Abstract
Approximately 50% of metastatic tumors contain Ras mutations. Ras proteins can activate at least three downstream signaling cascades mediated by the Raf–MEK–extracellular signal-regulated kinase family, phosphatidylinositol-3 (PI3) kinase, and Ral-specific guanine nucleotide exchange factors (RalGEFs). Here we investigated the contribution of RalGEF and ERK activation to the development of experimental metastasis in vivo and associated invasive properties in vitro. Each pathway contributes distinct properties to the metastatic phenotype. Following lateral tail vein injection, 3T3 cells transformed by constitutively active Raf or MEK produced lung metastasis that displayed circumscribed, noninfiltrating borders. In contrast, 3T3 cells transformed by Ras(12V,37G), a Ras effector mutant that activates RalGEF but not Raf or P13 kinase, formed aggressive, infiltrative metastasis. Dominant negative RalB inhibited Ras(12V,37G)-activated invasion and metastasis, demonstrating the necessity of the RalGEF pathway for a fully transformed phenotype. Moreover, 3T3 cells constitutively expressing a membrane-associated form of RalGEF (RalGDS-CAAX) formed invasive tumors as well, demonstrating that activation of a RalGEF pathway is sufficient to initiate the invasive phenotype. Despite the fact that Ras(12V,37G) expression does not elevate ERK activity, inhibition of this kinase by a conditionally expressed ERK phosphatase demonstrated that ERK activity was necessary for Ras(12V,37G)-transformed cells to express matrix-degrading activity in vitro and tissue invasiveness in vivo. Therefore, these experiments have revealed a hitherto-unknown but essential interaction of the RalGEF and ERK pathways to produce a malignant phenotype. The generality of the role of the RalGEF pathway in metastasis is supported by the finding that Ras(12V,37G) increased the invasiveness of epithelial cells as well as fibroblasts.
The signaling pathways that regulate progression and acquisition of the metastatic phenotype are mostly undefined. Transfected Ras oncogenes have been shown to induce metastatic properties in some cells, and activating mutations in Ras genes are commonly found in a variety of human tumors (4). In addition, many of the consequences of Ras expression have been detected in cells that become metastatic in the apparent absence of an altered Ras gene, suggesting that there are common biochemical changes that can lead to metastasis as a result of multiple signals mediating such changes. In its GTP-bound state, Ras activates multiple signaling pathways by interacting with distinct downstream effectors (40). Ras mutants containing point mutations in the Ras effector domain separate the ability of Ras to interact with its various targets (40). The most thoroughly characterized signaling pathways are those initiated by Ras binding to Raf protein kinases, type 1 phosphatidylinositol-3 (PI3) kinases, or Ral-specific guanine nucleotide exchange factors (RalGEFs) (16). Here, we investigate which Ras-mediated signaling pathways contribute to hematogenous metastasis, a complex process that requires cellular migration through blood vessels and tissue followed by clonal growth from a single cell.
Active Ras at the plasma membrane binds to and promotes activation of Raf, which phosphorylates and consequently activates MEK1 and MEK2, which in turn phosphorylate and activate extracellular signal-regulated kinases 1 and 2 (ERK1 and 2) (25). The ERK kinases are the only known substrates of MEK1 and -2. A substantial proportion of phosphorylated ERK translocates to the nucleus, where it functions to modify the activity of transcription factors, thereby regulating genetic programming (26). ERK activation has been found to be involved in proliferation and/or differentiation functions in multiple cellular systems.
Active Ras also binds to and activates PI3 kinases, which are lipid kinases that phosphorylate phosphoinositides at position 3 of the inositol ring, generating the second messengers PtdIns-3,4-P2 and PtdIns-3,4–5-P3 (36). Several effectors which have diverse effects on cellular physiology and function downstream of PI3 kinase have been identified, including the AKT kinase, p70-S6 kinase, certain isoforms of protein kinase C, and RacGEFs (8, 22).
RalGEFs, including Ral-GDS, RGL1, RGL2, and Rlf, interact with and are activated by Ras at the plasma membrane, leading to the formation of the GTP-bound state of the Ral-family GTPases (42). The activated Ras effector mutant Ras(12V,37G) stimulates endogenous RalGEFs but does not activate Raf or PI3 kinase (41). Ras-independent, calcium-dependent Ral activation also has been described (13, 44, 45). A few potential downstream effectors of the Ral GTPases have been identified, although their physiological significance is as yet unclear. In their GTP-bound state, Ral proteins bind RalBP1, a putative GTPase-activating protein for CDC42 and Rac GTPases (3). In addition, RalA constitutively binds phospholipase D (PLD) and enhances PLD activation by the Arf GTPase, implicating Ral in a scaffolding or targeting role in PLD activation (3, 23). RalA binds filamin in a GTP-dependent manner, leading to the induction of filopodia (30).
The physiological consequences of RalGEF activation in cells are outstanding issues to be resolved. One end point of the Ral pathway appears to be a transcriptional response, as ectopic expression of activated forms of RalGEFs stimulates transcription from the c-fos serum response element (31, 43), the cyclin D1 promoter (10), and the TATA-binding protein promoter (15). Several studies have contributed to an emerging picture implicating RalGEF activation in cellular transformation of rodent fibroblasts. Cells expressing constitutively active Rlf gain the ability to proliferate in low serum (43), and Ras(12V,37G)-expressing cells show enhanced growth rates and saturation densities and acquire an ability to form colonies in soft agar (18). Ras(12V,37G)-transformed cells form subcutaneous tumors in nude mice (18, 39). Importantly, a dominant negative Ral allele that is thought to act by sequestering RalGEF blocks experimental metastasis induced by v-src or v-ras (1), while another study failed to find evidence for a role for RalGEF in metastasis (39). Here, we investigated the contribution of two Ras-coupled signaling pathways, ERK and RalGEF, in the development of the metastatic and invasive phenotype. We used a tetracycline-inducible ERK phosphatase, PAC1, as a tool to manipulate ERK activity in vivo. PAC1 is one member of a family of phosphotyrosine/phosphothreonine phosphatases specific for the inactivation of mitogen-activated protein (MAP) kinases (5). PAC1, one of the most specific nucleus-localized MAP kinase phosphatases, preferentially inactivates ERK and has no apparent activity against JNK (5, 34).
We show here that activation of the RalGEF pathway as compared to activation of the ERK pathway leads to histologically distinctive types of metastasis. Of interest and unexpectedly, RalGEF-dependent transformation led to highly invasive metastasis. Surprisingly, we found that transformation mediated by elevated RalGEF activity required some ERK activity for the development of an infiltrative invasive phenotype even though RalGEF activation itself does not stimulate this kinase.
MATERIALS AND METHODS
Production of cell lines.
Cells inducible for PAC1 using a TET OFF system (11) were produced by cotransfecting NIH 3T3 cells with a hygromycin-selectable expression vector encoding a transactivator-tetracycline repressor fusion protein and with a G418-selectable plasmid containing the tetracycline operator linked to the PAC1 gene. Individual clones were selected using hygromycin (300 μg/ml) and Geneticin (400 μg/ml) and then screened by immunofluorescence for uniform and moderate expression of PAC1 in the absence of tetracycline. Clones exhibited optimal PAC1 levels 4 to 10 days following the withdrawal of tetracycline used at 1 μg/ml in the culture media.
Transformed cell lines containing a tetracycline-inducible PAC1 were made by infecting cells from clone K2F6 with various transforming retroviruses. Bosc 23 cells, plated on 60-mm tissue culture dishes at a concentration of 2.5 × 106 cells per plate, were transfected using a previously described procedure (32). MEK(218D,222D) (14), RafΔN (38), H-Ras(12V), H-Ras(12V,37G) and H-Ras(12V,40C) (40) were expressed from the pZIPneo and LC7ΔSX vectors. All subcloned inserts were checked by sequence analysis. Overexpression of H-Ras was confirmed by immunoblot analysis using anti-H-Ras monoclonal antibody (Transduction Labs). Alternatively, RalGEF-transformed lines were analyzed following cotransfection of K2F6 cells with a 5:1 ratio of pCIneo-RalGDS-CAAX (33) and pBabepuro followed by selection in media containing puromycin (10 μg/ml). Levels of RalGDS protein were determined by immunoblot analysis using polyclonal anti-RalGDS antibody (Santa Cruz Biotechnology).
K2F6 cells transformed with Ras(12V,37G) retroviruses were further modified to express hemagglutinin-tagged ERK2 or ERK2(D319N) by transfection and subsequent selection. Levels of transfected ERK were determined by Western blots with antihemagglutin antibodies. Isolated clones as well as polyclonal populations were analyzed for metastatic potential.
NIH 3T3 cells were transfected with pBabepuro vector, pBabepuro encoding either RalB(28N) or RalB(23V), or a 5:1 ratio of pRK5-RalB(39L) and pZipNeo using Lipofectamine Plus (Life Technologies). Transfected cells were selected in growth medium containing either G418 or puromycin (Sigma), and individual colonies were isolated using cloning rings. Ras(12V,37G) or MEK(218D,222D) was introduced, by retroviral infection, into cells transfected with empty vector or RalB(28N). Expression of RalB protein and levels of activated ERK were determined by immunoblot analysis using polyclonal anti-RalB antibody (Transduction Labs) and anti-phospho-ERK (Santa Cruz Biotechnology), respectively.
Detection of PAC1 expression by immunoprecipitation and Western blotting.
Cells were plated onto 100-mm tissue culture dishes (2 × 106 cells per plate) and grown overnight at 37°C in a 5% CO2 incubator. Cells were then washed with cold phosphate-buffered saline and harvested into 1 ml of lysis buffer (25 mM HEPES [pH 7.5], 10% glycerol, 1% Triton X-100, 150 mM NaCl, 10 μg each of leupeptin and aprotinin per ml, and 1 mM phenylmethylsulfonyl fluoride). Lysates were centrifuged at 20,000 × g for 20 min at 4°C. Soluble extracts, normalized for protein concentration, were precleared on protein G agarose and then immunoprecipitated with anti-PAC1 monoclonal antibody P9D10 (34) and protein G agarose (Life Technologies) at 4°C with rotation for 4 h. Washed immunoprecipitates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analysis with rabbit anti-PAC1 peptide (residues 279 to 291) antibody (34) and Supersignal chemiluminescent reagent (Pierce).
In vitro kinase assay.
Soluble lysates were prepared from tetracycline-inducible cells and precleared as described for immunoprecipitation of PAC1, except that phosphatase inhibitors (25 mM β-glycerophosphate, 1 mM vanadate, and 10 mM NaF) were also added to the lysis buffer. ERK2 was immunoprecipitated with polyclonal anti-ERK2 antibody (UB1) and protein G agarose (Life Technologies) for 2 h at 4°C. The immunocomplex kinase assay was performed as described previously (5) using myelin basic protein (1 μg) as the substrate.
Reporter assays.
The effect of PAC1 on Elk1-, Ets2-mediated transcription of a luciferase reporter gene was determined using a transient-transfection assay. Cells were cotransfected with 0.50 μg of an expression vector encoding a Gal4/Elk1 or Gal4/Ets2 fusion protein and 0.25 μg of a reporter plasmid (Gal-luciferase) which has five Gal4 DNA binding sites cloned upstream of the luciferase gene. Transfection efficiency was determined by cotransfection with a β-galactosidase expression vector. The data are presented as the ratio of luciferase activity (light units) to β-galactosidase activity (optical density units) in the cell extracts.
PAC1 staining in fixed tissues.
Immunohistochemistry was performed on sections cut from lungs that had been fixed overnight in 10% formalin and subsequently embedded in paraffin. Briefly, 5-μm paraffin sections were deparaffinized, dehydrated, treated for 30 min in methanol containing 0.5% H2O2, microwaved for 40 min in 10 mM citrate buffer, and then incubated for 1 h in 16% normal goat serum and overnight with P9D10 monoclonal antibody directed toward PAC1. The secondary antibody was then applied for 1 h at room temperature, followed by the Vectastain Elite ABC reagent (Vector Laboratories, Burlingame, Calif.) for 30 min. The peroxidase reaction was developed with diaminobenzidine and the slides were counterstained with hematoxylin.
Metastasis assay.
Cells were cultured either in the presence or absence of tetracycline for 7 days to optimize PAC1 expression. Three days prior to injection, 60-day time release, 42-mg tetracycline pellets (Innovative Research of America) were implanted under the skin of anesthetized NCR nu/nu mice that were to be injected with tetracycline-treated cells. Cells were trypsinized and resuspended in sterile saline (0.9%) to a concentration of 106 cells per ml. Then, 105 cells (0.1 ml) were injected into the lateral tail vein of each mouse using 27-gauge needles. Mice were sacrificed 6 to 8 weeks later, lungs were fixed in 10% formalin or inflated with Bouin's solution, and hematoxylin and eosin-stained sections were prepared (American Histolabs). Mice were routinely necropsied and examined for gross metastasis.
In vitro invasion assays.
A confluent culture of cells in a 75-cm2 tissue culture flask was trypsinized using 1 ml of trypsin solution and quenched with 11 ml of Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal calf serum. Following 2 h of recovery, cells were resuspended to a concentration of 106 cells/ml in 0.1% bovine serum albumin–DMEM. Invasion was assayed as described elsewhere (28).
RESULTS
The goal of the work presented here was to investigate the contribution of distinct Ras-initiated signaling pathways to metastasis and in vivo invasiveness. A hematogenous metastasis assay was used, a model which measures the ability of cells to survive in the bloodstream, extravasate from blood vessels, migrate through tissue, and expand to a tumor nodule from a single cell. In order to selectively stimulate two of the known Ras effector pathways, we employed either constitutively active upstream components of the ERK pathway, Raf and MEK, or Ras(12V,37G), which activates RalGDS but not Raf or PI3 kinase. In addition, to evaluate the contributions and importance of the ERK pathway for metastasis formation in vivo, we developed a 3T3 cell line in which an ERK-selective MAP kinase phosphatase, PAC1, could be conditionally induced. In the sections below, we describe the development and characterization of these cell lines, their in vitro transformation properties, and their use to evaluate specific pathways contributing to metastasis formation.
Characterization of cell lines with differentially expressed Ras effector pathways.
NIH 3T3 cell clones in which PAC1 expression is regulated by tetracycline were produced, and cells from one such clone, K2F6, were infected with transforming retroviruses in the absence of additional selection. The various retroviruses encoded constitutively activated forms of MEK1 [MEK(218D,222D)], an N-terminal deletion of c-Raf (RafΔN), oncogenic H-Ras(12V), and the effector mutant H-Ras(12V,37G). Ras protein levels were determined to be similar in Ras(12V,37G) and Ras(12V) cells (data not shown).
As shown in Fig. 1A, in the presence of tetracycline, PAC1 protein expression is undetectable in K2F6 cells and the retrovirally transformed populations of K2F6. Withdrawal of tetracycline results in PAC1 expression which is relatively similar in the different populations and consistently two- to threefold greater in cells transformed with Ras(12V). PAC1 expression is freely reversible following multiple rounds of induction and repression (data not shown). ERK2 activity as determined by an immune complex kinase assay, measured in the presence and absence of PAC1, is shown in Fig. 1A. ERK kinase activity was increased relative to the K2F6 level in the presence of MEK(218D,222D), RafΔN, and Ras(12V), but not Ras(12V,37G), and was significantly inhibited in all cells in the presence of PAC1. In PAC1-expressing Ras(12V) cells, ERK activity was greatly reduced but still detectable and comparable to that in nontransformed parental K2F6 cells.
FIG. 1.
(A) PAC1 expression results in down regulation of ERK2 in normal and transformed NIH 3T3 fibroblasts. Proteins in soluble cell extracts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and PAC1 expression was detected in each cell line by immunoblot analysis. A representative experiment of ERK2 activity in cell extracts as determined by an in vitro kinase assay using myelin basic protein (MBP) and [γ32-P]ATP as substrates for the phosphoryl transfer. The degree of MBP phosphorylation by ERK2 was detected by autoradiography. Parallel Western blot analyses showed equivalent ERK2 expression in the various cell lines (not shown). (B) PAC1 specifically inhibits nuclear ERK activity. Elk1- and Ets2-mediated transcription was determined by cotransfecting the various cell lines with either the Gal/Elk1 or Gal/Ets2 expression plasmid, respectively, and the Gal4 reporter.
To verify that nuclear ERK activity was inhibited by PAC1 expression, we performed multiple transcription-based reporter assays for Elk1 and Ets2, whose transactivation potential is dependent upon ERK phosphorylation (9, 12). Elk1- and Ets2-dependent transactivation was greatly increased relative to K2F6 in MEK(218D,222D)-, RafΔN-, and Ras(12V)-transformed cells, but not in Ras(12V,37G) cells. Reporter activity was inhibited approximately 90% following PAC1 expression in the various transformed cells (Fig. 1B). The reporter activity in Ras(V12) PAC1-expressing cells decreased to within twofold of the activity in the nontransformed parental cells. Thus, these cell lines afford the opportunity to evaluate the role of the ERK pathway in the context of MEK(218D,222D)-, Raf-, Ras-, and RalGEF-initiated signaling.
In regard to the other classes of MAP kinases, there was no indication that p38 was constitutively activated upon transformation by the oncogenes used here (data not shown). However, consistent with previous reports, Jun kinase activity was increased in Ras(12V,37G) cells (data not shown), confirming a known functional readout of this Ras effector mutant. PAC1 expression did not reduce Ras(12V,37G)-stimulated JNK activity, consistent with the specificity of PAC1 for ERK.
Ras effector pathways initiating in vitro transformation.
Retrovirus-infected cells were evaluated for in vitro properties of transformation, morphology, and clonagenic growth in soft agar, and the requirement for ERK activation in the maintenance of these properties was determined (Table 1). MEK(218D,222D)-, RafΔN-, and Ras(12V)-transformed cells displayed, in the absence of PAC1, the expected refractile, spindle-shaped morphology and propensity to form foci. Following PAC1 induction, MEK(218D,222D) and RafΔN cells gradually flattened and became contact inhibited, demonstrating reversion of their transformed morphology in the absence of ERK activation. In contrast, Ras(12V) cells were morphologically unchanged by PAC1 expression. Similarly, MEK(218D,222D)- and RafΔN- but not Ras(12V)-transformed cells were highly dependent upon elevated ERK activity in order to form colonies in soft agar, suggesting cellular transformation. Thus, MEK- and Raf-transformed cells appear to require higher levels of activated ERK to promote transformation than do Ras-transformed cells. Ras(12V,35S)-transformed cells were indistinguishable in phenotype from RafΔN cells (not shown).
TABLE 1.
Transforming properties of cell lines in the presence and absence of PAC1
| Mutant used to transform K2F6 | PAC1 | Morphologya | Growth in soft agar (no. of colonies/103 cells)b |
|---|---|---|---|
| None | − | Flat | 0 |
| + | Flat | 0 | |
| MEK(218D,222D) | − | Spindle | 42 |
| + | Flat | 0.43 | |
| RafΔN | − | Spindle | 22 |
| + | Flat | 14 | |
| Ras(12V) | − | Spindle | 147 |
| + | Spindle | 147 | |
| Ras(12V,37G) | − | Flat | 2.0 |
| + | Flat | 0.72 |
Morphology of cells was observed in DMEM supplemented with 10% calf serum.
Determination of growth in soft agar was performed as outlined previously (7). The results are averages for triplicate samples and are representative of at least three independent experiments.
Ras(12V,37G)-transformed K2F6 cells remained flat in the absence of PAC1 but did form compact nonrefractile foci at high cell densities and colonies in soft agar. These soft-agar colonies were reduced by about 65% upon PAC1 induction, implying a partial dependence upon ERK for this transformation property. PAC1 expression did not inhibit the growth of any of the above-cited cell lines in culture (data not shown) but either fully or partially reverted the properties of in vitro transformation for MEK(218D,222D)-, Raf-, and Ras(12V,37G)-transformed cells.
Ras effector pathways mediating experimental metastasis.
A hematogenous metastasis assay was used to investigate the tumorigenic and metastatic potential of the oncogene-transformed cells and to define ERK-dependent functions. Tetracycline administration to or withdrawal from the mice readily manipulated PAC1 expression in the introduced tumor cells (see Fig. 2 and explanation below). As shown in Table 2, MEK(218D,222D)-, RafΔN-, and Ras(12V)-transformed cells were able to form metastatic nodules in the lungs of mice following injection of cells into the lateral tail vein. PAC1 expression reduced tumor formation by MEK(218D,222D) and RafΔN cells by at least 90%, demonstrating that PAC1 effectively reverts highly ERK-dependent transformation in vivo. Consistent with the finding that Ras(12V)-mediated transformation in vitro is resistant to reversion by PAC1, induction of PAC1 in vivo did not affect quantitatively or qualitatively (see below) metastasis formation by these cells. As discussed more fully later, these data show that Ras-initiated metastasis apparently proceeds with significantly reduced ERK activity. Of particular interest, Ras(12V,37G)-transformed cells were highly effective at forming experimental lung metastasis. Even though Ras(12V,37G) does not stimulate ERK activation itself, PAC1 induction inhibited by more than 90% macroscopic lung metastasis formation by Ras(12V,37G)-transformed cells. This suggests a cooperation of Ras(12V,37G)-initiated and ERK pathways in metastasis formation. By contrast, Ras(12V,40C)-transformed cells, which demonstrated levels of Ras protein equivalent to those in Ras(12V,37G)-transformed cells, formed no tumors in the hematogenous metastasis assay (data not shown).
FIG. 2.
PAC1 expression is controlled by tetracycline in mice. Representative section of tumor (T) and normal lung (NL) tissue stained to demonstrate PAC1 expression. (Top) Ras(12V) tumors formed in mice without tetracycline pellets had a high level of nuclear PAC1 expression. (Bottom) PAC1 expression was completely suppressed by subcutaneous time-release tetracycline pellets. A MEK(218D,222D) tumor is shown and is representative of tumors initiated by other transforming retroviruses.
TABLE 2.
Metastasis formation by cell lines in the presence and absence of PAC1a
| Mutant used to transform K2F6 | PAC1 | Avg. no. of tumors/mouseb | % of mice with tumors | Range of tumor no. |
|---|---|---|---|---|
| None | − | 0 | 0 | 0 |
| + | 0 | 0 | 0 | |
| MEK(218D,222D) | − | 13 | 93 | 0–20 |
| + | 0.1 | 12 | 0–1 | |
| RafΔN | − | 7 | 68 | 0–20 |
| + | 0.3 | 21 | 0–2 | |
| Ras(12V) | − | 13 | 78 | 0–20 |
| + | 12 | 62 | 0–20 | |
| Ras(12V,37G) | − | 43 | 100 | 16–104 |
| + | 1.7 | 35 | 0–16 | |
| Ras(12V,37G)/ERK2 | − | 20 | 100 | 15–25 |
| + | 4 | 20 | 0–20 | |
| Ras(12V,37G)/ERK2(D319N) | − | 18 | 100 | 2–20 |
| + | 17 | 83 | 0–20 |
Athymic mice were injected intravenously with 105 parental or transformed K2F6 cells possessing inducible PAC1.
Average number of macroscopic tumors per mouse was determined 6 weeks following injection. At least 20 mice were injected with each cell line. Data are representative of at least three independent experiments.
In order to verify that during metastasis formation, ERK was the relevant target of PAC1-mediated inactivation, we transfected Ras(12V,37G)-transformed K2F6 cells with either wild-type ERK2 or ERK2(D319N), a PAC1-resistant form of ERK2 (5). Individual clones and polyclonal populations of the above cells were assayed for tumor nodules in the hematogenous metastasis assay. As shown in Table 2, injection of Ras(12V, 37G)-, ERK2-transformed cells led to tumor formation that was reduced 80% by PAC1 expression. By contrast, Ras(12V,37G)-, ERK2(D319N)-transformed cells formed equivalent numbers of tumors that were resistant to PAC1 expression. These data strongly suggest that ERK is the primary target of PAC1-mediated metastasis inhibition.
To ensure that PAC1 was regulated as expected by tetracycline in vivo, we applied PAC1-specific immunohistochemical analyses to sections of lung containing tumors. PAC1 expression was assayed and found to be repressed in tumors arising from MEK(218D,222D) cells (Fig. 2) and RafΔN, Ras(12V,37G), and Ras(12V) cells (not shown) in the presence of tetracycline. Importantly, tumors arising from Ras(12V) cells in the absence of tetracycline displayed strong nuclear PAC1 staining in the majority of tumor cells (Fig. 2). In addition, Ras(12V)-transformed cells from tumors formed in the presence and absence of PAC1 expression were cultured, and we found that in the presence of PAC1, ERK activity was reduced to levels near those of parental 3T3 cells (data not shown), similarly to cells prior to in vivo passage. Therefore, although we do not exclude the necessity for a low level of ERK activity in Ras-initiated metastasis, it appears that the level expressed is substantially greater than that which is required.
The different Ras-initiated pathways contribute distinct properties to experimental metastasis.
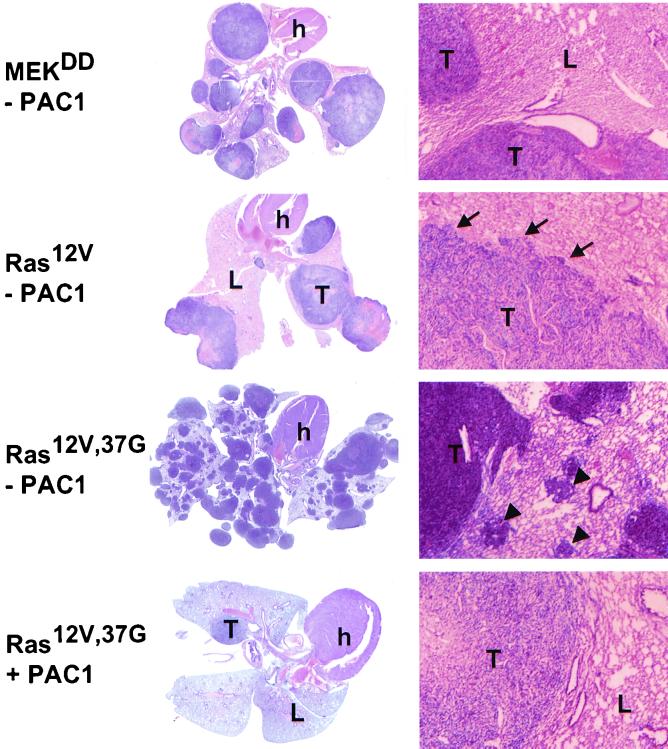
Histological analysis of lung tissues from the various experimental groups revealed morphological features that presumably reflect differences in the invasive nature of the transformed cells (Fig. 3). Metastasis of MEK(218D,222D) and RafΔN-transformed cells appeared as well-circumscribed, compact cell masses with discrete borders, and large tumors showed evidence of central necrosis. Therefore, direct elevation of ERK activity appears to result in cells that are sufficiently invasive to allow migration within the lung parenchyma and initiation of tumor formation, but the tumors so formed were encapsulated and rarely showed intermixing with lung parenchyma. By comparison, tumors composed of Ras(12V)-transformed cells had a highly malignant, well-vascularized character with a cartwheel pattern characteristic of many fibrosarcomas and frequent foci of direct invasion into the adjacent lung parenchyma, bronchioles, and vasculature. There was no histopathological difference between metastatic nodules that did and did not express PAC1.
FIG. 3.
Histology of metastatic nodules. (Left panels) Representative sections through the entire organ block composed of the heart and tumor-bearing lungs (hematoxylin and eosin stain, magnification, ×2); (right panels) corresponding histologies (hematoxylin and eosin stain; magnification, ×55). Note irregular border with invasion towards surrounding lung parenchyma (arrows) in tumors of Ras(12V) cells. Tumors of Ras(12V;37G) cells have multiple satellite lesions, composed of tumor cells (arrowheads). Tumors of Ras(12V,G37) cells expressing PAC1 were rare and rich in stroma. h, heart; T, tumor; L, normal lung.
Of particular interest, Ras(12V,37G) tumors also were highly invasive into adjacent tissues, and additional histological features distinguished them not only from MEK(218D,222D) and RafΔN-transformed cells but also from the oncogenic Ras-transformed cells. Ras(12V,37G) cells formed multiple macroscopic nodules or colonies composed of cells that were less spindle shaped and more orderly than oncogenic Ras-transformed cells. A unique feature of the Ras(12V,37G) colonies was their high frequency of adjacent, microscopic satellite tumors and nests of tumor cells within the lung parenchyma, which probably represent secondary metastasis. PAC1-expressing Ras(12V,37G) cells formed few tumors, and those which did develop had lost their infiltrating phenotype. Such tumors had more discrete borders and no or infrequent satellite tumors and revealed low cell density and a moderate amount of extracellular material. In order to address the possibility that the phenotypes observed were due to a clonal characteristic of K2F6 cells, we performed an independent infection of parental 3T3 cells with Ras(12V,37G) viruses. Following their injection, such cells formed metastatic tumors that were quantitatively and histologically identical to those of the infected K2F6 cells (not shown).
ERK activation influences two aspects of metastasis: initiation of experimental metastasis and development of an infiltrative invasive phenotype.
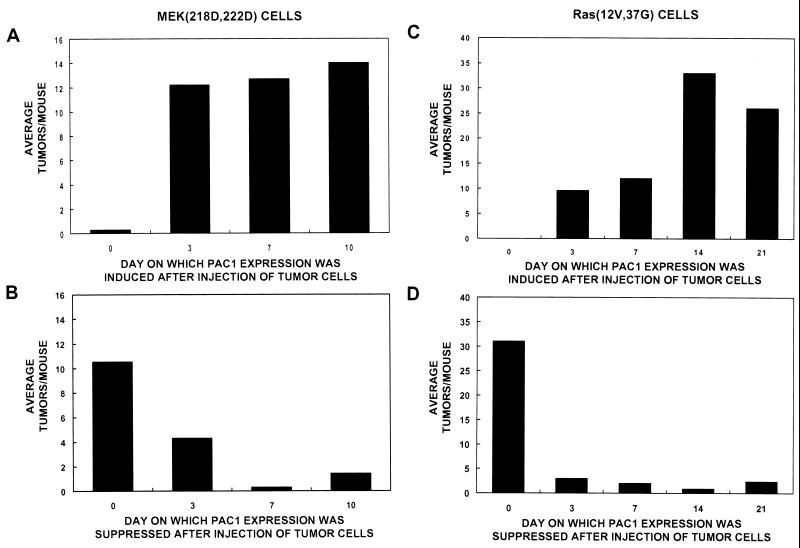
We used the reversible nature of PAC1 induction to investigate during which time period and for how long ERK function was required for tumorigenesis and invasion. Because of the distinct pathological nature of their tumors, the ERK-dependent processes of metastasis formation were determined in MEK(218D,222D)- and Ras(12V,37G)-transformed cells. Inducing PAC1 at various times after injection of MEK(218D,222D) cells allowed us to evaluate the length of time that cells require functional ERK to establish a tumor. Induction of PAC1 at the time of injection (day 0) almost entirely eliminated the tumorigenicity of the MEK(218D,222D) cells (Fig. 4A; Table 3), as expected from earlier results. By comparison, induction of PAC1 at 3 or more days postinjection had no measurable effect on the number of tumors or their pathologic appearance (Fig. 4A). Therefore, it appears that once the MEK(218D,222D)-transformed cells have migrated into the tissue, they require little or no ERK activity for clonal expansion, possibly because cells receive sufficient ERK-independent growth stimuli from their surrounding environment. This is consistent with the ability of MEK(218D,222D) cells expressing PAC1 to grow in vitro normally for several generations in complete media with 10% fetal calf serum (data not shown).
FIG. 4.
Kinetic analyses of PAC1 expression and metastasis development: increased ERK activity is required only for initiation of tumors from MEK(218D,222D)-transformed 3T3 cells, whereas a fully functional ERK pathway is necessary for both the initiation of tumors and the development of an infiltration pathology from Ras(12V, 37G)-transformed fibroblasts. (A and C) Athymic mice had time-release tetracycline pellets implanted under the skin 3 days prior to injection. Lateral tail veins were injected with MEK(218D, 222D)-transformed (A) or Ras(12V, 37G)-transformed (C) 3T3 cells, cultured in tetracycline. At various time points following injection, tetracycline pellets were removed from several mice. (B and D) The lateral tail veins of athymic mice were injected with either MEK(218D,222D)-transformed (B) or Ras (12V, 37G)-transformed (D) cells cultured in the absence of tetracycline. At various time points following the injections, tetracycline pellets were implanted under the skin of several mice. All of the animals were sacrificed 4 weeks [for Ras(12V,37G) cells] or 6 weeks [for MEK(218D,222D) cells] after injection, lung tumors were quantitated, and histological specimens were prepared. At least 10 mice were used for each time point, and the data are pooled from three independent experiments. The ranges of tumor number and the percentages of mice that developed tumors are shown in Table 3.
TABLE 3.
Effect of PAC1 on tumor formation in mice
| Pathway and day | PAC1 suppression
|
PAC1 induction
|
||
|---|---|---|---|---|
| No. of tumors | % of mice with tumors | No. of tumors | % of mice with tumors | |
| MEK(218D,222D) | ||||
| 0 | 0–20 | 80 | 0–1 | 30 |
| 3 | 0–10 | 67 | 1–20 | 100 |
| 7 | 0–3 | 10 | 1–20 | 100 |
| 10 | 0–7 | 50 | 2–10 | 100 |
| Ras(12V,37G) | ||||
| 0 | 20–50 | 100 | 0 | 0 |
| 3 | 0–18 | 51 | 1–39 | 68 |
| 7 | 0–7 | 67 | 1–50 | 83 |
| 14 | 0–4 | 42 | 5–50 | 100 |
| 21 | 0–9 | 58 | 2–50 | 100 |
Inhibiting PAC1 expression at various times after injection of MEK(218D,222D) confirmed that ERK is required for the initiation of experimental metastasis. As shown in Fig. 4B, PAC1 expression was silenced in cells starting at the time of injection (day 0) or after 3 or 7 days postinjection until the mice were sacrificed and analyzed at 6 weeks. When PAC1 was silenced at the time of injection, MEK(218D,222D)-transformed cells formed tumors in numbers and of the histological type observed previously. When PAC1 was expressed in the MEK(218D,222D) cells during the first 3 or 7 days following introduction of the cells, metastatic lung colonization was reduced by 60 and 90%, respectively. These data again suggest that ERK is important for an initiating event in the metastatic process such as extravasation, migration within the lung parenchyma, or early cell division events.
Induction of PAC1 at various times after the injection of Ras(12V,37G) tumor cells revealed that ERK activation was required for at least 2 weeks in order to fully develop the highly invasive character of these particular transformed cells. This confirms the synergy of Ras(12V,37G) and ERK pathways in mediating invasion. Ras(12V, 37G) cells demonstrated a gradual increase in tumor number and in the invasive character of those tumors formed with increasing time that ERK remained active between 0 and 14 days (Fig. 4C). The increased colony number may therefore be associated with a more extended period of migration to form satellite tumors and/or ERK-dependent growth in vivo. Similar to the finding for MEK(218D,222D) cells, PAC1 silencing at various times following injection of Ras(12V,37G) cells showed that ERK is required during an initiating event in tumor formation (Fig. 4D). When PAC1 was expressed in Ras(12V,37G) cells during the first 3 days after their injection, nodule formation was decreased by 90%.
The Ral pathway is required for hematogenous metastatic nodule formation by Ras(12V,37G)-transformed cells.
RalGEFs are well-established effectors of the Ras(12V,37G) mutant (42). In order to determine whether the Ral pathway was indeed functional and critical in the hematogenous metastasis assay, we transfected 3T3 cells with empty vector or an expression vector encoding a dominant negative allele of Ral, RalB(28N), and the levels of RalB expression in the selected polyclonal population and isolated clones are shown in Fig. 5. These cells were then infected with Ras(12V,37G) retroviruses. Ras(12V, 37G) cells transfected with empty vector were highly metastatic and invasive, identical to the Ras(12V,37G) cells described above (Fig. 3 and 4). By comparison, Ras(12V,37G) cells expressing the dominant negative Ral, as either isolated clones or a polyclonal population, showed a significant reduction of more than 90% in the number of metastatic nodules developing in the hematogenous metastasis assay, and there were several animals that were tumor free (Fig. 5). Compared to tumors formed from Ras(12V,37G) cells, histological analysis of the rare tumors that did form in the RalB(28N)-expressing cells revealed a less invasive phenotype, such that margins of the tumors had very few foci of direct invasion into adjacent tissue and there were no accumulations of microsatellite tumors (data not shown).
FIG. 5.
Dominant negative Ral inhibits metastasis formation by Ras(12V,37G). NIH 3T3 fibroblasts were transfected with dominant negative RalB(28N), and subsequently isolated polyclonal populations or selected clones were infected with Ras(12V,37G) retrovirus. A Western blot indicating RalB expression in the various cell lines is shown. The hematogenous metastasis assay was carried out as described in the text, and results are presented as the average number of tumors found in each mouse.
To address whether the inhibition of invasiveness by Ral(28N) expression was specific for Ras(12V,37G)-transformed cells, Ral(28N) and MEK(218D,222D) were coexpressed. No differences between Ral(28N)-expressing and non-Ral(28N)-expressing MEK(218D,222D) cells were observed with regard to metastasis formation (Table 4) or levels of Ras or activated ERK (data not shown). Results similar to those for MEK(218D,222D)-transformed cells were obtained for RafΔN- and Ral(28N)-cotransfected cells. Therefore, the RalGEF-Ral pathway appears to be specifically involved in invasiveness initiated by Ras(12V,37G) and not constitutively required at elevated levels for the survival or functionality of cells in the experimental metastasis assay.
TABLE 4.
Metastasis formation by cell lines with modified RalGEF-Ral pathways
| Cell line and pathway(s) | PAC1 | Avg no. of tumors/mousea | % of mice with tumors | Range of tumor no. |
|---|---|---|---|---|
| 3T3, MEK(218D,222D) | NA | 6 | 60 | 0–20 |
| 3T3 MEK(218D,222D) + Ral(28N) | NA | 9 | 75 | 0–20 |
| K2F6, RalGDS-CAAX | − | 7 | 100 | 2–20 |
| + | 3 | 100 | 1–5 | |
| 3T3, Ral(23V) | NA | 0 | 0 | 0 |
| 3T3, Ral(39L) | NA | 0 | 0 | 0 |
Athymic mice were injected intravenously with 105 cells as described in Materials and Methods. The average number of macroscopic tumors per mouse was determined 6 weeks following injection. At least 20 mice were injected with each cell line.
To determine whether activation of the RalGEF pathway is sufficient to confer an invasive phenotype, we transfected K2F6 cells with a membrane associated form of RalGEF, RalGDS-CAAX (33). As shown in Table 4, tail vein injection of a polyclonal population of selected cells produced multiple, well-vascularized lung tumors that were partially inhibited by ERK inactivation. Histological analyses revealed that the tumors were invasive although somewhat distinct in appearance from Ras(12V,37G) cells, in that fewer microsatellite colonies arose from the RalGDS-CAAX-transformed cells (data not shown). Therefore, activation of RalGEF is sufficient to initiate an invasive phenotype, but some properties of the invasiveness may be modified by the level of RalGEF expression or other Ras(12V,37G) effectors.
Although GTPase-deficient forms of Ral often do not substitute in function for the RalGEFs (27, 41, 43), we nevertheless evaluated whether overexpression of GTPase-deficient or putative fast-cycling alleles of RalB [RalB(23V) and RalB(39L), respectively] were sufficient to mediate experimental metastasis. 3T3 cells were transfected with an expression vector encoding RalB(23V) or RalB(39L). Polyclonal populations and five different clones for each form of activated Ral overexpressing between 5- and 10-fold were each independently assayed in at least five animals, and no tumor nodules were found (Table 4). Therefore, constitutively GTP-bound Ral does not mimic activated RalGEF function in the metastasis assay. It may be necessary for the Ral proteins to cycle through GDP- and GTP-bound states to optimally mediate downstream signaling.
ERK and Ral pathways synergize to produce an activity that stimulates extracellular matrix invasion.
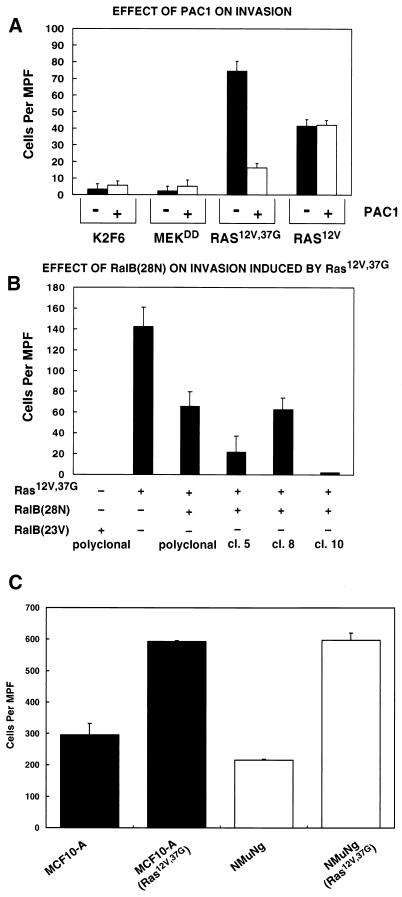
ERK inactivation and dominant negative Ral expression substantially inhibited the invasive phenotype of Ras(12V,37G) tumor cells in vivo, and therefore, we investigated the invasive activity in vitro to determine if this property was cell autonomous and sensitive to the status of ERK and Ral activation. Invasion is a function of both motility and matrix proteolysis. We used lysophosphatidic acid as a chemotactic factor because it is equally active in stimulating motility of cells regardless of PAC1 expression (data not shown), allowing an ERK-dependent component in matrix-degrading activity to be observed. As shown in Fig. 6A, K2F6, and MEK(218D,222D)-transformed cells were minimally invasive through Matrigel in vitro, while Ras(12V,37G)- and Ras(12V)-transformed cells were 8- to 15-fold more invasive. The in vivo pattern of sensitivity to PAC1 was duplicated in vitro; Ras(12V,37G) cells were inhibited in their invasiveness by ERK inactivation; while Ras(12V) cells were not affected. The results obtained by inhibiting ERK activity with PAC1 expression were duplicated with the MEK inhibitor PD98059 (not shown). Invasiveness is also a cell-autonomous activity of the Ral pathway. Ras(12V,37G) cells expressing dominant negative Ral in a selected polyclonal population or in isolated clones were inhibited between 60 and 95% in their ability to invade extracellular matrix (Fig. 6B). The motility of Ras(12V,37G) cells was unaffected by dominant negative RalB expression (data not shown), implying that inhibition of matrix proteolysis was responsible for decreased invasiveness. Consistent with the metastasis results, RalB(23V)-expressing cells were not invasive in vitro (Fig. 6B). Therefore, it appears as if the ability to proteolyze matrix is an intrinsic property of Ras(12V,37G) cells that requires ERK and Ral pathway activation.
FIG. 6.
(A) PAC1 inhibits invasion of Ras(12V,37G)-transformed cells in vitro. The chemoattractant lysophosphatidic acid (10 μg/ml) was loaded into the bottom wells of a BioCoat Matrigel invasion chamber, and cells were placed in the top well over an 8-μm-pore Matrigel-coated membrane. Following 18 h of incubation at 37°C and 5% CO2, the cells that had not invaded were removed and the cells that had invaded to the lower surface of the membrane were fixed, stained, and counted as described in Materials and Methods. (B) Ral activity is required for invasion by Ras(12V,37G)-transformed cells but it is not sufficient for this process. Cells expressing dominant negative RalB(28N) or empty vector in addition to Ras(12V,37G) were used in the in vitro invasion assay as described for panel A except that 10% fetal calf serum was used as the chemoattractant. (C) Expression of Ras(12V,37G) in immortalized human breast epithelia cells and murine breast cancer cells results in increased invasiveness. The chemoattractant used to stimulate invasion was complete medium (50% DMEM, 50% Ham's F-12, 5% horse serum, 20 ng of epidermal growth factor per ml, 10 μg of insulin per ml, 100 μg of gentamicin per ml, and 0.5 μg of hydrocortisone per ml), but otherwise the protocol was the same as that for panel A.
Expression of Ras(12V,37G) increases the invasiveness of breast epithelial cells.
In order to establish whether the Ras effector mutant Ras(12V,37G) activated a signal transduction pathway(s) leading to increased invasiveness in cell types other than 3T3 cells, we infected human immortalized breast epithelial cells, MCF10A, and murine breast cancer cells, NMuNg, with Ras(12V,37G)-expressing retroviruses. As shown in Fig. 6C, the in vitro invasiveness of the Ras(12V,37G)-expressing cells increased two- to threefold relative to that of uninfected cells, although the motility response to the chemotactic factors shown was unaffected by Ras(12V,37G) expression (data not shown).
DISCUSSION
The Ras oncogene induces metastatic ability in several model systems (4). We describe here induction of a RalGEF-dependent invasive phenotype using either Ras(12V,37G)- or RalGDS-CAAX-transformed cells. By contrast, activation of the Raf-ERK pathway leads to experimental metastasis formed from cells that are sufficiently invasive to allow migration within the lung parenchyma and initiation of tumor formation, but the tumors so formed are encapsulated and minimally invasive thereafter. Moreover, induction of invasiveness by the Ras(12V,37G) pathway requires what appears to be a nonelevated or basal level of ERK even though this MAP kinase is not specifically stimulated by Ras(12V,37G). Importantly, expression of PAC1 or a dominant negative Ral allele does not affect the growth of Ras(12V,37G) cells in vitro (data not shown) but does inhibit their intrinsic invasiveness, i.e., their ability to migrate in vitro through extracellular matrix (Fig. 6). Therefore, we suggest that the ERK and RalGEF pathways necessarily interact to produce a genetic program leading to a metastatic phenotype by coordinately regulating an essential gene(s) and/or by regulating distinct sets of genes that contribute different features to the metastatic phenotype. Interestingly, RalGEF and ERK previously have been found to synergize in Ras-induced differentiation of F9 embryonal carcinoma cells in vitro (37).
An unexpected result of the study presented here is the complete lack of effect of ERK downregulation on Ras(12V) transformation as assayed by cell morphology, growth in soft agar, metastatic potential, and in vitro invasion through Matrigel. It is important to note that ERK activity in Ras(12V)-transformed cells expressing PAC1 was reduced to levels similar to nontransformed cells based on enzymatic activity as well as various ERK-sensitive reporter assays (Fig. 1). Why do Ras(12V) and Ras(12V,37G) cells appear to have different requirements for ERK? One possibility is that both cells require some low level of ERK and that PAC1 reduces the ERK activity in Ras(12V,37G) cells below this threshold while leaving higher residual activity in Ras(12V) cells. Since the constitutive ERK activity is at least 25 times greater in Ras(12V) cells than in Ras(12V,37G) cells, PAC1 cannot completely shut off ERK activity in the former cells. An important alternative to consider is that the additional signaling pathways that function in Ras(12V) cells compared to Ras(12V,37G) cells may alter the requirement for ERK.
The critical involvement of the Raf-MEK-ERK cascade in mediating Ras transformation is supported by various experiments utilizing dominant interfering mutants (6, 17, 20, 35). However, the potential indirect effects of such mutants resulting from irreversible protein-protein interactions complicate interpretation. Although it is likely that many properties associated with Ras transformation have a requirement for at least some level of ERK activity, the system presented here has allowed a refinement in quantifying that requirement. We conclude that minimal levels of ERK activity but not necessarily continually activated levels of ERK are sufficient to maintain the transformed and metastatic phenotype of 3T3 cells in the context of other pathways stimulated by Ras. Furthermore, RalGEF activation appears to lead to the induction of metastasis on a background of minimal ERK activity.
Analyses of the kinetic requirements for ERK activation in the experimental metastasis model for MEK(218D,222D)- and Ras(12V,37G)-transformed cells revealed at least two separate phases during which ERK activity contributes. For both cell types, ERK activation was required during the initiation phase of the experimental metastatic process which is expected to encompass extravasation from the lung capillary bed or establishment of micrometastasis but not growth to macroscopic tumors (24). Although it has been shown that parental 3T3 and Ras-transformed 3T3 cells extravasate equally well in a chicken embryo chorioallantoic membrane model (21), the relevancy to our findings is unclear. It would be of interest to directly assay the extravasation capability of the various transformed lines described here. ERK is required for the cellular motility in some systems (2, 19, 29, 46) and therefore may be necessary for extravasation and/or for migration to a supportive environment in the lung.
For the MEK-transformed cells, it appears that once micrometastasis is established, diminished levels of ERK are required for their noninvasive, “benign-like” clonal expansion. This is consistent with our finding that down regulation of ERK did not affect the growth, in complete media, of the parental K2F6 cells expressing PAC1 (data not shown), suggesting that a rich environment can stimulate sufficiently redundant pathways to eliminate or greatly reduce the requirement for ERK-mediated signaling. By contrast, a second phase during which ERK contributes is the development of the invasive phenotype by the Ras(12V,37G) cells. Both tumor number and pathological characteristics indicated that ERK is required for approximately the first 2 weeks of tumor establishment and growth. ERK becomes dispensable once the metastatic colony density has become sufficiently large, implying a change in the interaction of the tumors with the surrounding parenchyma or in selective changes in the tumors.
The generality of increased invasiveness mediated by the Ras(12V,37G) signaling pathway is an important question. A previous report did not find primary metastasis induction by Ras(12V,37G) in 3T3 cells (39). However, variation in the response of 3T3 cells to Ras effector mutants has been previously noted (18) and suggests that subtle genetic differences may provide a permissive background. Alternatively, the level of RalGEF activation might need to reach a threshold not obtained in the previous study. Importantly, two separate breast epithelial cell lines, MCF10A and NMuNg, showed increased invasiveness in vitro following expression of Ras(12V,37G), supporting the notion that RalGEF activation stimulates physiological changes applicable to epithelial cancers as well as fibroblasts. The data presented here suggest that the RalGEF pathway significantly contributes to Ras-initiated metastasis. In addition, Ras-independent activation pathways to Ral exist (13, 45), suggesting that the frequency with which activation of the RalGEF pathway plays a role in RAS-dependent and -independent tumorigenesis and/or progression merits further investigation.
ACKNOWLEDGMENTS
We thank Channing Der for providing the RafΔN, Gal4-Elk, and Gal4-Jun constructs. We also gratefully acknowledge Michael White for the H-Ras(12V), H-Ras(12V,40C), H-Ras(12V,37G), H-Ras(12V,35S), RalGDS-CAAX, RalB(23V), RalB(39L), and RalB28N constructs, Nadeem Moghul for the MEK(218D,222D) plasmid, and Craig Hauser for the Gal4-Ets reporter. We thank Uli Siebenlist for his suggestions on improving the manuscript.
REFERENCES
- 1.Aguirre-Ghiso J A, Frankel P, Farias E F, Lu Z, Jiang H, Olsen A, Feig L A, de Kier Joffe E B, Foster D A. RalA requirement for v-Src- and v-Ras-induced tumorigenicity and overproduction of urokinase-type plasminogen activator: involvement of metalloproteases. Oncogene. 1999;18:4718–4725. doi: 10.1038/sj.onc.1202850. [DOI] [PubMed] [Google Scholar]
- 2.Anand-Apte B, Zetter B R, Viswanathan A, Qiu R G, Chen J, Ruggieri R, Symons M. Platelet-derived growth factor and fibronectin-stimulated migration are differentially regulated by the Rac and extracellular signal-regulated kinase pathways. J Biol Chem. 1997;272:30688–30692. doi: 10.1074/jbc.272.49.30688. [DOI] [PubMed] [Google Scholar]
- 3.Cantor S B, Urano T, Feig L A. Identification and characterization of Ral-binding protein 1, a potential downstream target of Ral GTPases. Mol Cell Biol. 1995;15:4578–4584. doi: 10.1128/mcb.15.8.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chambers A F, Tuck A B. Ras-responsive genes and tumor metastasis. Crit Rev Oncog. 1993;4:95–114. [PubMed] [Google Scholar]
- 5.Chu Y, Solski P A, Khosravi-Far R, Der C J, Kelly K. The mitogen-activated protein kinase phosphatases PAC1, MKP-1, and MKP-2 have unique substrate specificities and reduced activity in vivo toward the ERK2 sevenmaker mutation. J Biol Chem. 1996;271:6497–6501. doi: 10.1074/jbc.271.11.6497. [DOI] [PubMed] [Google Scholar]
- 6.Cowley S, Paterson H, Kemp P, Marshall C J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 7.Cox A D, Der C D. Biological assays for cellular transformation. Methods Enzymol. 1994;238:277–294. doi: 10.1016/0076-6879(94)38026-0. [DOI] [PubMed] [Google Scholar]
- 8.Downward J. Role of phosphoinositide-3-OH kinase in Ras signaling. Adv Second Messenger Phosphoprot Res. 1997;31:1–10. doi: 10.1016/s1040-7952(97)80004-3. [DOI] [PubMed] [Google Scholar]
- 9.Galang C K, Der C J, Hauser C A. Oncogenic Ras can induce transcriptional activation through a variety of promoter elements, including tandem c-Ets-2 binding sites. Oncogene. 1994;9:2913–2921. [PubMed] [Google Scholar]
- 10.Gille H, Downward J. Multiple ras effector pathways contribute to G(1) cell cycle progression. J Biol Chem. 1999;274:22033–22040. doi: 10.1074/jbc.274.31.22033. [DOI] [PubMed] [Google Scholar]
- 11.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill C S, Marais R, John S, Wynne J, Dalton S, Treisman R. Functional analysis of a growth factor-responsive transcription factor complex. Cell. 1993;73:395–406. doi: 10.1016/0092-8674(93)90238-l. [DOI] [PubMed] [Google Scholar]
- 13.Hofer F, Berdeaux R, Martin G S. Ras-independent activation of Ral by a Ca(2+)-dependent pathway. Curr Biol. 1998;8:839–842. doi: 10.1016/s0960-9822(98)70327-6. [DOI] [PubMed] [Google Scholar]
- 14.Huang W, Kessler D S, Erikson R L. Biochemical and biological analysis of Mek1 phosphorylation site mutants. Mol Biol Cell. 1995;6:237–245. doi: 10.1091/mbc.6.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson S A, Mandavia N, Wang H D, Johnson D L. Transcriptional regulation of the TATA-binding protein by ras cellular signaling. Mol Cell Biol. 2000;20:5000–5009. doi: 10.1128/mcb.20.14.5000-5009.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joneson T, Bar-Sagi D. Ras effectors and their role in mitogenesis and oncogenesis. J Mol Med. 1997;75:587–593. doi: 10.1007/s001090050143. [DOI] [PubMed] [Google Scholar]
- 17.Khosravi-Far R, Solski P A, Clark G J, Kinch M S, Der C J. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol Cell Biol. 1995;15:6443–6453. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khosravi-Far R, White M A, Westwick J K, Solski P A, Chrzanowska-Wodnicka M, Van Aelst L, Wigler M H, Der C J. Oncogenic Ras activation of Raf/mitogen-activated protein kinase-independent pathways is sufficient to cause tumorigenic transformation. Mol Cell Biol. 1996;16:3923–3933. doi: 10.1128/mcb.16.7.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klemke R L, Cai S, Giannini A L, Gallagher P J, de Lanerolle P, Cheresh D A. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolch W, Heidecker G, Lloyd P, Rapp U R. Raf-1 protein kinase is required for growth of induced NIH/3T3 cells. Nature. 1991;349:426–428. doi: 10.1038/349426a0. [DOI] [PubMed] [Google Scholar]
- 21.Koop S, Schmidt E E, MacDonald I C, Morris V L, Khokha R, Grattan M, Leone J, Chambers A F, Groom A C. Independence of metastatic ability and extravasation: metastatic ras-transformed and control fibroblasts extravasate equally well. Proc Natl Acad Sci USA. 1996;93:11080–11084. doi: 10.1073/pnas.93.20.11080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leevers S J, Vanhaesebroeck B, Waterfield M D. Signalling through phosphoinositide 3-kinases: the lipids take centre stage. Curr Opin Cell Biol. 1999;11:219–225. doi: 10.1016/s0955-0674(99)80029-5. [DOI] [PubMed] [Google Scholar]
- 23.Luo J Q, Liu X, Frankel P, Rotunda T, Ramos M, Flom J, Jiang H, Feig L A, Morris A J, Kahn R A, Foster D A. Functional association between Arf and RalA in active phospholipase D complex. Proc Natl Acad Sci USA. 1998;95:3632–3637. doi: 10.1073/pnas.95.7.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luzzi K J, MacDonald I C, Schmidt E E, Kerkvliet N, Morris V L, Chambers A F, Groom A C. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol. 1998;153:865–873. doi: 10.1016/S0002-9440(10)65628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marais R, Marshall C J. Control of the ERK MAP kinase cascade by Ras and Raf. Cancer Surv. 1996;27:101–125. [PubMed] [Google Scholar]
- 26.Marshall C J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 27.Miller M J, Prigent S, Kupperman E, Rioux L, Park S H, Feramisco J R, White M A, Rutkowski J L, Meinkoth J L. RalGDS functions in Ras- and cAMP-mediated growth stimulation. J Biol Chem. 1997;272:5600–5605. doi: 10.1074/jbc.272.9.5600. [DOI] [PubMed] [Google Scholar]
- 28.Mulvaney P T, Stracke M L, Nam S W, Woodhouse E, O'Keefe M, Clair T, Liotta L A, Khaddurah-Daouk R, Schiffmann E. Cyclocreatine inhibits stimulated motility in tumor cells possessing creatine kinase. Int J Cancer. 1998;78:46–52. doi: 10.1002/(sici)1097-0215(19980925)78:1<46::aid-ijc9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen D H, Catling A D, Webb D J, Saukovic M, Walker L A, Somlyo A V, Weber M J, Gonias S L. Myosin light chain kinase functions downstream of Ras/ERK to promote migration of urokinase-type plasminogen activator-stimulated cells in an integrin-selective manner. Cell Biol. 1999;146:149–164. doi: 10.1083/jcb.146.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohta Y, Suzuki N, Nakamura S, Hartwig J H, Stossel T P. The small GTPase RalA targets filamin to induce filopodia. Proc Natl Acad Sci USA. 1999;96:2122–2128. doi: 10.1073/pnas.96.5.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okazaki M, Kishida S, Hinoi T, Hasegawa T, Tamada M, Kataoka T, Kikuchi A. Synergistic activation of c-fos promoter activity by Raf and Ral GDP dissociation stimulator. Oncogene. 1997;14:515–521. doi: 10.1038/sj.onc.1200860. [DOI] [PubMed] [Google Scholar]
- 32.Pear W S, Nolan G P, Scott M L, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramocki M B, White M A, Konieczny S F, Taparowsky E J. A role for RalGDS and a novel Ras effector in the Ras-mediated inhibition of skeletal myogenesis. J Biol Chem. 1998;273:17696–17701. doi: 10.1074/jbc.273.28.17696. [DOI] [PubMed] [Google Scholar]
- 34.Rohan P J, Davis P, Moskaluk C A, Kearns M, Krutzsch H, Siebenlist U, Kelly K. PAC-1: a mitogen-induced nuclear protein tyrosine phosphatase. Science. 1993;259:1763–1766. doi: 10.1126/science.7681221. [DOI] [PubMed] [Google Scholar]
- 35.Schaap D, van der Wal J, Howe L R, Marshall C J, van Blitterswijk W J. A dominant-negative mutant of raf blocks mitogen-activated protein kinase activation by growth factors and oncogenic p21ras. J Biol Chem. 1993;268:20232–20236. [PubMed] [Google Scholar]
- 36.Vanhaesebroeck B, Leevers S J, Panayotou G, Waterfield M D. Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem Sci. 1997;22:267–272. doi: 10.1016/s0968-0004(97)01061-x. [DOI] [PubMed] [Google Scholar]
- 37.Verheijen M H, Wolthuis R M, Defize L H, den Hertog J, Bos J L. Interdependent action of RalGEF and Erk in Ras-induced primitive endoderm differentiation of F9 embryonal carcinoma cells. Oncogene. 1999;18:4435–4439. doi: 10.1038/sj.onc.1202834. [DOI] [PubMed] [Google Scholar]
- 38.Wasylyk C, Wasylyk B, Heidecker G, Huleihel M, Rapp U R. Expression of raf oncogenes activates the PEA1 transcription factor motif. Mol Cell Biol. 1989;9:2247–2250. doi: 10.1128/mcb.9.5.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Webb C P, Van Aelst L, Wigler M H, Woude G F. Signaling pathways in Ras-mediated tumorigenicity and metastasis. Proc Natl Acad Sci USA. 1998;95:8773–8778. doi: 10.1073/pnas.95.15.8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White M A, Nicolette C, Minden A, Polverino A, Van Aelst L, Karin M, Wigler M H. Multiple Ras functions can contribute to mammalian cell transformation. Cell. 1995;80:533–541. doi: 10.1016/0092-8674(95)90507-3. [DOI] [PubMed] [Google Scholar]
- 41.White M A, Vale T, Camonis J H, Schaefer E, Wigler M H. A role for the Ral guanine nucleotide dissociation stimulator in mediating Ras-induced transformation. J Biol Chem. 1996;271:16439–16442. doi: 10.1074/jbc.271.28.16439. [DOI] [PubMed] [Google Scholar]
- 42.Wolthuis R M, Bos J L. Ras caught in another affair: the exchange factors for Ral. Curr Opin Genet Dev. 1999;9:112–117. doi: 10.1016/s0959-437x(99)80016-1. [DOI] [PubMed] [Google Scholar]
- 43.Wolthuis R M, de Ruiter N D, Cool R H, Bos J L. Stimulation of gene induction and cell growth by the Ras effector Rlf. EMBO J. 1997;16:6748–6761. doi: 10.1093/emboj/16.22.6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolthuis R M, Franke B, van Triest M, Bauer B, Cool R H, Camonis J H, Akkerman J W, Bos J L. Activation of the small GTPase Ral in platelets. Mol Cell Biol. 1998;18:2486–2491. doi: 10.1128/mcb.18.5.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolthuis R M, Zwartkruis F, Moen T C, Bos J L. Ras-dependent activation of the small GTPase Ral. Curr Biol. 1998;8:471–474. doi: 10.1016/s0960-9822(98)70183-6. [DOI] [PubMed] [Google Scholar]
- 46.Xie H, Turner T, Wang M H, Singh R K, Siegal G P, Wells A. In vitro invasiveness of DU-145 human prostate carcinoma cells is modulated by EGF receptor-mediated signals. Clin Exp Metastasis. 1995;13:407–419. doi: 10.1007/BF00118180. [DOI] [PubMed] [Google Scholar]