogdanović O., de la Calle-Mustienes E., Domínguez O., Taher L., et al. 2011. Genome-wide CTCF distribution in vertebrates defines equivalent sites that aid the identification of disease-associated genes. Nat. Struct. Mol. Biol. 18:708–714. 10.1038/nsmb.2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe, R.J., Sleckman B.P., Monroe B.C., Khor B., Claypool S., Ferrini R., Davidson L., and Alt F.W.. 1999. Developmental regulation of TCR delta locus accessibility and expression by the TCR delta enhancer. Immunity. 10:503–513. 10.1016/S1074-7613(00)80050-3 [DOI] [PubMed] [Google Scholar]
- Naik, A.K., Hawwari A., and Krangel M.S.. 2015. Specification of Vδ and Vα usage by Tcra/Tcrd locus V gene segment promoters. J. Immunol. 194:790–794. 10.4049/jimmunol.1402423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima, P.B., Menetski J.P., Roth D.B., Gellert M., and Bosma M.J.. 1995. V-D-J rearrangements at the T cell receptor δ locus in mouse thymocytes of the α β lineage. Immunity. 3:609–621. 10.1016/1074-7613(95)90132-9 [DOI] [PubMed] [Google Scholar]
- Neuwirth, E. 2014. RColorBrewer: ColorBrewer Palettes. https://CRAN.R-project.org/package=RColorBrewer (accessed March 14, 2021).
- Petrie, H.T., Livak F., Schatz D.G., Strasser A., Crispe I.N., and Shortman K.. 1993. Multiple rearrangements in T cell receptor α chain genes maximize the production of useful thymocytes. J. Exp. Med. 178:615–622. 10.1084/jem.178.2.615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie, H.T., Livak F., Burtrum D., and Mazel S.. 1995. T cell receptor gene recombination patterns and mechanisms: cell death, rescue, and T cell production. J. Exp. Med. 182:121–127. 10.1084/jem.182.1.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto, F.L., and Lindblad P.. 2010. A guide for in-house design of template-switch-based 5′ rapid amplification of cDNA ends systems. Anal. Biochem. 397:227–232. 10.1016/j.ab.2009.10.022 [DOI] [PubMed] [Google Scholar]
- Quigley, M.F., Almeida J.R., Price D.A., and Douek D.C.. 2011. Unbiased molecular analysis of T cell receptor expression using template-switch anchored RT-PCR. Curr. Protoc. Immunol. 94:10.33.1–10.33.16. 10.1002/0471142735.im1033s94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/.
- Roy, S., Moore A.J., Love C., Reddy A., Rajagopalan D., Dave S.S., Li L., Murre C., and Zhuang Y.. 2018. Id proteins suppress E2A-driven invariant natural killer T cell development prior to TCR selection. Front. Immunol. 9:42. 10.3389/fimmu.2018.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz, D.G., and Ji Y.. 2011. Recombination centres and the orchestration of V(D)J recombination. Nat. Rev. Immunol. 11:251–263. 10.1038/nri2941 [DOI] [PubMed] [Google Scholar]
- Shih, H.-Y., and Krangel M.S.. 2010. Distinct contracted conformations of the Tcra/Tcrd locus during Tcra and Tcrd recombination. J. Exp. Med. 207:1835–1841. 10.1084/jem.20100772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih, H.-Y., Verma-Gaur J., Torkamani A., Feeney A.J., Galjart N., and Krangel M.S.. 2012. Tcra gene recombination is supported by a Tcra enhancer- and CTCF-dependent chromatin hub. Proc. Natl. Acad. Sci. USA. 109:E3493–E3502. 10.1073/pnas.1214131109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shugay, M., Bagaev D.V., Turchaninova M.A., Bolotin D.A., Britanova O.V., Putintseva E.V., Pogorelyy M.V., Nazarov V.I., Zvyagin I.V., Kirgizova V.I., et al. 2015. VDJtools: unifying post-analysis of T cell receptor repertoires. PLOS Comput. Biol. 11:e1004503. 10.1371/journal.pcbi.1004503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, P., Schimenti J.C., and Bolcun-Filas E.. 2015. A mouse geneticist’s practical guide to CRISPR applications. Genetics. 199:1–15. 10.1534/genetics.114.169771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleckman, B.P., Khor B., Monroe R., and Alt F.W.. 1998. Assembly of productive T cell receptor δ variable region genes exhibits allelic inclusion. J. Exp. Med. 188:1465–1471. 10.1084/jem.188.8.1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, S.D., Pelkonen J., and Hurwitz J.L.. 1990. First T cell receptor alpha gene rearrangements during T cell ontogeny skew to the 5′ region of the J alpha locus. J. Immunol. 145:2347–2352. [PubMed] [Google Scholar]
- Wang, F., Huang C.Y., and Kanagawa O.. 1998. Rapid deletion of rearranged T cell antigen receptor (TCR) Valpha-Jalpha segment by secondary rearrangement in the thymus: role of continuous rearrangement of TCR α chain gene and positive selection in the T cell repertoire formation. Proc. Natl. Acad. Sci. USA. 95:11834–11839. 10.1073/pnas.95.20.11834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnes, G.R., Bolker B., Bonebakker L., Gentleman R., Huber W., Liaw A., Lumley T., Maechler M., Magnusson A., Moeller S., et al. 2009. gplots: Various R programming tools for plotting data. https://CRAN.R-project.org/package=gplots (accessed March 14, 2021).
- Yoshida, H., Lareau C.A., Ramirez R.N., Rose S.A., Maier B., Wroblewska A., Desland F., Chudnovskiy A., Mortha A., Dominguez C., et al. Immunological Genome Project . 2019. The cis-Regulatory Atlas of the Mouse Immune System. Cell. 176:897–912.e20. 10.1016/j.cell.2018.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, B., Wu J., Jiao Y., Bock C., Dai M., Chen B., Chao N., Zhang W., and Zhuang Y.. 2015. Differential requirements of TCR signaling in homeostatic maintenance and function of dendritic epidermal T cells. J. Immunol. 195:4282–4291. 10.4049/jimmunol.1501220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, L., Frock R.L., Du Z., Hu J., Chen L., Krangel M.S., and Alt F.W.. 2016. Orientation-specific RAG activity in chromosomal loop domains contributes to Tcrd V(D)J recombination during T cell development. J. Exp. Med. 213:1921–1936. 10.1084/jem.20160670 [DOI] [PMC free article] [PubMed] [Google Scholar]