Abstract
Objective: To examine the association of objective sleep and fatigue in the early postpartum period with postpartum depression in Japanese primiparas intending to establish breastfeeding.
Materials and Methods: The participants were 34 primiparas who were in the postnatal ward after vaginal delivery and responsively breastfeeding their rooming-in baby. Actigraphy data for objective sleep were collected for three consecutive days starting from the first day postpartum. Fatigue and postpartum depression were assessed using the Postpartum Fatigue Scale and Edinburgh Postnatal Depression Scale, respectively, on numerous days between the first day postpartum and the one-month checkup. Breastfeeding and rooming-in data were also collected.
Results: The mean total sleep time was 252.0 ± 73.1 min/day. Mean breastfeeding frequency was 12.4 ± 3.2 times/day and mean total breastfeeding time was 247.4 ± 101.8 min/day. Among the participants, 67.6% were exclusively breastfeeding on the discharge day. Fatigue scores were significantly higher during the hospital stay, compared with one month postpartum. Multiple regression analysis showed that sleep frequency on the third day postpartum and mean total breastfeeding time and fatigue on the fifth day postpartum were factors affecting the Edinburgh Postnatal Depression Scale score.
Conclusion: The association between postpartum depression among breastfeeding primiparas in the early postpartum period and objective sleep, fatigue, and total breastfeeding time per day was suggested. An environment wherein breastfeeding mothers can rest and sleep without hesitation will be beneficial. Moreover, the importance of sleep during pregnancy and the early postpartum period must be highlighted. Midwifery and/or nursing care starting while the mother is in a postnatal hospital stay can play a key role in preventing postpartum depression.
Keywords: postpartum depression, postpartum sleep, postpartum fatigue, responsive breastfeeding
Introduction
Postpartum depression (PPD) has increasingly been recognized as a serious issue in Japan because social awareness of maternal suicides has increased in recent years. Takeda et al.1) reported that the maternal suicide rate over a decade, from 2005 to 2014, in 23 wards in Tokyo was 8.7 per 100,000 live births, which was higher than the maternal mortality rate during the same period2). The leading cause was PPD, which was estimated to account for 33% of maternal suicides1). The prevalence of PPD among women in Japan is estimated to be in the range of approximately 10–17%3, 4), which is comparable to the reported levels in other countries.
PPD onset occurs between two weeks and one month after delivery4). An assessment of Japanese mothers using the Edinburgh Postnatal Depression Scale (EPDS) on the fifth day postpartum showed that a quarter of them were at a high risk of PPD and primiparas had a higher risk than multiparas5). Many postpartum women experienced sleep deprivation and strong fatigue, as well as experienced difficulty with resting6). A study reported that the daily total sleep time of Japanese primiparas who stayed with the baby in their ward was as short as approximately four hours in the early postpartum period, which was significantly shorter than that of multiparas7). In their systematic review, Bhati and Richards8) noted a relationship between sleep disturbance and PPD.
Most (93.4%) Japanese mothers intend to breastfeed9), and Japanese hospitals generally endeavor to follow the WHO/UNICEF guidelines10) for successful breastfeeding, wherein mothers are encouraged to stay with their babies at all times and breastfeed responsively. However, to ensure the successful establishment of lactation, responsive breastfeeding indicates that eight to twelve breastfeeding sessions must occur each day11). If mothers prioritize their sleep, the possibility of not initiating or giving up breastfeeding may increase.
Although numerous studies have focused on the association between sleep disturbance and PPD, very few studies have focused on the early postpartum period. According to the aforementioned systematic review, only two studies have measured the objective sleep time of mothers from the first day postpartum12, 13). These studies have revealed a relationship between subjective sleep and mood, but noted no correlation between objective sleep time and mood. The measured mean sleep times (358.28 min and 372.57 min, respectively) were notably longer than those of Japanese mothers staying with their babies in the postnatal ward, as examined by Yamazaki et al7). Moreover, research has yet to include breastfeeding as a variable while examining the factors affecting maternal sleep in the early postpartum period.
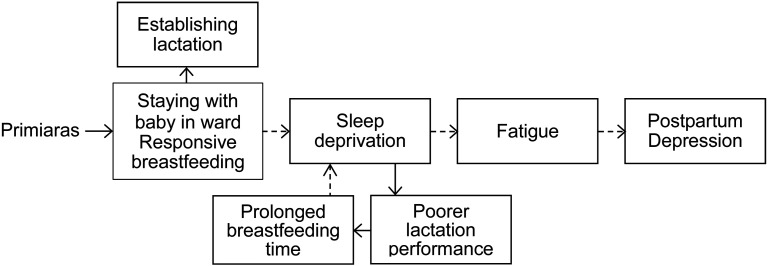
To summarize, research has yet to determine how responsive breastfeeding and the mother-and-baby staying together in the ward would affect maternal sleep and fatigue, as well as how sleep and fatigue are related to PPD. Based on this research background, a conceptual framework was developed for this study (Figure 1). Therefore, the primary purpose of the current study was to analyze objective sleep and fatigue characteristics in the early postpartum period of Japanese primiparas staying with their babies in the ward and intending to establish breastfeeding. Moreover, this study aimed to evaluate the relationship between these factors and PPD.
Figure 1.
Conceptual framework.
In Japan, the postnatal hospital stay is longer, compared to other countries; Japanese primiparas usually remain in the hospital for four to six days after vaginal delivery. Therefore, midwives and obstetric nurses are able to engage with new mothers starting from the early days of postnatal hospital stay, observe maternal sleep and fatigue, and ensure that mothers rest as much as possible while trying to initiate and establish breastfeeding. However, clinical guidelines have yet to be established regarding the sleep and rest of breastfeeding mothers during the early postpartum period. Midwifery textbooks suggest that mothers should rest between breastfeeding sessions, but no specific advice has been provided regarding sleep. Consequently, a standard protocol has yet to be established for bedside care and support by midwives for these exhausted and vulnerable mothers. Therefore, this study also offers clinical suggestions for midwives and obstetric nurses regarding effective care, advice, and interventions that may be provided to breastfeeding primiparas in the early postpartum period, starting immediately after delivery, to minimize the risk of PPD.
Materials and Methods
Research design and data collection
Data from a sample of 42 primiparas were collected between June 2015 and April 2016 and analyzed using a descriptive and prospective design. Participants were recruited from a general hospital in Japan by the authors and midwives working there. They were provided information about the study on the delivery day or first day postpartum. The hospital has a breastfeeding policy that encourages mothers to stay with their babies at all times and breastfeed responsively immediately after childbirth. When a mother wants to spend time by herself, she may leave her baby with a midwife in the nursery room. The day of discharge was the fifth day postpartum in primiparas.
The eligibility criteria were as follows: (i) continuous postnatal hospital stay after vaginal delivery, with the mother and baby staying together in the ward starting from the day of delivery; (ii) the mother must not be younger than 20 years of age; (iii) the mother must have no history of mental illnesses, and (iv) the baby must be born after a full-term without complications. The participants’ age, delivery duration, education, amount of bleeding during delivery, and hemoglobin level on the second day postpartum, as well as the baby’s sex, birth weight, weight loss rate, and other delivery and background information were collected from the clinical records.
Postpartum sleep
Each participant was asked to wear a wrist actigraph (MicroMini RC, Ambulatory Monitoring, Inc., Ardsley, NY, USA) on the non-dominant wrist starting from the first to fifth days postpartum to obtain objective measurements of sleep-wake activity, except while taking a shower and bathing the baby. Data were collected in one-minute epochs in the zero-crossing mode. AW2 software (Ambulatory Monitoring, Inc., USA) was used to calculate three sleep measures by using the algorithm of Cole et al.: cumulative length of sleep episodes (“total sleep time”), the longest sleep episode, and the number of sleep episodes lasting for twenty minutes or more (“sleep frequency”).
Every participant was awake and wore a wrist actigraph at 18:00. Therefore, actigraphy data for three consecutive days starting at 18:00 on the first day postpartum were extracted for analysis, whereas data recorded while the actigraph was removed for showering and bathing the baby were excluded. The concordance between wrist actigraphy and polysomnography has been proven14).
Postpartum fatigue
Postpartum fatigue was assessed using the Postpartum Fatigue Scale (PFS) developed by Yamazaki et al.15) on the first, third, and fifth days postpartum, and the day of the one-month checkup. Participants used a four-point scale to respond to the PFS questionnaire, which included 36 question items, with nine questions for four subscales—physical stress, mental stress, sleep deprivation, and difficulties in caring for the baby (hereafter “baby care difficulties”). The maximum score is 144, and higher scores indicate a more severe postpartum fatigue level. The reliability of PFS was confirmed by the Cronbach’s alpha score (0.938), with alpha scores of subscales ranging from 0.810 to 0.909. The validity of PFS has also been demonstrated15).
Postpartum depression
PPD was assessed using the Japanese language edition of EPDS16) on the first and fifth days postpartum, and the day of the one-month checkup. Participants were asked to answer ten questions using a four-point scale. The cut-off point in Japan is 8/917) and a score of nine or higher indicates a high risk of PPD. EPDS has been translated into many languages and is used as a screening test for PPD worldwide. This scale does not contain questions about physical symptoms, thus the score is not affected by the changing nature of postpartum physical conditions16). In Japan, EPDS is widely used for screening purposes at maternity wards and during home visits by public health visitors. Cronbach’s alpha score was 0.78. At a cut-off point of 8/9, the sensitivity was 0.75, and the specificity was 0.93, which was higher than at other cut-off points17).
Breastfeeding and rooming-in
The number (“breastfeeding frequency”) and cumulative length (“total breastfeeding time”) of breastfeeding sessions were collected from activity logs that every mother at the participating hospital was asked to fill in. Breastfeeding referred only to the act of the baby sucking breastmilk and did not include feeding bottled milk or pumped breastmilk. Rooming-in data (time periods when the mother and the baby stayed together in the ward and the baby was left with nursery room staff) were collected from the records filled in by midwives.
Statistical analysis
Descriptive statistics were used to describe sample characteristics, actigraphy data, PFS and EPDS scores, and breastfeeding and rooming-in data. A one-factor repeated-measures analysis of variance or the Freidman test was conducted to assess time-dependent changes in sleep parameters, PFS, and EPDS, and when significant differences were observed, the Bonferroni method was used for multiple comparisons. The impact of sleep deprivation on fatigue and PPD was examined by dividing participants into two groups using the mean of total sleep time on the third day postpartum as a cut-off point, and comparing PFS and EPDS scores using the Mann-Whitney test. Based on the results of the Mann-Whitney test and research findings, a multiple regression analysis was performed to determine the influencing factors of PPD by using the EPDS score on the fifth day postpartum as an independent variable. IBM SPSS Statistics 24 was employed for the analysis, and the significance level was set at 0.05.
Ethical considerations
Participants were informed about the study both orally and in writing by the authors and midwives working at the hospital. They were informed that participation in the study was voluntary and that refusal or cessation afterward would not jeopardize the hospital care provided to them. Only the participants who signed a letter of consent were recruited. The study was approved by the ethical committees of the authors’ institution (approval No. 15-9) and the participating hospital.
Results
Sample characteristics
Among the 42 mothers who consented to participate in the study, eight were excluded for not wearing the wrist actigraph as prescribed (four), removing the actigraph for more than three hours (two), or failing to answer some items of the EPDS/PFS questionnaires (two). The characteristics of the remaining 34 participants are listed in Table 1. The mean age was 29.5 (range, 21–41), with four participants (11.8%) aged 35 or above. None of the participants had experienced an epidural birth because the participating hospital did not offer this option.
Table 1. Characteristics of participants and their babies (n=34).
| Variables | ||
|---|---|---|
| Age (years) | 29.5 ± 4.7 | |
| Over 35 years of age: N (%) | 4 (11.8) | |
| Married/having a partner: N (%) | 34 (100.0) | |
| Educational background: N (%) | ||
| Junior or high school | 11 (32.4) | |
| Vocational school or junior college | 12 (35.3) | |
| University or graduate school | 11 (32.4) | |
| BMI before pregnancy | 20.6 ± 2.8 | |
| Increased body mass in pregnancy (kg) | 10.4 ± 4.6 | |
| Total delivery time (min) | 942.7 ± 871.0 | |
| Second stage of labor duration (min) | 127.3 ± 198.6 | |
| Amount of bleeding during delivery (g) | 576.8 ± 345.8 | |
| Hemoglobin level on day 2 (g/dL) | 9.6 ± 1.5 | |
| Induction of delivery: N (%) | 4 (11.8) | |
| Labor inducing drug used: N (%) | 8 (23.5) | |
| Vacuum extraction: N (%) | 3 (8.8) | |
| Episiotomy: N (%) | 10 (29.4) | |
| Gestation (weeks) | 39.4 ± 1.0 | |
| Birthweight (g) | 3,012.7 ± 354.1 | |
| Low-birth-weight (less than 2,500 g) baby: N (%) | 1 (2.9) | |
| Sex of baby: N (%) | ||
| Male | 12 (35.3) | |
| Female | 22 (64.7) | |
| Rate of baby’s weight loss (%) | 8.6 ± 1.7 | |
| Baby’s lowest weight (day of life) | 2.4 ± 0.6 | |
| Phototherapy: N (%) | 3 (8.8) | |
| Mothers who never left baby with nursery room staff: N (%) | 10 (29.4) | |
| No formula used during postnatal hospital stay: N (%) | 8 (23.5) | |
| Exclusive breastfeeding on the day of discharge: N (%) | 23 (67.6) | |
Data are presented as the mean ± standard deviation or N (%).
State of rooming-in
Ten mothers (29.4%) never left their babies with a midwife in the nursery room, and stayed together with the baby at all times during the postnatal hospital stay. Other participants left their babies in the care of nursery room staff at some time, and this number decreased over time, from ten on the first day to seven on the second day, six on the third day, and four on the fourth day postpartum. Figure 2 shows the mean total time they stayed with their babies each day.
Figure 2.
Total length of time rooming-in and in nursery room for babies.
( ) Length of time mothers and babies
stayed together; (
) Length of time mothers and babies
stayed together; ( ) Length of time the
baby was left with nursery room staff, excluding mothers who stayed with the baby at
all times.
) Length of time the
baby was left with nursery room staff, excluding mothers who stayed with the baby at
all times.
State of breastfeeding
Eight mothers (23.5%) did not use formula milk during the postnatal hospital stay. On the day of discharge, there were 23 (67.6%) exclusively breastfeeding mothers and 11 (33.4%) partially breastfeeding mothers, with no exclusively bottle-feeding mothers. At one month postpartum, 19 mothers (55.9%) were exclusively breastfeeding, 14 mothers (41.2%) were partially breastfeeding, and one mother (2.9%) was exclusively bottle-feeding.
Two participants who could not breastfeed due to nipple pain, and another participant who did not have complete breastfeeding time records were excluded from the breastfeeding analysis. Among the remaining 31 mothers, the mean breastfeeding frequency per 24 hours was 12.7 times (SD 3.3) and the mean total breastfeeding time per 24 hours was 247.4 min (SD 101.8). Significant chronological differences were not observed starting from the first to the fourth day postpartum, in terms of total breastfeeding time and frequency. The maximum frequency among participants was 26 times, which was recorded on the third day, and the maximum total breastfeeding time in a day was 705 min recorded on the second day postpartum by a different mother. The results are summarized in Figures 3 and 4
Figure 4.
Changes in total breastfeeding time (n=31).
.
Figure 3.
Changes in breastfeeding frequency (n=31).
State of postpartum sleep
Table 2 presents the sleep parameters of participants. The mean value of total sleep time per day over three days of actigraphic data was 252.0 min (SD 73.1, range 158.7–456.0). Sleep episodes lasting for 20 min or more (sleep frequency) occurred 4.2 times (SD 1.6, range 1.7–8.7) per day on average, and the mean value of the longest sleep episode was 84.3 min (SD 33.7, range 35.0–164.7). The total sleep time and longest sleep were shorter and the sleep frequency was higher on the second day postpartum than the other days, but no significant differences were noted on a day-to-day basis. A positive correlation was observed between total sleep time and sleep frequency each day (Day 1; r=0.665, P<0.000, Day 2; r=0.693, P<0.000, Day 3; r=0.643, P<0.000).
Table 2. Comparison of maternal sleep parameters of two groups divided by the mean of total sleep time on Day 3.
Association between sleep and fatigue
The PFS and subscale scores of participants are presented in Table 3. The PFS score, the total of four subscale scores, peaked on the third day postpartum and declined constantly thereafter. Among the subscales, the scores for baby care difficulties and physical stress gradually reduced over a one-month period, but those for sleep deprivation and mental stress showed minimal change even at one month postpartum.
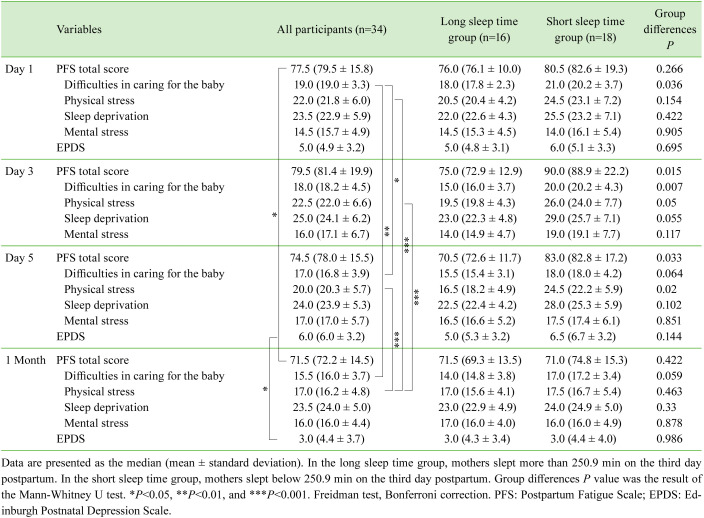
Table 3. Comparison of PFS and EPDS scores of two groups divided by the mean of total sleep time on Day 3.
A negative correlation was noted between total sleep time and the baby care difficulties subscale score (r=−0.438, P=0.010), as well as between sleep frequency and the same subscale score on the third day postpartum (r=−0.371, P=0.031). Based on these findings, we analyzed the impact of sleep on fatigue by dividing participants into two groups using the sleep data from the third day and comparing their sleep parameters and respective PFS scores. The results are presented in Tables 2 and 3.
Postpartum depression
Table 3 presents the mean EPDS scores of participants. Three participants (8.8%) scored nine points or more on the first day and six participants (17.6%) scored the same points on the fifth day as well as one month postpartum. A positive correlation was found between the EPDS score on the fifth day and that at one month postpartum (r=0.513, P=0.002). A negative correlation was also noted between sleep frequency on the third day and the EPDS score on the fifth day postpartum (r=−0.405, P=0.018).
Total sleep time of the low-risk group (EPDS scores of 8 or less on the fifth day postpartum) were 261.3 ± 95.1 min on Day 1, 246.7 ± 95.6 min on Day 2, and 258.8 ± 93.2 min on Day 3. On the other hand, the total sleep time of the high-risk group (scores of 9 or more) were 264.7 ± 99.4 min on Day 1, 227.2 ± 65.9 min on Day 2, and 214.5 ± 147.9 min on Day 3. Total sleep time of the high-risk group reduced over three days starting from the first to third days postpartum, albeit no significant difference was observed between the two groups on a day-to-day basis.
Factors associated with EPDS on Day 5
Based on the aforementioned findings, a forced entry multiple regression analysis was conducted to determine factors influencing the EPDS score on the fifth day, using sleep frequency on the third day and total breastfeeding time and PFS total score on the fifth day postpartum, as well as total sleep time on Day 3, as dependent variables. Data normality was confirmed using the Shapiro-Wilk test. The adjusted coefficient of determination was 0.443, and the significance level was set at 1%. The test results (Table 4) show that sleep frequency on the third day as well as total breastfeeding time and PFS total score on the fifth day were significantly associated with PPD, as represented by the EPDS score, on the fifth day postpartum.
Table 4. Results of multiple regression analysis for factors associated with EPDS on Day 5.
| Independent variables | Partial regression coefficient (B) | Standardized regression coefficient (β) | t-ratio | P | 95% CI for B | R2 | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Constant | 0.524 | 0.203 | 0.841 | –4.761 | 5.808 | 0.511 | |
| Sleep frequency on Day 3 | –0.963 | –0.536 | –3.043 | 0.005 | –1.61 | –0.316 | |
| Mean total breastfeeding time | 0.011 | 0.383 | 2.844 | 0.008 | 0.003 | 0.019 | |
| PFS total score on Day 5 | 0.072 | 0.347 | 2.594 | 0.015 | 0.015 | 0.129 | |
| Total sleep time on Day 3 | 0.005 | 0.148 | 0.824 | 0.417 | –0.007 | 0.016 | |
EPDS: Edinburgh Postnatal Depression Scale; CI: Confidence Interval; PFS: Postpartum Fatigue Scale.
Discussion
In this study, a relationship was noted between total sleep time and fatigue of primiparas who intended to establish breastfeeding in the early postpartum period. The results (Table 3) highlight that participants experienced difficulties in caring for their babies for a few days after giving birth, which resulted in shorter sleep time on the third day postpartum, and in turn led to fatigue and physical stress on the fifth day postpartum. Multiple regression analysis revealed that the factors affecting the EPDS score on the day of discharge (fifth day postpartum) were (i) sleep frequency on the third day; (ii) average daily total breastfeeding time during postnatal hospital stay; and (iii) fatigue represented by the PFS score on the fifth day postpartum. Therefore, an association was noted between objective sleep and PPD in the early postpartum period. The results show that cumulative breastfeeding time per day was an additional factor influencing PPD.
Nearly a quarter (23–24%) of Japanese primiparas had an EPDS score of nine or higher on the day of discharge and were classified as having a high risk of experiencing PPD5). Another study reported that the prevalence of maternal depression among primiparas peaked at two weeks postpartum18). In the current study, three (50%) out of six mothers who had an EPDS score of nine or higher on the day of discharge (fifth day postpartum) also scored nine or higher on the day of the one-month checkup, thereby indicating a correlation between the EPDS scores of these two days. This indicates that nursing care designed to minimize the risk of PPD should begin during the postnatal hospital stay.
Rooming-in
Participants started to stay with their babies immediately after delivery and spent more than 22 hours together per day on average. This finding can be compared with the rooming-in times of 13.9 hours on the first day and 12.3 hours on the second day postpartum after vaginal delivery at baby-friendly hospitals in Taiwan that keenly encouraged mothers to establish breastfeeding during 48 hours of postnatal hospital stay19). The authors suggest that the long period during which mothers stayed with their babies contributed to sleep shortage and fatigue in this study. Moreover, participants’ fatigue levels were higher during the postnatal hospital stay than at one month postpartum, and peaked on or around the third day postpartum. The authors suggest that this occurs because the mothers started rooming-in with the baby and responsive breastfeeding when they had not yet recovered from delivery fatigue, which resulted in poor quality sleep. Bozoky and Corwin20) reported that fatigue during the first week postpartum is predictive of depression on the 28th postpartum day, but the present study findings suggest that fatigue as early as the fifth day postpartum is indicative of PPD risk.
Postpartum sleep
The participants’ total sleep time per day was approximately four hours, which is similar to the results of a previous Japanese study that conducted an actigraphic sleep analysis of primiparas staying together with the baby in the ward7). Four hours of sleep represents a sudden drop from seven hours in late pregnancy, as reported by Shinkoda et al21). In this study, the longest sleep episode was only 80 min, recorded on the second and third days postpartum. These findings indicate that breastfeeding primiparas had to manage with poor sleep during the early postpartum period.
Subjective awareness of sleep shortage remained high among participants even at one month postpartum. By considering the current findings along with those of past research showing that the maternal sleep cycle was noticeably irregular one month after childbirth21), we can predict that maternal sleeping behavior remains unchanged at one month postpartum and sleep shortage continues for more than a month. In the past, the post-delivery sleep shortage of mothers was not considered a serious issue. Horiuchi22), for instance, observed a significant decrease in the ratio of sleep time and an increase in the ratio of wake time in the maternal sleep-wake cycle, but suggested that mothers should maintain sleep quality to compensate for sleep shortage.
According to studies on sleep, having as little as four hours of sleep a day would induce a strongly drowsy state that could lead to acute sleep disorders23), and having a short sleep lasting for four hours maximum for five continuous days would intensify the anxiety and depression states24). The present study supports these results because it showed that the total sleep time of mothers with high PPD risk was shorter than those in the low-risk group.
Breastfeeding mothers may have to wake up at any time to breastfeed and look after their baby, but there should be awareness that continuous sleep shortage (four hours of sleep per day) lasting more than a month will increase the risk of PPD.
Postpartum sleep and breastfeeding
Participants breastfed the baby more than 12 times per day on average, spending more than four hours in total for breastfeeding. They spent 20 min out of every two hours on breastfeeding. As noted by Shinkoda et al.25), nearly 75% of sleep disruption in mothers was caused by breastfeeding. Participants sacrificed their sleep for their babies, and breastfeeding was an influential factor for sleep disruption.
Research has widely recognized that mothers who intend to establish breastfeeding should be given support in line with Ten steps to successful breastfeeding10), and one study reported that the mother-and-baby staying together and initiation of responsive breastfeeding starting from the delivery day resulted in higher rates of breastfeeding26). However, there are no guidelines or evidence regarding how breastfeeding mothers who stay with their babies should spend their time during the early postpartum period, and there has been a long-standing perception that postpartum women could do with short sleep. Nighttime waking and sleep shortages were regarded as a necessity by Japanese mothers27) and midwives. The concentration of prolactin, a lactogenic hormone, increases during sleep28), thus sleep deprivation can reduce prolactin concentration in the blood29) and negatively impact lactation, thereby extending the breastfeeding time. Sacrificing sleep for breastfeeding can eventually lead to reduced lactation performance, thereby causing a spiral of longer breastfeeding time and sleep shortage for mothers.
Clinical suggestions
The current study findings show that sleep, breastfeeding, and fatigue can impact PPD in the early postpartum days. When this is combined with previous findings showing that on or around the fifth day postpartum, nearly a quarter of primiparas were evaluated to be at high risk of PPD5) and the EPDS score peaked at two weeks postpartum18), it becomes clear that midwifery and/or nursing care starting while mothers are in the postnatal hospital stay can play a key role in the prevention of PPD. Clinicians need to monitor the total length and frequency of mothers’ sleep and breastfeeding, as well as their fatigue levels. Noticing signs of baby care difficulties and providing appropriate support in the first two–three days after childbirth are particularly important. Postpartum women should be educated about the importance of sleep for both the prevention of PPD and the establishment of breastfeeding, and should be encouraged to take every opportunity to sleep even for a short time period. Provisions should be in place to ensure that any mother who cannot sleep between breastfeeding sessions or is extremely tired can rest even for a short time. Potential arrangements include allowing the baby to stay with a midwife in the nursery room in a manner that would not affect breastfeeding, tailoring the timing of necessary nursing/hospital duties (such as routine nursing care and discharge consultation and advice) to the condition of individual mothers, and ensuring that hospital visits should not prevent mothers from resting. If a mother spends too much time breastfeeding, nursing interventions should be considered to discuss the situation with her, determine the reason, and provide the necessary support.
Limitations and future agenda
The sample size was small, with 34 participants from a single hospital, thus the current study findings should be validated by larger and more diverse samples. Although no correlation was noted between the PFS score and hemoglobin level or amount of bleeding during delivery, some participants had lower hemoglobin levels due to a loss of large quantities of blood during delivery, which may have affected the fatigue data. The research period should be extended to include a pre-delivery period, and additional data on subjective sleep should be collected. Experiencing difficulties while caring for the baby in the early postpartum period was found to be one of the key factors affecting PPD; therefore, future studies must identify the causes of these difficulties. The long-term goal is to aid in developing nursing and midwifery care guidelines that start as early as possible after delivery and help mothers successfully establish breastfeeding and secure sufficient sleeping time, thereby minimizing the risk of PPD.
Conclusion
The results of this study suggest that PPD in breastfeeding primiparas in the early postpartum period is associated with objective sleep, fatigue, and total breastfeeding time per day. Midwives who provide post-delivery care to new mothers in hospitals can play a key role in the prevention of PPD. Effective interventions may include monitoring the total length and frequency of mothers’ sleep and breastfeeding, assessing their fatigue level, helping them to deal with, and hopefully solve, baby care difficulties in the first few days after delivery, creating an environment wherein they can rest and sleep without hesitation, and educating women about the importance of sleep during pregnancy and the early postpartum period.
Acknowledgment
The authors would like to thank all the mothers who participated in this study and the midwives who worked in the participating hospital.
References
- 1.Takeda S, Takeda J, Murakami K, et al. Annual Report of the Perinatology Committee, Japan Society of Obstetrics and Gynecology, 2015: Proposal of urgent measures to reduce maternal deaths. J Obstet Gynaecol Res 2017; 43: 5–7. doi: 10.1111/jog.13184 [DOI] [PubMed] [Google Scholar]
- 2.Ministry of Health, Labor, Welfare. Population Survey Report. 2017. https://www.mhlw.go.jp/english/database/db-hw/populate/dl/E01.pdf. (Accessed Mar. 16, 2021)
- 3.Kitamura T, Yoshida K, Okano T, et al. Multicentre prospective study of perinatal depression in Japan: incidence and correlates of antenatal and postnatal depression. Arch Women Ment Health 2006; 9: 121–130. doi: 10.1007/s00737-006-0122-3 [DOI] [PubMed] [Google Scholar]
- 4.Yamashita H, Yoshida K, Nakano H, et al. Postnatal depression in Japanese women. Detecting the early onset of postnatal depression by closely monitoring the postpartum mood. J Affect Disord 2000; 58: 145–154. doi: 10.1016/S0165-0327(99)00108-1 [DOI] [PubMed] [Google Scholar]
- 5.Mori E, Maehara K, Iwata H, et al. Physical and psychosocial wellbeing of older primiparas during hospital stay after childbirth: A comparison of four groups by maternal age and parity. Boei Eisei 2016; 56: 558–566(Japanese journal of Maternal health). [Google Scholar]
- 6.Kurth E, Spichiger E, Zemp Stutz E, et al. Crying babies, tired mothers - challenges of the postnatal hospital stay: an interpretive phenomenological study. BMC Pregnancy Childbirth 2010; 10: 21. doi: 10.1186/1471-2393-10-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamazaki K, et al. Factors affecting mothers’ sleep after starting rooming-in—study focusing on elderly primiparas—. Nihon Boshi Kangogakkaishi 2015; 8: 21–30(Journal of the Japan Maternal and Infant Caring Association). [Google Scholar]
- 8.Bhati S, Richards K. A systematic review of the relationship between postpartum sleep disturbance and postpartum depression. J Obstet Gynecol Neonatal Nurs 2015; 44: 350–357. doi: 10.1111/1552-6909.12562 [DOI] [PubMed] [Google Scholar]
- 9.Ministry of Health, Labor, Welfare. National nutrition survey on preschool children 2015. (in Japanese). https://www.mhlw.go.jp/file/06-Seisakujouhou-11900000-Koyoukintoujidoukateikyoku/0000134207.pdf (Accessed Mar. 16 2021)
- 10.World Health Organization UNICEF. Protecting, promoting and supporting breast-feeding: the special role of maternity services/a joint WHO/UNICEF statement. 1989. http://apps.who.int/iris/handle/10665/39679. (Accessed Mar. 16, 2021)
- 11.Gartner LM, Morton J, Lawrence RA, et al. American Academy of Pediatrics Section on Breastfeeding.Breastfeeding and the use of human milk. Pediatrics 2005; 115: 496–506. doi: 10.1542/peds.2004-2491 [DOI] [PubMed] [Google Scholar]
- 12.Bei B, Milgrom J, Ericksen J, et al. Subjective perception of sleep, but not its objective quality, is associated with immediate postpartum mood disturbances in healthy women. Sleep 2010; 33: 531–538. doi: 10.1093/sleep/33.4.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coo Calcagni S, Bei B, Milgrom J, et al. The relationship between sleep and mood in first-time and experienced mothers. Behav Sleep Med 2012; 10: 167–179. doi: 10.1080/15402002.2012.668147 [DOI] [PubMed] [Google Scholar]
- 14.Morgenthaler T, Alessi C, Friedman L, et al. Standards of Practice CommitteeAmerican Academy of Sleep Medicine.Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep 2007; 30: 519–529. doi: 10.1093/sleep/30.4.519 [DOI] [PubMed] [Google Scholar]
- 15.Yamazaki K, Takagi H, Saito M. Development of a scale for a “feeling of postpartum fatigue” evoked in early puerperium and verification of the reliability and validity. Boei Eisei 2015; 55: 711–720(Japanese Journal of Maternal Health). [Google Scholar]
- 16.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry 1987; 150: 782–786. doi: 10.1192/bjp.150.6.782 [DOI] [PubMed] [Google Scholar]
- 17.Okano T, Murata M, Masuji F, et al. Validation and reliability of Japanese version of the EPDS. Seishinka Shindangaku 1996; 7: 525–533(Archives of Psychiatric Diagnosis and Clinical Evaluation). [Google Scholar]
- 18.Takehara K, Tachibana Y, Yoshida K, et al. Prevalence trends of pre- and postnatal depression in Japanese women: a population-based longitudinal study. J Affect Disord 2018; 225: 389–394. doi: 10.1016/j.jad.2017.08.008 [DOI] [PubMed] [Google Scholar]
- 19.Lai YL, Hung CH, Stocker J, et al. Postpartum fatigue, baby-care activities, and maternal-infant attachment of vaginal and cesarean births following rooming-in. Appl Nurs Res 2015; 28: 116–120. doi: 10.1016/j.apnr.2014.08.002 [DOI] [PubMed] [Google Scholar]
- 20.Bozoky I, Corwin EJ. Fatigue as a predictor of postpartum depression. J Obstet Gynecol Neonatal Nurs 2002; 31: 436–443. doi: 10.1111/j.1552-6909.2002.tb00066.x [DOI] [PubMed] [Google Scholar]
- 21.Shinkoda H, Matsumoto K, Park YM. Changes in sleep-wake cycle during the period from late pregnancy to puerperium identified through the wrist actigraph and sleep logs. Psychiatry Clin Neurosci 1999; 53: 133–135. doi: 10.1046/j.1440-1819.1999.00518.x [DOI] [PubMed] [Google Scholar]
- 22.Horiuchi S. [Changes in sleep parameters of young women from late pregnancy to postpartum]. Nihon Kango Kagakkaishi 1994; 14: 38–47(in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 23.Carskadon MA, Dement WC. Nocturnal determinants of daytime sleepiness. Sleep 1982; 5(Suppl 2): S73–S81. doi: 10.1093/sleep/5.S2.S73 [DOI] [PubMed] [Google Scholar]
- 24.Motomura Y, Kitamura S, Oba K, et al. Sleep debt elicits negative emotional reaction through diminished amygdala-anterior cingulate functional connectivity. PLoS One 2013; 8: e56578. doi: 10.1371/journal.pone.0056578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shinkoda H, Matsumoto K, Mishima M. Changes of Primipara and Multipara Mother’s Sleep-wake. Journal of Japan Academy of Nursing Science 2001; 21: 1–11(in Japanese, Abstract in English). doi: 10.5630/jans1981.21.2_1 [DOI] [Google Scholar]
- 26.Murai F, Saitoh S, Nonoyama M, et al. Examination of breastfeeding with UNICEF/WHO “The ten steps to successful breastfeeding” at childbirth institutions of 6 prefectures in the Kanto area—part 2: the influence of breastfeeding care on breastfeeding rate—. Boei Eisei 2008; 48: 505–513(Japanese Journal of Maternal Health). [Google Scholar]
- 27.Horiuchi S, Kondo J, Koyama M, et al. Subjective estimation of sleep patterns during postpartum period compared with pregnancy periods. Sei Ruka Kango Daigaku kiyō (Bulletin of St Luke’s College of Nursing) 1990; 16: 49–59 (in Japanese, Abstract in English). [Google Scholar]
- 28.Sassin JF, Frantz AG, Weitzman ED, et al. Human prolactin: 24-hour pattern with increased release during sleep. Science 1972; 177: 1205–1207. doi: 10.1126/science.177.4055.1205 [DOI] [PubMed] [Google Scholar]
- 29.Baumgartner A, Dietzel M, Saletu B, et al. Influence of partial sleep deprivation on the secretion of thyrotropin, thyroid hormones, growth hormone, prolactin, luteinizing hormone, follicle stimulating hormone, and estradiol in healthy young women. Psychiatry Res 1993; 48: 153–178. doi: 10.1016/0165-1781(93)90039-J [DOI] [PubMed] [Google Scholar]