Abstract
The Caenorhabditis elegans GATA transcription factor genes elt-1 and elt-3 are expressed in the embryonic hypodermis (also called the epidermis). elt-1 is expressed in precursor cells and is essential for the production of most hypodermal cells (22). elt-3 is expressed in all of the major hypodermal cells except the lateral seam cells, and expression is initiated immediately after the terminal division of precursor lineages (13). Although this expression pattern suggests a role for ELT-3 in hypodermal development, no functional studies have yet been performed. In the present paper, we show that either elt-3 or elt-1 is sufficient, when force expressed in early embryonic blastomeres, to activate a program of hypodermal differentiation even in blastomeres that are not hypodermal precursors in wild-type embryos. We have deleted the elt-3 gene and shown that ELT-3 is not essential for either hypodermal cell differentiation or the viability of the organism. We showed that ELT-3 can activate hypodermal gene expression in the absence of ELT-1 and that, conversely, ELT-1 can activate hypodermal gene expression in the absence of ELT-3. Overall, the combined results of the mutant phenotypes, initial expression times, and our forced-expression experiments suggest that ELT-3 acts downstream of ELT-1 in a redundant pathway controlling hypodermal cell differentiation.
There is now substantial understanding of the way in which maternal genes establish the anterior-posterior axis and specify the fates of early blastomeres in the Caenorhabditis elegans embryo (3, 29). In contrast, much less is known about the way in which particular tissue types are produced by these blastomeres later in embryogenesis. The major tissue types of the worm (with the exception of the clonally derived gut and germline) arise from a variety of different cell lineages produced by several different early embryonic blastomeres. It might be anticipated that formation of these tissues will be a complex process that is likely to involve combinatorial use of a number of transcription factors.
The nematode hypodermis (also referred to as the epidermis) comprises the outermost layer of cells of the worm. By completion of embryogenesis, there are 85 hypodermal nuclei (including specialized nuclei of the head and tail but excluding the rectum) deriving from four different blastomeres of the 12-cell embryo. Each of these blastomeres undergoes a complex series of cell divisions that produces a variety of cell types in addition to the hypodermis. Only one gene has been shown to be required for the specific formation of hypodermal tissue i.e., that which encodes GATA transcription factor ELT-1 (22, 31) (for reviews of research on this family of zinc finger transcription factors, see references 6 and 21). ELT-1 acts early in hypodermal precursor cells and appears to specify hypodermal cell fate, as opposed to (or possibly in addition to) being directly involved in hypodermal differentiation. In elt-1 loss-of-function mutants, precursor cells fail to produce hypodermal cells but, instead, produce an excess of cell types that are normally produced by the sister lineage (at least in two of the three major hypodermal lineages) (22).
The identification of genes acting downstream of the elt-1 gene is an important step in understanding how ELT-1 specifies hypodermal cell fate. One such downstream gene is likely to be that which encodes zinc finger candidate transcription factor LIN-26 (17, 19). However lin-26 appears to be more important in the maintenance, rather than the establishment, of hypodermal cell properties and has functions in cell types besides strictly hypodermis cells (e.g., neuronal support cells) (17, 19). We have previously described the isolation and characterization of ELT-3, a second GATA transcription factor expressed in the C. elegans hypodermis (13). Several aspects of the expression pattern of the gene for ELT-3 make it a likely candidate for a gene involved, downstream of ELT-1, in the control of hypodermal cell differentiation. The initiation of elt-3 expression is largely dependent on the presence of elt-1 but appears to be independent of the presence of lin-26. elt-3 is first expressed immediately following the terminal division of cell lineages that give rise to hypodermal cells and is expressed in the hypodermal daughter cell but not in the nonhypodermal daughter cell of such divisions. elt-3 expression begins shortly before the onset of hypodermal cell differentiation, and it is possible that ELT-3 is a direct regulator of hypodermal structural genes, such as the collagen gene dpy-7 (12). elt-3 is expressed exclusively in hypodermal cells, except for a few cells of the digestive tract later in development. Indeed, elt-3 is expressed in all of the major hypodermal cells of the embryo except the lateral seam cells, a specialized subset of hypodermal cells that remain as blast cells throughout embryonic and postembryonic development. In the current study, we investigated the function of elt-3 and the relationship between elt-1 and elt-3 as a first step toward working out the transcription factor hierarchy that controls hypodermal differentiation.
MATERIALS AND METHODS
Forced expression of elt-3 and elt-1.
Forced expression of GATA transcription factor genes was performed using transgenic strains containing chromosomally integrated plasmids in which the corresponding cDNA had been cloned downstream of a C. elegans heat shock promoter. The details of these plasmid constructs can be found in reference 10. The heat shock promoter used was hsp16-2, which is inactive at 25°C but at 33.5°C is expressed at a high level in most tissues from early embryogenesis onward (32). We have used several independently chromosomally integrated transgenic strains (generously supplied by T. Fukushige) in these experiments. JM53 cals4, JM54 cals5, and JM55 cals6 all contain the hsp16-2::elt-1 construct; JM57 cals8 contains the hsp16-2::elt-2 construct; and JM58 cals9, JM59 cals10, and JM60 cals11 all contain the hsp16-2::elt-3 construct. Most experiments were conducted with all of these strains to rule out possible variability due to factors such as chromosomal location and copy number of the integrated constructs; in fact, no significant differences were found among the three hsp16-2::elt-1 lines or among the three hsp16-2::elt-3 lines. The designations hs-elt-3, hs-elt-2, and hs-elt-1 will be used to describe animals following induction of expression of the corresponding GATA transcription factor by heat shock. The precise experimental conditions used are described in the text as appropriate. We stained hs-elt-3-arrested embryos (JM58, JM59, and JM60) with an ELT-3-specific antibody (13) and detect widespread ELT-3 expression throughout the embryo (data not shown). Although we did not quantitate the level of ELT-3 expression in these embryos, the intensity of staining was not dramatically different than that seen with endogenous ELT-3 in the hypodermal cells of control embryos. Similarly, ELT-2 levels detected when hs-elt-2 embryos are stained with ELT-2-specific antibody are not dramatically different than normal ELT-2 levels in the guts of control embryos (T. Fukushige, personal communication).
Microscopy and counting of cell nuclei.
Microscopy and image processing were performed essentially as previously described (11). Embryos were optically sectioned on the z axis at 1-μm intervals throughout the embryo, and stacked, deconvolved images were analyzed using NIH Image (Scion Corporation).
Laser ablations.
One-cell embryos were mounted in M9 on gelatin-coated slides (7). Blastomeres were ablated by pulsing the nuclei with a laser beam from a model VSL-337 nitrogen laser generator (Laser Science Inc.) until nuclear breakdown and scarring were visible. The subsequent two rounds of embryonic cell division were observed to ensure that the appropriate blastomere(s) had been successfully ablated. Embryos operated on were then subjected to the heat shock regimen as appropriate (40 min at 33.5°C beginning at 1 h after laser ablation) and then incubated at 20°C for 14 to 18 h before being observed under UV illumination for green fluorescent protein (GFP) fluorescence.
Isolation of a deletion in the elt-3 gene.
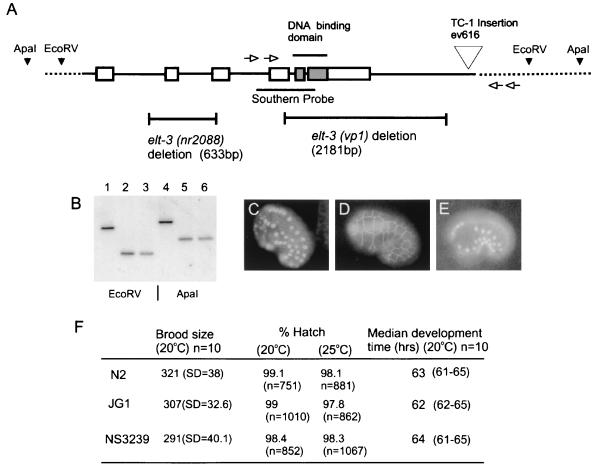
Strain NW1122 (ev616::Tc1), which is homozygous for a Tc1 insertion 1,352 bp downstream of the elt-3 polyadenylation site (1,843 bp downstream of the T of the stop codon) was kindly provided by J. Culotti (Samuel Lunenfeld Research Institute, Toronto, Ontario, Canada). (This strain had a UNC phenotype that could be segregated from ev616::Tc1 by backcrossing against N2 [data not shown].) A PCR-based strategy was used to screen for imprecise excisions of the Tc1 insertion as described in reference 25. Briefly, 200 plates were each seeded with four or five NW1122 L4 hermaphrodites and after several weeks of growth, with the plates nearing starvation, half of the worms were washed off and genomic DNA was prepared (35). A two-dimensional matrix of pooled samples (10 samples per pool) was screened by a single round of PCR using primers flanking the insertion site; primers JG8 5′GTCAGCGGCAGCTGATTGAGTATCG3′ and JG13 5′CAACGATGAACGATTATCGAGTGG3′ are separated by 3,639 bp of the wild-type genomic sequence (see Fig. 6A). A 1.4-kb fragment was detected in one of these pools, and a single positive DNA sample was identified by repeating the PCR on individual samples contributing to the positive pool. A dilution method was used to confirm that this PCR product reflected a germline rather than a somatic excision; a 1-μl aliquot of the original 200-μl DNA sample was diluted 500-fold, and PCR was performed on 15 1-μl aliquots of this diluted sample using two sets of nested primers flanking the insert. The first-round PCR used primers JG8 and JG13 (see above), and the second-round PCR used nested primers JG7 (5′GCTCTTAAATGACATTACGGATCGG3′) and JG14 (5′CAACTTGACCAACCGACCTATGCGG3′). The amplification of the product from all 15 aliquots demonstrated that many more template molecules were present in the original DNA sample than could be accounted for by a somatic excision event. Sib selection was then performed on the positive plate by picking 1,000 worms onto 200 plates (i.e., 5 worms per plate). Following growth on these plates, genomic DNA was prepared and screened by PCR exactly as for the first round; three samples yielding the 1.4-kb product were identified. From one of these positive plates, 500 individual worms were picked to separate plates, allowed to lay eggs, and then removed to prepare DNA for PCR. Nine of these worms were positive for the 1.4-kb product. The amplified PCR fragment was sequenced, and a 2,181-bp deletion with a 6-bp GGAAAT insertion was identified. A homozygous strain was isolated by identifying single worms in which the deletion was present in 100% of the progeny tested by PCR. As shown below, the chromosomal deletion was confirmed by Southern blotting.
FIG. 6.
Chromosomal deletion of the elt-3 gene. (A) Schematic representation of the elt-3 genomic locus. Exons are shown as boxes, and noncoding sequence are shown as lines. Strain NW1122 (ev616) was screened for deletions induced by imprecise Tc1 excision using primers at the positions indicated by open arrowheads. The elt-3(vp1) deletion was isolated and shown by direct sequencing to remove the elt-3 DNA binding domain. A homozygous strain was isolated by sib selection and backcrossed 15 times against N2. The elt-3(nr2088) deletion was isolated following chemical mutagenesis (Axys Pharmaceuticals, San Francisco, Calif.). (B) Southern blot of N2 worms (lanes 1 and 4), JG1 worms before backcrossing (lanes 2 and 5), and JG1 worms after 15 backcrosses (lanes 3 and 6). Genomic DNA was digested with EcoRV (lanes 1, 2, and 3) or ApaI (lanes 4, 5, and 6) and probed with the 858-bp fragment indicated in panel A. The positions of the EcoRV and ApaI sites are also indicated in panel A. The blot shows that the JG1 strain is homozygous for the elt-3(vp1) deletion. (C, D, and E) Comma stage JG1(vp1) embryos stained with LIN-26 antibody and MH27 antibody and expressing dpy-7::GFP, respectively. The expression of these markers and the morphology of the hypodermis appear indistinguishable from those of the wild type. This was also true for pretzel stage embryos and L1 larvae (data not shown). (F) Summary of reproduction and development parameters from JG1(vp1) and NS3239(nr2088) worms. The final column shows median development times with the ranges in parentheses.
A second deletion in the elt-3 gene (nr2088) was isolated at our request by Nemapharm (Axys Pharmaceuticals) by PCR screening of populations of chemically mutagenized worms. We used PCR screening and sib selection as described above to isolate a strain homozygous for this deletion. Direct sequencing of the PCR product revealed a 666-bp deletion (with a 19-bp insertion CGTGGCCCCTTTTGCTAGG) extending from the middle of the first intron to a point near the end of the third exon.
A multiplex PCR was used to genotype animals at the elt-3 locus in some of the genetic crosses using the JG1 strain. Upstream primer JG20 (5′CAAACTTCGCAACATTCCAACCAGC3′) was used in conjunction with downstream primers JG8c (5′CAGTGCTTGTTATGTCTTTCTCGG3′) and ELT3/1a (5′CCATCTTTTCTAAGAGACAGTGGACG3′). Downstream primer ELT3/1a, which was complementary to the sequence removed by the elt-3(vp1) deletion, amplified a 400-bp product from the wild-type elt-3 allele but no product from the elt-3(vp1) allele. Primer JG8c, which was complementary to the sequence 2,874 bp downstream of JG20 in the wild-type DNA, amplified a 699-bp product from the elt-3(vp1) allele but no band from the wild-type allele under the PCR conditions used (1-min extension time). This multiplex PCR distinguished among the genotypes elt-3(+)/elt3(+) (400-bp product), elt-3(vp1)/elt-3(vp1) (699-bp product), and elt-3(+)/elt-3(vp1) (699- and 400-bp products).
Phenotypic analysis.
Brood size and embryonic mortality (at 20 and 25°C) were determined by placing L4 hermaphrodites (10 animals of each strain) singly onto plates and transferring them daily for 4 days. Unhatched eggs and hatched larvae were counted on each plate 16 h after removal of the adult worms. The rate of development at 20°C was estimated by placing freshly laid eggs singly onto plates and then, after 60 h at 20°C, examining the plates hourly to determine the time at which egg laying began. The median development time for a group of 10 animals from each strain was determined.
RNAi.
RNA-mediated interference (RNAi) was performed essentially as described in reference 8. RNA was transcribed in vitro separately from the two complementary strands of the appropriate cloned cDNA using either T3 or T7 polymerase. The plasmids used as templates were pBluescript plasmids containing either the full-length elt-3 cDNA (13), an expressed sequence tag corresponding to the elt-5 gene (pYK446E9), or an expressed sequence tag corresponding to the elt-6 gene (pYK173E7). Following in vitro synthesis, the two strands were denatured and then annealed at 37°C in injection buffer (8). Double-stranded RNA was injected into the hermaphrodite gonad at a final concentration of 150 ng/μl. Injected animals were allowed to recover for 6 h and then transferred to fresh plates every 10 to 14 h; the progeny produced during the 36 h following injection were examined.
General worm culture, strains, and genetics.
C. elegans culture and genetic methods were as described in reference 4. The worm strains used were IA105 ijls12, JG5 vpls1, NW1229 evls111, PD4251 ccls4251(I), J1129 elt-1 (zu180) unc-43(e408)/unc-24(e138) dpy-20(e1282) IV, JM53 cals4, JM54 cals5, JM55 cals6, JM57 cals8, JM58 cals9, JM59 cals10, JM60 cals11, NW1122 elt-3(ev616::Tc1), JG1 elt-3(vp1), and NS3239 elt-3(nr2088).
The integrated transgenes were crossed into various hs-GATA strains. ijls12 (dpy-7::GFP) and vpls1 (elt-3::GFP) were crossed into JM53, JM54, JM55, JM57, JM58, JM59, and JM60. evls111 (F25B3.3::GFP) and ccls4251 (myo-3::GFP) were crossed into JM53, JM55, JM57, JM58, and JM60. Hence, for each reporter gene, the ability of forced expression to activate marker expression was assayed using at least two, and in some cases three, independently integrated hs-elt-3 or hs-elt-1 transgenes.
Animals with the genotype elt-1(zu180) unc-43(e408)/+; elt-3(vp1)/elt-3(vp1) were constructed in the following manner. elt-1(zu180) unc-43(e408)/unc-24(e138) dpy-20(e1282) IV hermaphrodites (strain J1129) were crossed with elt-3(vp1) X males, and F1 animals with the genotype elt-1(zu180) unc-43(e408)/+; elt-3(vp1)/+ were identified by progeny testing for the segregation of arrested embryos (along with failure to segregate Dpy Uncs) combined with a multiplex PCR using primers that distinguish elt-3 loci with and without deletions (see above). We then picked F2 progeny individually to plates and identified elt-1(zu180) unc-43(e408)/+; elt-3(vp1)/elt-3(vp1) animals by observing the segregation of dead embryos and confirming elt-3(vp1) homozygosity by multiplex PCR.
RESULTS
Forced ectopic expression of either elt-1 or elt-3 results in widespread ectopic expression of hypodermal marker genes.
In order to investigate the function of the elt-1 and elt-3 genes (and their relationship to each other), we determined the effects of ectopically expressing these genes in the pluripotent blastomeres of the early embryo.
Several independent C. elegans transgenic strains have been produced with chromosomally integrated arrays containing either elt-1 or elt-3 cDNA cloned downstream of the heat shock promoter gene hsp16-2 (10). These strains allow elt-1 (strains JM53, JM54, and JM55) or elt-3 (strains JM58, JM59, and JM60) to be expressed throughout the embryo in response to the following heat shock regimen. Two cell embryos are collected, allowed to develop for 1 h at 20°C, incubated at 33.5°C for 40 min, and then returned to 20°C for 16 to 20 h before assay of marker expression. (As described in Materials and Methods, we used ELT-3-specific antibody to show that ELT-3 is indeed expressed throughout the JM58, JM59, and JM60 embryos in response to heat shock and the intensity of staining was not dramatically different than that seen with endogenous ELT-3 in the hypodermal cells of control embryos). The heat shock regimen results in essentially 100% of hs-elt-3 (strains JM58, JM59, and JM60) or hs-elt-1 (strains JM53, JM54, and JM55) embryos being arrested in development as a “ball of cells” with no visible signs of morphogenesis (Fig. 1A and B). As negative controls, the same heat shock regimen was performed on wild-type N2 worms and on a strain carrying a chromosomally integrated construct in which the heat shock promoter drives a cDNA of the endoderm-specific GATA transcription factor gene elt-2 but with a frameshift mutation introduced upstream of the DNA binding domain (10). In both of these cases, fewer than 5% of the embryos were arrested in development. Furthermore, control embryos that were arrested (presumably because of nonspecific effects of heat shock) underwent various degrees of morphogenesis and did not resemble the ball-of-cells phenotype. We concluded that the arrest phenotype is due to ectopic elt-1 or elt-3 expression.
FIG. 1.
(A) DAPI staining of embryos arrested by forced ectopic expression of elt-3 (A), elt-1 (B), or elt-2 (C) performed at 60 min of development. Such arrested embryos appear as a ball of cells and show no visible signs of morphogenesis.(B) Expression of hypodermal cell markers following forced ectopic expression of elt-3 (JM60), elt-1 (JM53), or elt-2 (JM57). Shown is the expression of four hypodermal cell markers, dpy-7::GFP (ijls12) (A, B, C, and D), LIN-26 antibody (E, F, G, and H), elt-3::GFP (vpls1) (I, J, K, and L), and MH27 antibody (M, N, O, and P), in embryos arrested by forced ectopic expression of elt-3 (B, F, J, and N), elt-1 (C, G, K, and O), or elt-2 (D, H, L, and P). The expression of these markers in wild-type comma stage embryos is shown in parts A, E, I, and M.
Forced ectopic expression of either elt-1 (strains JM54 and JM55) or elt-3 (strains JM59 and JM60) produces widespread expression of three quite different hypodermal markers, dpy-7::GFP (integrated transgene ijls12), elt-3::GFP (integrated transgene vpls1), and LIN-26, which was assayed by a LIN-26-specific polyclonal antibody (17) (Fig. 1B). As shown in Fig. 1B, expression of these markers was detected in a large number of cells throughout the embryo and at a level equal to or greater than that of wild-type expression. As a control for the specificity of these forced-expression experiments, we repeated the experiment with endodermal GATA transcription factor ELT-2 (10). In embryos arrested by hs-elt-2, expression of hypodermal markers was generally suppressed; only small numbers of cells in each embryo expressed these hypodermal markers at or near wild-type levels (Fig. 1B, parts D, H, and L). The great majority of cells in hs-elt-2-arrested embryos showed no detectable expression of lin-26 or elt-3::GFP. Although dpy-7::GFP expression was seen throughout hs-elt-2-arrested embryos, the level of this expression was dramatically lower than the levels of expression seen in response to hs-elt-1 or hs-elt-3 or in hypodermal cells of wild-type embryos (Fig. 1B, part D).
An interesting demonstration of the cell type specificity of these ectopic expression experiments emerged when we used the monoclonal antibody MH27 as a marker (Fig. 1B, parts M, N, O, and P). This antibody recognizes a component of adherens junctions in the cell membranes of hypodermal, gut, and pharyngeal cells and has become a standard marker for studying cell morphology in these tissues during C. elegans development (9, 26). A large number of cell boundaries stain with MH27 throughout hs-elt-3-arrested (JM59 and JM60), hs-elt-1-arrested (JM54 and JM55), and hs-elt-2-arrested (JM57) embryos (Fig. 1B, parts N, O, and P). However, the morphology of cells outlined by MH27 is quite different in hs-elt-3- and hs-elt-1-arrested embryos compared to hs-elt-2-arrested embryos. The large, flat MH27-positive cells in the hs-elt-3- and hs-elt-1-arrested embryos are clearly reminiscent of hypodermal cells, particularly close to the surface of the arrested embryos (Fig. 1B, parts N and O). In contrast, the distribution of MH27 staining in hs-elt-2-arrested embryos shows small rings of fluorescence, quite unlike the cells of hypodermal morphology (Fig. 1B, part P).
To demonstrate that forced ectopic expression of elt-3 or elt-1 results in larger numbers of cells expressing hypodermal cell markers than normally occur in wild-type embryos, we used confocal sections to count total nuclei (4′,6′-diamidino-2-phenylindole [DAPI] stained), dpy-7::GFP-expressing nuclei, and elt-3::GFP-expressing nuclei. The counts are collected in Table 1 and show that there was an increase in the number of cells expressing the dpy-7::GFP and elt-3::GFP reporter genes relative to wild-type embryos and at the same time there was a reduction in the total number of nuclei. We examined a substantially greater number of heat-shocked embryos without making accurate cell counts, and the same conclusion was drawn, namely, that additional cells expressing hypodermal cell markers are formed in response to forced ectopic elt-3 and elt-1 expression.
TABLE 1.
Numbers of nuclei expressing dpy-7::GFP and elt-3::GFP reporter gene markers in embryos arrested by forced expression of elt-3 (JM60), elt-1(JM53), or elt-2 (JM57)a
| Embryo | Total no. of nuclei (DAPI stained) | dpy-7::GFP | ELT-3::GFP |
|---|---|---|---|
| Wild type | 558 | 71 | 51 |
| hs-elt-3 | 294 (21.9) | 88.1 (19.1) [118] | 63 (12.2) [82] |
| hs-elt-1 | 313.4 (27) | 103.6 (23.5) [139] | 76.8 (11.5) [92] |
| hs-elt-2 | ND | 2.5 (2.9) [7] | 1.5 (1.6) [4] |
Two-cell embryos were allowed to develop at 20°C for 1 h and then heat shocked at 33.5°C for 40 min. Accurate counts of nuclei were performed by reconstruction of a series of 1-μm optical sections taken through each embryo examined (see Materials and Methods). The mean numbers of nuclei (DAPI stained or dpy-7::GFP or elt-3::GFP positive) are shown (n = 6), with the SD in parentheses. The maximum number of nuclei counted in a single embryo is shown in brackets. We defined GFP-positive nuclei as those showing expression levels equal to or greater than wild-type levels; faintly fluorescent nuclei were treated as negative. Hence, the values represent the minimum numbers of cells expressing these markers. The number of nuclei in a wild-type embryo, as determined previously for dpy-7::GFP (12) and elt-3::GFP (13), is indicated for comparison. ND, not done.
We also found that the two hs-elt-1 transgenic lines (JM54 and JM55) consistently produce arrested embryos with a greater number of dpy-7::GFP- and elt-3::GFP-expressing cells than do the hs-elt-3 transgenic lines (JM58, JM59, and JM60) (Table 1 and data not shown), suggesting that ELT-1 is in some way more effective than ELT-3 in activating hypodermal marker expression. One possibility is that ELT-1 activates hypodermal gene expression in some lineages in which ELT-3 does not.
Forced expression of elt-3 or elt-1 in nonhypodermal precursor cells can activate hypodermal marker gene expression.
The above-described experiments have shown that forced ectopic expression of elt-3 or elt-1 results in additional cells expressing hypodermal cell markers. Although this result suggests that ELT-3 and ELT-1 are sufficient to activate a program of hypodermal cell differentiation, these additional hypodermis-like cells could arise in several ways. For example, hypodermal cells or lineages could undergo additional divisions or expression of hypodermal markers could be induced in the immediate lineal neighbors of hypodermal cells. Hence, we wished to determine whether at least some of the additional cells that express hypodermal markers in hs-elt-1 and hs-elt-3 embryos were derived from precursor cells that do not give rise to hypodermis cells in wild-type embryos. We approached this problem by ectopically expressing elt-3 and elt-1 in blastomeres isolated by laser ablation.
We first examined the effect of forced elt-3 or elt-1 expression in embryos following isolation of the P1 blastomere. The dpy-7::GFP reporter gene is expressed exclusively in hypodermal cells, and only 13 of these are derived from the P1 blastomere. Forced ectopic expression of elt-3 or elt-1 causes the isolated P1 blastomere to produce greater than 13 dpy-7::GFP-positive cells (Fig. 2B and D). A variety of controls were performed to rule out artifacts due to incomplete killing of the AB blastomere and/or nonspecific effects of heat shock (Fig. 2A, C, E, and F).
FIG. 2.
Activation of dpy-7::GFP and elt-3::GFP by forced expression of elt-3 or elt-1 in the isolated P1 blastomere (A to F) or in the isolated EMS blastomere (G to P). The P1 or EMS blastomere was isolated by laser ablation in JM60 (hs-elt-3), JM55 (hs-elt-1), and N2 embryos carrying chromosomally integrated dpy-7::GFP (A to L) and elt-3::GFP (M to P) reporter genes. One hour later, embryos were heat shocked at 33.5°C for 40 min and then allowed to recover for 16 to 20 h. For each experiment, control embryos were allowed to develop following laser ablation without the application of heat shock. Each panel shows a typical embryo, and the values below are the mean number of positive nuclei, the SD, the maximum number of nuclei counted in a single embryo, and the total number of embryos examined.
We next examined the effect of forced elt-3 or elt-1 expression in embryos following the isolation of the EMS blastomere. In the wild-type embryo, the isolated EMS blastomere does not give rise to any hypodermal cells (33) (Fig. 2K and L). However, following forced ectopic expression of elt-3 or elt-1, the isolated EMS blastomere produces a significant number of dpy-7::GFP-positive cells (Fig. 2G to J). We also tested the ability of ELT-3 and ELT-1 to activate the expression of a second hypodermal cell marker, elt-3::GFP, in the isolated EMS blastomere. In later wild-type embryos, this reporter is expressed in a small number of cells in addition to hypodermal cells, probably two of the pharyngeal-intestinal valve cells (vpi3 cells, both derived from the MS lineage) and five intestinal rectal valve cells and rectal epithelial cells (derived from the AB lineage) (13). As expected, the elt-3::GFP reporter gene is expressed in a maximum of two cells following isolation of the EMS blastomere by laser ablation (Fig. 2M and O). In contrast, forced ectopic expression of either elt-3 or elt-1 causes the isolated EMS blastomere to produce additional elt-3::GFP-positive cells (Fig. 2N and P).
Consistent with the experiments with intact embryos described earlier, forced ectopic expression of elt-1 induces both P1 (Fig. 2B and D) and EMS (Table 2) to produce a greater number of dpy-7::GFP-positive and elt-3::GFP-positive cells than does forced ectopic expression of elt-3.
TABLE 2.
Activation of the dpy-7::GFP reporter gene following EMS blastomere isolation in several independent transgenic lines containing chromosomally integrated hs-elt-3 or hs-elt-1 constructsa
| Strain | Mean no. of dpy-7::GFP-positive nuclei from isolated EMS (SD) [no. of embryos examined] |
|---|---|
| hs-elt-3 | |
| JM58 | 4.5 (2.9) [6] |
| JM59 | 6.1 (2.1) [19] |
| JM60 | 8.3 (3.5) [11] |
| hs-elt-1 | |
| JM54 | 13.8 (4.6) [9] |
| JM55 | 15.7 (3.9) [10] |
One hour after EMS isolation, embryos were heat shocked at 33.5°C for 40 min and then allowed to recover for 16 to 20 h. For each strain, control embryos were allowed to develop following laser ablation without the application of heat shock; none of these control embryos produced dpy-7::GFP-positive nuclei (data not shown).
Forced expression of elt-3 or elt-1 represses the expression of nonhypodermal cell markers.
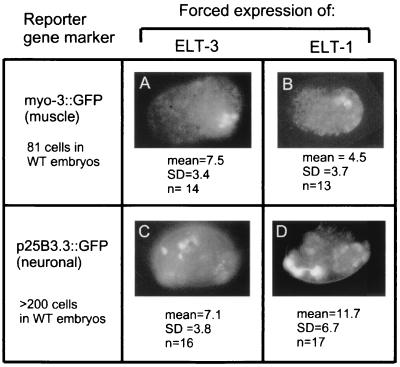
Does forced ectopic expression of elt-3 or elt-1 simply activate hypodermal marker gene expression in inappropriate cells, or does it lead to additional hypodermis like cells being formed as a result of cell fate transformation? One prediction of the latter model is that forced ectopic expression of elt-3 and elt-1, in addition to activating hypodermal marker gene expression, should concomitantly extinguish the expression of marker genes from nonhypodermal lineages (Fig. 3). myo-3::GFP is expressed in the 81 bodywall muscle cells of wild-type embryos (8), but myo-3::GFP is only expressed in a mean of 7.5 and 4.5 cells of embryos arrested by forced expression of elt-3 and elt-1, respectively (Fig. 3A and B). Similarly, the marker pF25B3.3::GFP is expressed in more than 200 neuronal cells in wild-type embryos (D. Pilgrim, personal communication) but is only expressed in a mean of 7.1 and 11.7 cells of embryos arrested by forced expression of elt-3 and elt-1, respectively (Fig. 3C and D). Hence, in contrast to the widespread expression of hypodermal markers, few cells in the arrested embryos express neuronal and muscle cell markers. Expression of gut cell markers (MH33 and gut granules) is likewise suppressed in hs-elt-3- and hs-elt-1-arrested embryos (10) (J. S. Gilleard, data not shown). Overall, our results are best interpreted in terms of a model in which forced ectopic expression of elt-3 or elt-1 results in additional cells being transformed into hypodermal cells.
FIG. 3.
Expression of body wall muscle marker myo-3::GFP and neuronal marker F25B3.3::GFP following forced ectopic elt-3 or elt-1 expression. Transgenes from strains ccls4215 (myo-3::GFP) and evls111 (F25B3.3::GFP) were crossed into strains JM60 (hs-elt-3) and JM53 (hs-elt-1). The doubly transgenic embryos were then subjected to a 40-min heat shock of 33.5°C at 1 h of development and examined 16 h later. Each panel shows a typical embryo, together with the mean number of GFP-positive nuclei observed and the total number of embryos examined (n). Panels A and B show the expression of the myo-3::GFP marker, and panels C and D show the F25B3.3::GFP marker in embryos following forced expression of either elt-3 (A and C) or elt-1 (B and D). WT, wild type.
ELT-3 and ELT-1 can only activate ectopic hypodermal marker gene expression during a restricted time period in early embryogenesis.
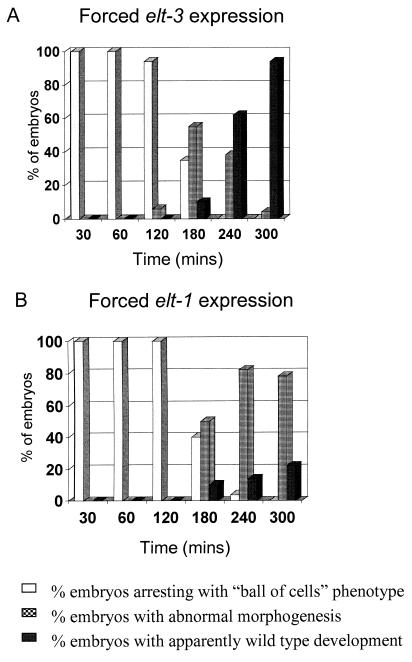
We investigated whether the ability of ELT-3 and ELT-1 to induce the formation of additional hypodermis-like cells was restricted to the time of embryogenesis when blastomeres were still pluripotent. Embryos were harvested at the two-cell stage, allowed to develop for defined periods of time at 20°C, and then heat shocked at 33.5°C for 40 min. Following heat shock, embryos were allowed to develop overnight at 20°C before being assayed for marker expression. As described above, forced expression of elt-3 or elt-1 during the first 2 h of development causes developmental arrest in essentially 100% of the embryos with a ball-of-cells phenotype (Fig. 4). Essentially all of these arrested embryos show widespread expression of the dpy-7::GFP, elt-3::GFP, LIN-26, and MH27 hypodermal markers (data not shown). This arrest phenotype is not seen when either elt-1 or elt-3 expression is induced later than 3 h beyond the two-cell stage of development (Fig. 4). Furthermore, accurate counts show that only embryos arrested with the ball-of-cells phenotype contain more cells expressing hypodermal markers than occur in wild-type embryos (data not shown). During the first 2 h of C. elegans embryogenesis, most blastomeres are pluripotent. However, inspection of the embryonic lineage (33) suggests that after 3 h of development, the majority of cells are committed to adopting a particular tissue or organ fate. Hence, we concluded that forced ectopic expression of elt-3 or elt-1 can induce the production of additional hypodermis-like cells only from pluripotent cells and not from cells that are already involved in alternative differentiation programs. Forced expression of either elt-1 or elt-3 later than 3 h does, however, produce a variety of morphological abnormalities in the embryos, with elt-1 being significantly more effective than elt-3 (Fig. 4 and data not shown).
FIG. 4.
Results of forced ectopic expression of (A) elt-3 (JM60) and (B) elt-1 (JM53) performed at different times of embryogenesis. The x axis shows the time (in minutes) elapsed between the isolation of two cell embryos and the beginning of a 40-min period of heat shock (at 33.5°C). Embryos were examined 16 h after the heat shock period. The y axis shows the percentage of embryos in each of three phenotypic categories: ball of cells, i.e., embryos that undergo developmental arrest without any visible signs of morphogenesis; abnormal morphogenesis, i.e., embryos in which morphogenesis proceeds but in which a variety of abnormalities (including “lumpy-dumpy” phenotypes and the presence of vacuoles) are visible in late embryos or hatched larvae; and embryos with apparently wild-type development.
ELT-3 can activate hypodermal gene expression in the absence of ELT-1 function.
If ELT-3 acts downstream of ELT-1 in the pathway that controls hypodermal differentiation, we would predict that ELT-3 could still activate hypodermal gene expression in the absence of ELT-1 function. The availability of the elt-1(zu180) mutant allowed us to test this prediction. Although Page et al. (22) have reported that elt-1(zu180) mutant embryos lack any cells with hypodermal morphology, the existence of some hypodermal cells in elt-1(zu180) mutant embryos is suggested by the presence of a small number of elt-3::GFP-positive cells (13). Indeed, we measured a mean of 11.9 (standard deviation [SD], 3.7; n = 31) dpy-7::GFP-positive cells (maximum, 16) in arrested embryos segregating from strain JG42 elt-1(zu180) unc-43(e408)lunc-24(e138) dpy-20(e1282) IV; ijls12. We do not know the lineal origin of this small number of hypodermal cells in elt-1(zu180) mutant embryos, but some of them may be the “minor” hypodermal cells of the head and tail in which elt-3 and dpy-7 are expressed (13) but elt-1 is not (22). However, the important conclusion with respect to the following experiment is that a maximum of 16 dpy-7::GFP-expressing cells are formed in elt-1(u180) mutant embryos.
To test if ELT-3 can activate hypodermal gene expression in the absence of ELT-1 function, we constructed strain JG48, with the genotype elt-1(zu180) unc-43(e408)lunc-24(e138) dpy-20(e1282) IV; cals10; ijls12. As expected, in the absence of heat shock, approximately 25% of JG48 embryos were arrested in development and all showed fewer than 17 dpy-7::GFP-expressing cells (Fig. 5A). In contrast, heat shock induction of elt-3 expression in JG48 embryos caused widespread dpy-7::GFP expression in almost all of the embryos examined, 25% of which were predicted to be elt-1(zu180) homozygotes (Fig. 5B). Indeed, comparison of heat-shocked JG48 embryos with heat-shocked JG10 embryos (genotype cals10 ijls12 but wild type for the elt-1 gene) revealed no differences either in the proportion of embryos with widespread dpy-7::GFP expression (Fig. 5B and C) or in the number of dpy-7::GFP-expressing cells in individual arrested embryos (data not shown). Thus, we concluded that forced expression of elt-3 can activate hypodermal gene expression in the absence of elt-1 function.
FIG. 5.
Forced expression of elt-3 in elt-1(zu180) mutant embryos. Each panel shows a typical arrested embryo and the number of embryos that were arrested with either fewer than 17, between 18 and 40, or greater than 40 GFP-positive nuclei, respectively.(A) Control JG48 elt-1(zu180) unc-43(e408)lunc-24(e138) dpy-20(e1282) IV; cals10; ijls12 embryos examined for dpy-7::GFP expression in the absence of heat shock. The photomicrograph shows two typical elt-1(zu180) embryos. The number of embryos arrested with fewer than 17 GFP-positive nuclei is shown; the remaining embryos, which hatched as L1s with the wild-type dpy-7 expression pattern, are shown in the >40 column.(B and C) JG48 elt-1(zu180) unc-43(e408)lunc-24(e138) dpy-20(e1282) IV; cals10; ijls12 and JG10 (cals10 ijls12) embryos examined for dpy-7::GFP expression after heat shock induction of elt-3 expression. Two-cell embryos were allowed to develop at room temperature for 1 h, heat shocked at 33.5°C for 40 min, and examined for GFP expression 16 to 20 h later. The photomicrographs show representative embryos following heat shock.
The elt-3 gene is not essential for hypodermal differentiation or for viability of C. elegans.
Having shown that ELT-3 is sufficient to activate a program of hypodermal differentiation, we next determined whether ELT-3 is necessary for this program. We used the method of imprecise excision of a Tc1 transposon (25, 38) to disrupt the elt-3 gene. Strain NW1122 (ev616::Tc1), isolated in the laboratory of J. Culotti, contains a Tc1 insertion 1,352 bp downstream of the elt-3 polyadenylation site (1,843 bp downstream of the T of the stop codon). We screened populations of worms from this strain by PCR and isolated a strain that contains a deletion of 2,181 bp, accompanied by a 6-bp GGAAAT insertion. This deletion removes the entire DNA binding domain (Fig. 6A). The strain was backcrossed 15 times against N2, resulting in strain JG1, which is homozygous for the deletion allele elt-3(vp1) as assessed by PCR and by genomic Southern blotting (Fig. 6B and data not shown). Homozygous elt-3(vp1) animals were morphologically indistinguishable from the wild type, had a normal brood size with ∼100% of the embryos hatching (at either 20 or 25°C), and had a normal rate of development at 20°C (Fig. 6F). The expression of hypodermal cell markers (LIN-26, dpy-7::GFP, and MH27) was also indistinguishable from that in the wild type (Fig. 6C, D, and E). At our request, Nemapharm (Axys Pharmaceuticals) has isolated a second deletion in the elt-3 gene using PCR screening of chemically mutagenized worms. Strain NS3239 is homozygous for elt-3(nr2088), a 666-bp deletion (with an insertion of 19 bp [CGTGGCCCCTTTTGCTAGG]) that extends from the middle of the first intron to a point inside the third exon. There are no identifiable 3′ splice acceptor sites downstream of the deletion that might allow variant splicing to maintain the reading frame, and thus, this deletion is likely to result in a truncated polypeptide lacking the DNA binding domain. elt-3(nr2088)-homozygous animals (after backcrossing three times against N2) are also viable and have a normal brood size, morphology, hatching frequency, development time, and expression of hypodermal markers (Fig. 6F and data not shown). Finally, injection of in vitro-synthesized double-stranded RNA corresponding to the full-length elt-3 transcript (elt-3 RNAi) (8) also failed to produce a visible phenotype. Hence, we concluded that the elt-3 gene is not essential for C. elegans viability or, within the limits of our phenotypic analysis, normal hypodermal differentiation and development.
In order to test the ability of ectopic elt-1 to activate hypodermal gene expression in the absence of ELT-3 function, we constructed strain JG49, with the genotype cals5; ijls12; elt-3(vp1). Heat shock induction of elt-1 expression in JG49 embryos (33.5°C for 40 min at 1 h after the two-cell stage) resulted in widespread expression of dpy-7::GFP at levels comparable to those obtained in the same experiment done with elt-3+ embryos (data not shown). In other words ELT-1 can activate hypodermal gene expression in the absence of ELT-3 function, a result consistent with the lack of phenotype of the elt-3 deletion mutants. Presumably, ectopic elt-1 expression activates hypodermal gene expression either directly or through genes redundant with elt-3.
Testing for functional redundancy between ELT-3 and other members of the C. elegans GATA transcription factor family.
The C. elegans genomic sequence is essentially complete (2) and reveals a total of 11 genes clearly identifiable as genes that encode GATA-type transcription factors. The expression of seven of these genes appears to be confined to the endoderm and/or mesoderm (10, 14, 37) (M. Maduro and J. L. Rothman, personal communication), and their products would not be expected to have embryonic functions that overlap those of ELT-3. The remaining two genes (in addition to elt-1 and elt-3) are elt-5 (F55A8.1) and elt-6 (F52C12.5). These form an apparent discistronic unit that is expressed throughout the ectoderm but at a particularly high level in lateral seam cells (K. Koh and J. L. Rothman, personal communication). These two genes appear to have overlapping (i.e., mutually redundant) functions that are critical to lateral seam cell development (Koh and Rothman, personal communication). We found that reduction of elt-5 gene function using RNAi in N2 worms gave a highly penetrant larval arrest phenotype but that reduction of elt-6 function by RNAi had no visible effect (in agreement with the unpublished results of Koh and Rothman; elt-5 is the upstream gene of the apparent dicistron, and RNAi of elt-5 appears to interfere with the expression of both elt-5 and elt-6). The result from RNAi with these two genes, performed either separately or combined, is indistinguishable whether performed in N2 or JG1 elt-3(vp1) worms (data not shown). In other words, RNAi experiments provide no evidence for redundancy among elt-3, elt-5, and elt-6. Because RNAi is not effective for all zygotically expressed genes, confirmation of these results must await the production of elt-5 and elt-6 deletion mutants.
Because elt-1 mutant embryos arrest development before elt-3 is first expressed, neither RNAi nor the available loss-of-function mutants can be used to investigate possible functional redundancy between elt-3 and elt-1. As an alternative, we have tested the effect of reducing the copy number of the elt-1 wild-type allele in an elt-3 homozygous mutant background. The progeny of seven hermaphrodites with the genotype elt-1(zu180) unc-43(e408)/+; elt-3(vp1)/elt-3(vp1) were examined; 24.3% of the embryos were arrested (464 arrested embryos from 1,909 total progeny examined), but the remaining viable progeny showed no visible abnormalities. Since two-thirds of the viable progeny are predicted to be heterozygous for elt-1(zu180), we concluded that reducing the copy number of the wild-type elt-1 allele in elt-3 null mutant animals does not produce a detectable phenotype. Further investigation of possible redundancy between elt-1 and elt-3 must await the isolation of a conditional elt-1 mutant or the development of inducible inhibition.
DISCUSSION
The role of ELT-3 and ELT-1 in hypodermal development.
The GATA transcription factor ELT-1 has been shown to be required for hypodermal precursor cells to produce hypodermal cell fates (22). We have previously described a second hypodermal GATA transcription factor, ELT-3 (13). elt-3 is first expressed later than elt-1, immediately before the onset of hypodermal differentiation. In the present paper, we have shown that either elt-1 or elt-3, when force expressed in early blastomeres, is sufficient to activate the expression of a collection of hypodermal markers, even in blastomeres that would ordinarily never produce hypodermal cells in wild-type embryos. This set of hypodermal markers comprises two transcription factors, a cuticle collagen gene and an adherens junction component. Forced ectopic ELT-1 and ELT-3 expression also represses the expression of muscle, neuron, and endoderm markers. Overall, the expression of such a range of functionally unrelated markers, together with the repression of nonhypodermal marker genes, suggests that either ELT-1 or ELT-3 is able to activate a significant portion of the hypodermal differentiation program in nonhypodermal cells. This may actually constitute a change in cell fate.
ELT-3 can activate hypodermal gene expression in the absence of a functional elt-1 gene, showing that ELT-3 does not activate hypodermal differentiation simply by activating elt-1 expression. These results, within the limitations of ectopic-expression experiments, are also consistent with the idea that ELT-3 acts downstream of ELT-1 or in an independent pathway. Since ELT-1 is essential for the formation of the majority of hypodermal cells, the existence of such a pathway seems unlikely. It is not yet known whether ELT-1 directly activates elt-3 expression, but this is a distinct possibility; there are nine WGATAR consensus motifs within the 1.3 kb upstream of the elt-3 gene. Indeed, one particular 240-bp region contains seven of these sites, six of which have the sequence TGATAA. This sequence is possibly a preferred ELT-1 binding site, since an A residue immediately 3′ to the GATA core motif was found to be essential for ELT-1 binding as assayed by activation of reporter gene expression in yeast (30).
We have shown that ELT-3 function is not essential, either for hypodermal differentiation or for C. elegans viability, suggesting that at least one other gene acts redundantly with elt-3 downstream of elt-1. This suggestion is consistent with the results of a deficiency screen (covering approximately 75% of the genome) in which a number of C. elegans loci were identified that, when deleted, resulted in morphological abnormalities of the hypodermis (5). However, only one deficiency (covering the elt-1 locus) resulted in a specific failure in hypodermal cell formation. Other deficiencies that produced a significant loss of hypodermal cells also resulted in a low total embryonic cell number, suggesting a general role in cell proliferation rather than a specific role in hypodermal differentiation. Furthermore, a number of forward genetic screens have failed to identify mutations (other than elt-1) that result in a specific reduction in hypodermal cell number (I. L. Johnstone and M. Labouesse, personal communications). Although there are classes of genes that might have been missed in such screens, e.g., genes acting maternally or genes with additional early embryonic functions, the simplest interpretation is that the core program of hypodermal cell differentiation is controlled in a redundant manner. The identification of elt-3 as a gene that is sufficient but not essential for activation of the hypodermal differentiation program supports this conclusion.
Two other GATA factors (in addition to elt-1 and elt-3) are known to be expressed in ectodermal tissues, elt-5 and elt-6 (Koh and Rothman, personal communication). However, RNAi with these genes in the elt-3 mutant background did not unveil any enhanced phenotype that would indicate functional overlap with elt-3. This is not entirely surprising, since the predominant function of the genes for these two factors, as determined by RNAi (Koh and Rothman, personal communication), is in lateral seam cells, a specialized subset of hypodermal cells in which elt-3 is not expressed.
In spite of the fact that deletion of elt-3 causes no apparent phenotype, it must be recalled that a gene highly similar to elt-3 (100% amino acid identity within the predicted DNA binding domain) exists in the related nematode Caenorhabditis briggsae, suggesting that elt-3 does indeed play an evolutionarily significant role (13).
General principles of tissue and organ development.
The results of our experiments with elt-1 and elt-3 are generally consistent with the framework of tissue and organ development described by Labouesse and Mango (18), who have suggested that genes involved in controlling the development of organs and tissues in C. elegans can be divided into organ and tissue identity genes and organ and tissue differentiation genes. An organ and tissue identity gene is defined as a gene that acts before terminal differentiation in precursor cells to specify the fate of cells that will comprise a particular organ or tissue. In contrast, a differentiation gene activates the process of differentiation itself and controls, either directly or indirectly, the expression of terminal differentiation markers. Labouesse and Mango (18) defined tissue and organ identity genes by three characteristics (see also reference 15). First, absence of the gene leads to the loss of a tissue due to a cell fate transformation. Second, the gene is able to activate the tissue developmental program in naive blastomeres. Third, the gene is expressed early in precursor cells, before the onset of terminal differentiation. The first and third of these characteristics have already been demonstrated for elt-1 (22). Our results now show that elt-1 can indeed activate a program of hypodermal cell differentiation in naive blastomeres.
Our experiments also show that elt-3 broadly fits the definition of a tissue differentiation gene, as defined by the following three characteristics. First, mutations in differentiation genes, unlike those in identity genes, do not prevent the formation of an organ or tissue. Second, ectopic expression of differentiation genes leads to ectopic expression of appropriate terminal differentiation markers. Third, expression begins after expression of the identity gene (in this case, elt-1) but before terminal differentiation. elt-3 fulfills all of these criteria.
Although the distinction between identity and differentiation genes is useful as a general framework, it is likely to be an oversimplification. Our results suggest that there is a large degree of functional overlap between elt-1 (identity gene) and elt-3 (differentiation gene), in that they produce similar effects when force expressed in naive blastomeres. We cannot distinguish between activation of a tissue developmental program and activation of appropriate terminal differentiation markers because the markers are generally the same. We will be able to make such a distinction only when we know the direct downstream targets of ELT-1 and ELT-3.
Our results have also revealed a marked difference in the effectiveness with which the endodermal GATA factors and the hypodermal GATA factors induce marker gene expression in naive blastomeres. Forced expression of the endodermal GATA factors end-1 and elt-2 causes essentially 100% of the cells in the early embryo to express endodermal markers (10, 36). In contrast, forced expression of elt-1 or elt-3 causes a maximum of 30 to 40% of embryonic cells to express hypodermal markers. The explanation for this may lie in differences between the embryonic origins of hypodermal and endodermal cells. Endoderm is a simple clonal lineage; end-1 and elt-2 are expressed early in the lineage, and perhaps they are able to issue a simple “become endoderm” command to all other embryonic blastomeres. In contrast, hypodermis is derived from several rather complex lineages and elt-1, in particular, is expressed in hypodermal precursor cells at a time when they will go on to produce a variety of other cell types in addition to hypodermis. Perhaps elt-1 (and elt-3) is not able to issue a simple “become hypodermis” command but must operate in a context of other factors that make hypodermal cell lineages distinct from nonhypodermal sister lineages such as nerves or muscles. Perhaps only those cells in the embryo that contain these additional factors are able to express hypodermal markers in response to ectopic hypodermal GATA factors.
Nematode GATA factors and redundancy.
We have found that the C. elegans GATA factor ELT-3 is not essential for either viability or, within the limits of our phenotypic analysis, normal development. There are now null mutations available for 3 of the 11 known C. elegans GATA factors in addition to the elt-3 deletions described here. Both elt-1 and elt-2 are essential genes involved in hypodermal and endodermal development, respectively (10, 22). A chromosomal deletion that removes the endodermal GATA factor gene end-1, along with a considerable number of other genes (or the elimination of end-1 together with a second GATA factor gene, end-3), results in failure of endoderm formation (37). However, a deletion that removes only the end-1 gene appears not to affect endoderm specification (Rothman, personal communication). RNAi experiments conducted on the remaining seven genes by a number of laboratories suggest that these may have redundant functions (K. Koh, M. Maduro, and J. L. Rothman, personal communication; J. S. Gilleard, T. Fukushige, and J. D. McGhee, unpublished data). Although confirmation of this statement must await deletion or mutation of the remaining family members, there is growing evidence that the majority of the C. elegans GATA transcription factors are functionally redundant. This is in marked contrast to the vertebrate GATA factors, all of which (GATA-1 to GATA-6) have been shown to have essential functions by gene knockout experiments with-mice or, in the case of GATA-5, zebra fish (16, 20, 23, 24, 27, 34). It is also noteworthy that in spite of the greater complexity of vertebrates, they appear to have only half of the number of GATA factors present in C. elegans. Although it is possible that additional vertebrate GATA factors await to be discovered, the recently completed Drosophila melanogaster genome sequence (1, 28) predicts only four GATA factors in the fly. Hence, the GATA factors represent an example of a gene family that is larger and more redundant in nematodes than in apparently more complex organisms.
ACKNOWLEDGMENTS
We thank J. Culotti (Toronto, Ontario, Canada) for strains NW1122 and NW1129, Axys Pharmaceuticals (San Francisco, Calif.) for strain NS3239, Andrew Fire (Baltimore, Md.) for providing convenient reporter vectors, Barbara Page (Seattle, Wash.) for strain J1129, Michel Labouesse (Strasbourg, France) for the LIN-26 antibody, R. Waterson (St. Louis, Mo.) for the MH27 antibody, Iain Johnstone (Glasgow, United Kingdom) for strain IA105, and Kyunghee Koh and Joel Rothman (Santa Barbara, Calif.) for generously providing information prior to publication. Particular thanks are due to Tetsunari Fukushige (Calgary, Alberta, Canada) for providing strains JM53 through JM60. Several strains in this work were supplied by the Caenorhabditis Genetics Centre, which is funded by the NIH Centre for Research Resources.
This work was funded by the Wellcome Trust by virtue of a Wellcome Trust Prize Traveling Fellowship (J.G.) and by the Medical Research Council of Canada (J.D.M.). J.D.M. is a Medical Scientist of the Alberta Heritage Foundation for Medical Research.
REFERENCES
- 1.Adams M D, Celniker S E, Holt R A, Evans C A, Gocayne J D, Amanatides P G, Scherer S E, Li P W, Hoskins R A, Galle R F, George R A, Lewis S E, Richards S, Ashburner M, Henderson S N, Sutton G G, Wortman J R, Yandell M D, Zhang Q, Chen L X, Brandon R C, Rogers Y H, Blazej R G, Champe M, Pfeiffer B D, Wan K H, Doyle C, Baxter E G, Helt G, Nelson C R, Gabor Miklos G L, Abril J F, Agbayani A, An H J, Andrews-Pfannkoch C, Baldwin D, Ballew R M, Basu A, Baxendale J, Bayraktaroglu L, Beasley E M, Beeson K Y, Benos P V, Berman B P, Bhandari D, Bolshakov S, Borkova D, Botchan M R, Bouck J, Brokstein P, Brottier P, Burtis K C, Busam D A, Butler H, Cadieu E, Center A, Chandra I, Cherry J M, Cawley S, Dahlke C, Davenport L B, Davies P, de Pablos B, Delcher A, Deng Z, Mays A D, Dew I, Dietz S M, Dodson K, Doup L E, Downes M, Dugan-Rocha S, Dunkov B C, Dunn P, Durbin K J, Evangelista C C, Ferraz C, Ferriera S, Fleischmann W, Fosler C, Gabrielian A E, Garg N S, Gelbart W M, Glasser K, Glodek A, Gong F, Gorrell J H, Gu Z, Guan P, Harris M, Harris N L, Harvey D, Heiman T J, Hernandez J R, Houck J, Hostin D, Houston K A, Howland T J, Wei M H, Ibegwam C, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. Genome sequence of the nematode C. elegans: a platform for investigating biology. The C. elegans Sequencing Consortium. Science. 1998;282:2012–2018. doi: 10.1126/science.282.5396.2012. [DOI] [PubMed] [Google Scholar]
- 3.Bowerman B. Maternal control of pattern formation in early Caenorhabditis elegans embryos. Curr Top Dev Biol. 1998;39:73–117. doi: 10.1016/s0070-2153(08)60453-6. [DOI] [PubMed] [Google Scholar]
- 4.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chanal P, Labouesse M. A screen for genetic loci required for hypodermal cell and glial-like cell development during Caenorhabditis elegans embryogenesis. Genetics. 1997;146:207–226. doi: 10.1093/genetics/146.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charron F, Nemer M. GATA transcription factors and cardiac development. Semin Cell Dev Biol. 1999;10:85–91. doi: 10.1006/scdb.1998.0281. [DOI] [PubMed] [Google Scholar]
- 7.Edgar L G, McGhee J D. Embryonic expression of a gut-specific esterase in Caenorhabditis elegans. Dev Biol. 1986;114:109–118. doi: 10.1016/0012-1606(86)90387-8. [DOI] [PubMed] [Google Scholar]
- 8.Fire A, Xu S, Montgomery M K, Kostas S A, Driver S E, Mello C C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 9.Francis G R, Waterston R H. Muscle organization in Caenorhabditis elegans: localization of proteins implicated in thin filament attachment and I-band organization. J Cell Biol. 1985;101:1532–1549. doi: 10.1083/jcb.101.4.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukushige T, Hawkins M G, McGhee J D. The GATA-factor elt-2 is essential for formation of the Caenorhabditis elegans intestine. Dev Biol. 1998;198:286–302. [PubMed] [Google Scholar]
- 11.Fukushige T, Hendzel M J, Bazett-Jones D P, McGhee J D. Direct visualization of the elt-2 gut-specific GATA factor binding to a target promoter inside the living Caenorhabditis elegans embryo. Proc Natl Acad Sci USA. 1999;96:11883–11888. doi: 10.1073/pnas.96.21.11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilleard J S, Barry J D, Johnstone I L. cis regulatory requirements for hypodermal cell-specific expression of the Caenorhabditis elegans cuticle collagen gene dpy-7. Mol Cell Biol. 1997;17:2301–2311. doi: 10.1128/mcb.17.4.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilleard J S, Shafi Y, Barry J D, McGhee J D. ELT-3: a Caenorhabditis elegans GATA factor expressed in the embryonic epidermis during morphogenesis. Dev Biol. 1999;208:265–280. doi: 10.1006/dbio.1999.9202. [DOI] [PubMed] [Google Scholar]
- 14.Hawkins M G, McGhee J D. elt-2, a second GATA factor from the nematode Caenorhabditis elegans. J Biol Chem. 1995;270:14666–14671. doi: 10.1074/jbc.270.24.14666. [DOI] [PubMed] [Google Scholar]
- 15.Kalb J M, Lau K K, Goszczynski B, Fukushige T, Moons D, Okkema P G, McGhee J D. pha-4 is Ce-fkh-1, a fork head/HNF-3alpha, beta, gamma homolog that functions in organogenesis of the C. elegans pharynx. Development. 1998;125:2171–2180. doi: 10.1242/dev.125.12.2171. [DOI] [PubMed] [Google Scholar]
- 16.Koutsourakis M, Langeveld A, Patient R, Beddington R, Grosveld F. The transcription factor GATA-6 is essential for early extraembryonic development. Development. 1999;126(9):723–732. doi: 10.1242/dev.126.9.723. . (Corrected and republished with original paging; originally, Development 126(4):723–732, 1999.) [DOI] [PubMed] [Google Scholar]
- 17.Labouesse M, Hartwieg E, Horvitz H R. The Caenorhabditis elegans LIN-26 protein is required to specify and/or maintain all non-neuronal ectodermal cell fates. Development. 1996;122:2579–2588. doi: 10.1242/dev.122.9.2579. [DOI] [PubMed] [Google Scholar]
- 18.Labouesse M, Mango S E. Patterning the C. elegans embryo: moving beyond the cell lineage. Trends Genet. 1999;15:307–313. doi: 10.1016/s0168-9525(99)01750-3. [DOI] [PubMed] [Google Scholar]
- 19.Labouesse M, Sookhareea S, Horvitz H R. The Caenorhabditis elegans gene lin-26 is required to specify the fates of hypodermal cells and encodes a presumptive zinc-finger transcription factor. Development. 1994;120:2359–2368. doi: 10.1242/dev.120.9.2359. [DOI] [PubMed] [Google Scholar]
- 20.Molkentin J D, Lin Q, Duncan S A, Olson E N. Requirement of the transcription factor GATA-4 for heart tube formation and ventral morphogenesis. Genes Dev. 1997;11:1061–1072. doi: 10.1101/gad.11.8.1061. [DOI] [PubMed] [Google Scholar]
- 21.Orkin S H. Hematopoiesis: how does it happen? Curr Orkin Cell Biol. 1995;7:870–877. doi: 10.1016/0955-0674(95)80072-7. [DOI] [PubMed] [Google Scholar]
- 22.Page B D, Zhang W, Steward K, Blumenthal T, Priess J R. ELT-1, a GATA-like transcription factor, is required for epidermal cell fates in Caenorhabditis elegans embryos. Genes Dev. 1997;11:1651–1661. doi: 10.1101/gad.11.13.1651. [DOI] [PubMed] [Google Scholar]
- 23.Pandolfi P P, Roth M E, Karis A, Leonard M W, Dzierzak E, Grosveld F G, Engel J D, Lindenbaum M H. Targeted disruption of the GATA-3 gene causes severe abnormalities in the nervous system and in fetal liver haematopoiesis. Nat Genet. 1995;11:40–44. doi: 10.1038/ng0995-40. [DOI] [PubMed] [Google Scholar]
- 24.Pevny L, Simon M C, Robertson E, Klein W H, Tsai S F, D'Agati V, Orkin S H, Costantini F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991;349:257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- 25.Plasterk R H A. Reverse genetics: from gene sequence to mutant worm. In: Epstein H F, Shakes D C, editors. Caenorhabditis elegans: modern biological analysis of an organism. Vol. 48. New York, N.Y: Academic Press, Inc.; 1995. pp. 59–80. [DOI] [PubMed] [Google Scholar]
- 26.Podbilewicz B, White J G. Cell fusions in the developing epithelial of C. elegans. Dev Biol. 1994;161:408–424. doi: 10.1006/dbio.1994.1041. [DOI] [PubMed] [Google Scholar]
- 27.Reiter J F, Alexander J, Rodaway A, Yelon D, Patient R, Holder N, Stainier D Y. GATA-5 is required for the development of the heart and endoderm in zebrafish. Genes Dev. 1999;13:2983–2995. doi: 10.1101/gad.13.22.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubin G M, Yandell M D, Wortman J R, Gabor Miklos G L, Nelson C R, Hariharan I K, Fortini M E, Li P W, Apweiler R, Fleischmann W, Cherry J M, Henikoff S, Skupski M P, Misra S, Ashburner M, Birney E, Boguski M S, Brody T, Brokstein P, Celniker S E, Chervitz S A, Coates D, Cravchik A, Gabrielian A, Galle R F, Gelbart W M, George R A, Goldstein L S, Gong F, Guan P, Harris N L, Hay B A, Hoskins R A, Li J, Li Z, Hynes R O, Jones S J, Kuehl P M, Lemaitre B, Littleton J T, Morrison D K, Mungall C, O'Farrell P H, Pickeral O K, Shue C, Vosshall L B, Zhang J, Zhao Q, Zheng X H, Zhong F, Zhong W, Gibbs R, Venter J C, Adams M D, Lewis S. Comparative genomics of the eukaryotes. Science. 2000;287:2204–2215. doi: 10.1126/science.287.5461.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schnabel R P., Jr . Specification of cell fates in the early embryo. In: Riddle D L, Blumenthal T, Meyer B J, Priess J R, editors. C. elegans II. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 361–382. [PubMed] [Google Scholar]
- 30.Shim Y H, Bonner J J, Blumenthal T. Activity of a C. elegans GATA transcription factor, ELT-1, expressed in yeast. J Mol Biol. 1995;253:665–676. doi: 10.1006/jmbi.1995.0581. [DOI] [PubMed] [Google Scholar]
- 31.Spieth J, Shim Y H, Lea K, Conrad R, Blumenthal T. ELT-1, an embryonically expressed Caenorhabditis elegans gene homologous to the GATA transcription factor family. Mol Cell Biol. 1991;11:4651–4659. doi: 10.1128/mcb.11.9.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stringham E G, Dixon D K, Jones D, Candido E P. Temporal and spatial expression patterns of the small heat shock (hsp16) genes in transgenic Caenorhabditis elegans. Mol Biol Cell. 1992;3:221–233. doi: 10.1091/mbc.3.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sulston J E, Schierenberg E, White J G, Thomson J N. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 34.Tsai F Y, Keller G, Kuo F C, Weiss M, Chen J, Rosenblatt M, Alt F W, Orkin S H. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371:221–226. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- 35.Williams B D. Genetic mapping with polymorphic sequence tagged sites. In: Epstein H F, Shakes D C, editors. Caenorhabditis elegans: modern biological analysis of an organism. Vol. 48. New York, N.Y: Academic Press, Inc.; 1995. pp. 81–96. [DOI] [PubMed] [Google Scholar]
- 36.Zhu J, Fukushige T, McGhee J D, Rothman J H. Reprogramming of early embryonic blastomeres into endodermal progenitors by a Caenorhabditis elegans GATA factor. Genes Dev. 1998;12:3809–3814. doi: 10.1101/gad.12.24.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu J, Hill R J, Heid P J, Fukuyama M, Sugimoto A, Priess J R, Rothman J H. end-1 encodes an apparent GATA factor that specifies the endoderm precursor in Caenorhabditis elegans embryos. Genes Dev. 1997;11:2883–2896. doi: 10.1101/gad.11.21.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zwaal R R, Broeks A, van Meurs J, Groenen J T, Plasterk R H. Target-selected gene inactivation in Caenorhabditis elegans by using a frozen transposon insertion mutant bank. Proc Natl Acad Sci USA. 1993;90:7431–7435. doi: 10.1073/pnas.90.16.7431. [DOI] [PMC free article] [PubMed] [Google Scholar]