Abstract
Attention-deficit/hyperactivity disorder (ADHD) is a neurobehavioral disorder affecting approximately 4-7% of children and persisting in 2-5% of adults. The core symptoms include pervasive inattention and inappropriate levels of hyperactivity-impulsivity. High-frequency gamma activity has been implicated in the temporal binding of stimulus properties across cortical areas, and is known to be crucial for complex information processing and attentional processes in particular. Thus, we evaluated the amplitude of gamma-frequency neural responses in adults with and those without ADHD, and tested whether stimulant medications, the most common treatment for ADHD, modulate gamma activity in affected adults. Participants underwent two sessions (~75 minutes apart) of auditory stimulation using stimuli known to elicit 40 Hz gamma-band responses as magnetoencephalography data were acquired. Between sessions, the ADHD group (who were in maintenance therapy) were administered their daily stimulant medication and both groups were told to relax. The primary results indicated that gamma activity was weaker in the ADHD group during session one (pre-drug), but not session two (post-drug), and that gamma activity significantly increased following stimulant administration in adults with ADHD. These results suggest that ADHD is associated with reduced cortical gamma activity and that stimulants may ameliorate this abnormality.
Keywords: attention, ADHD, 40 Hz, magnetoencephalography, stimulant, ADD, Adderall
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is the most common neurobehavioral disorder in children and adolescents (CDC, 2005). Although it is less common in adults, estimates of ADHD prevalence are still significant at 2-5% of adults in the United States (Kessler et al., 2006). The core symptoms of ADHD include inappropriate levels of hyperactivity-impulsivity and/or pervasive inattention, with the inattention component being the more frequent and critical feature in adult patients (Seidman et al., 2004a; Kessler et al., 2010). There is substantial evidence linking ADHD with structural, functional, and chemical brain deviations across widespread neurofunctional networks (Seidman et al., 2004b; Krain and Castellanos, 2006; Paloyelis et al., 2007). The brain regions commonly implicated include higher-order areas such as prefrontal and parietal cortices, as well as unisensory (i.e., all modalities), motor, cerebellar, and subcortical areas (Seidman et al., 2004b; Krain and Castellanos, 2006; Paloyelis et al., 2007; Vaidya and Stollstorff, 2008; Dockstader et al., 2009). The precise origin(s) of these abnormalities are not known, but environmental factors and genetics are likely central. It is also unclear whether these aberrations are intrinsic features of the pathophysiology, a long-term consequence of treatment, or lack thereof (e.g., see Shaw et al., 2009).
Recent electrophysiological studies have indicated that patients with ADHD have aberrant gamma-frequency neural activity compared with their non-ADHD peers (Lenz et al., 2008, 2010; Barry et al., 2009, 2010). Gamma-band (30-120 Hz) activity occurs across the neocortex and is crucially dependent upon the integrity of local interneuronal networks, which function as GABA-gated pacemakers for cortical oscillatory activity (Singer, 1999; Bartos et al., 2007; Fries et al., 2007; Uhlhaas et al., 2009). An organizing hypothesis supported by extensive neurophysiological data postulates that gamma oscillations are crucial to coordinating information processing (e.g., see Fries, 2009; Uhlhaas et al., 2009). Essentially, synchronous gamma oscillations are believed to be critical to the integration of distinct aspects of stimulus and/or intrinsic processes that are performed by separate regions of a distributed neural system (Singer, 1999; Fries, 2009; Uhlhaas et al., 2009). In patients with ADHD, gamma-band deficits have been observed during periods of eyes-closed rest and during various cognitive tasks (Lenz et al., 2008, 2010; Barry et al., 2009, 2010). These abnormalities appear to be closely linked to the expressed symptomatology, as a recent pediatric study indicated that inattention scores on the Conners’ Parent Rating Scale were negatively correlated with absolute gamma-band power (Barry et al., 2010). This higher-order connection between gamma activity and attentional control may reflect the role that high-frequency neuronal activity plays in serving the functional connectivity between distinct neural systems.
Although the specific neural basis of the disorder is not understood, there are several stimulant (e.g., methylphenidate) and non-stimulant (e.g., atomoxetine) medications that have proven effective for suppressing the symptomatology. To date, the overwhelming majority of clinical trials have focused on youth with ADHD (Biederman et al., 2003; Newcorn et al., 2008), but these medications have shown efficacy in studies of their adult counterparts as well (Faraone and Glatt, 2010). Stimulants represent the largest class of psychotropic medication prescribed to children in the United States (CDC, 2005), and are also the most common treatment for adults with ADHD (Coghill and Seth, 2006). While their clinical efficacy is clear, the mechanisms by which stimulants alleviate ADHD symptoms are only vaguely characterized. Largely from animal work, amphetamine-based stimulants are known to block the reuptake of norepinephrine and dopamine into the pre-synaptic neuron and increase the release of these monoamines into the extracellular space. Limited PET and SPECT ligand-labeling data indicate the same chemical systems are modulated in humans, although specificity in regard to site(s) and neurophysiologic mechanism are lacking (Vaidya and Stollstorff, 2008). Recent fMRI studies have provided some insight into the brain areas that are strongly modulated by stimulants in patients with ADHD, but thus far these studies have mostly focused on children, varied considerably in design, and have tended to be inconsistent (Paloyelis et al., 2007). The most pertinent fMRI findings include enhanced suppression (during the task phase) of neural activity in the ventral anterior cingulate node of the default-mode network following stimulant ingestion in adolescents with ADHD (Peterson et al., 2009). Rubia et al. (2009) found that methylphenidate modulated activity in many brain areas including anterior cingulate, caudate, cerebellum, and ventromedial frontal regions of children completing a rewarded continuous performance task. These studies have also shown enhanced functional interactivity in fronto-parietal networks, and between the anterior cingulate and prefrontal cortices in patients with ADHD after stimulant administration (Peterson et al., 2009; Rubia et al., 2009). In perhaps the most well-controlled study to date, Bush et al. (2008) demonstrated increased activation in the anterior cingulate cortices, prefrontal cortex, and parietal areas during an attention-demanding interference task in stimulant-medicated adults with ADHD compared with a matched group of ADHD adults receiving placebo. Finally, at least in healthy adults, the precise neural regions where stimulant medications act is likely contingent on the particular cognitive processes needed for successful task performance (Dodds et al., 2008).
In this study, we investigated whether adults with ADHD have reduced capacity to generate and/or maintain gamma-frequency activity relative to a matched group of adults without ADHD, and whether stimulant medications up-modulate this capacity in the group with ADHD. Participants in both groups listened to auditory stimuli that are known to elicit very strong gamma responses in two distinct blocks, which were separated by a ~75 minute rest/treatment-administration interval, as high-density magnetoencephalography (MEG) data were recorded. We localized the neural generators of these gamma-frequency responses and extracted their current-amplitude time series per participant and recording session. Our primary hypotheses were that adults with ADHD would have reduced gamma-band activity and that amphetamines would enhance these high-frequency responses.
Method
Subject Selection
We studied 13 adults (4 females) with inattentive-type ADHD and 12 adults (4 females) without ADHD. Mean ages were 41.62 years-old (ADHD; SD: 11.42 years) and 42.88 years-old (controls; SD: 11.8 years) at enrollment. All participants were right-handed, except one with ADHD who was left-handed. All participants with ADHD had shown a satisfactory clinical response to a mixture of dextroamphetamine salts, extended release formula (MAS-XR; Adderall XR), and been prescribed the same regularly-monitored dosage for at least 6 months prior to study enrollment. Diagnoses were based on a semi-structured comprehensive psychiatric assessment by a board certified psychiatrist (MWW) utilizing DSM-IV diagnostic criteria, the Adult ADHD Symptom Rating Scale (ASRS, v1.1; Kessler et al., 2005), and collateral history. Exclusionary criteria included any medical illness affecting CNS function, neurological disorder, history of head trauma, and current substance abuse. After complete description of the study to participants, written informed consent was obtained in accord with the Medical Center’s Institutional Review Board.
Experimental Paradigm
All participants were scheduled for MEG early in the morning (e.g., 07:30-08:00) and for the group with ADHD, a minimum of 18 hours since their last stimulant dosage. Upon arrival each participant completed one block of binaural stimulation, which consisted of ~150 trains of DC pulses (duration = 500 ms; inter-train interval = 1200 ms). Each pulse or “click” was of 5 ms duration and these were repeated at a rate of 40 Hz over the 500 ms pulse-train. Participants with ADHD were then orally administered their standard dosage of MAS-XR and moved to the patient waiting area, while those without ADHD were simply moved from the MEG room to the patient waiting area. Approximately 75 minutes later, all participants returned to the MEG room and completed a second (identical) block of 40 Hz stimulation. All stimuli were presented at 80 dB SPL using foam ear-inserts with 30 dB attenuation to exterior noise.
MAS-XR is an extended release amphetamine product FDA approved for the treatment of ADHD in adults. It combines neutral sulfate salts of dextroamphetamine and amphetamine, with the dextro isomer of amphetamine saccharate and d,l-amphetamine aspartate monohydrate. Upon administration, blood plasma concentration levels rise sharply then begin to asymptote toward a peak of 20-30 ng/mL at 6-8 hours post-administration. This is followed by a gradual decline in plasma concentration level over the next 16-18 hours. However, the degree to which the brain’s response curve follows the plasma concentration curve is entirely unknown.
Structural Magnetic Resonance Imaging (MRI)
High-resolution neuroanatomic images were acquired using a Philips Achieva 3T X-series scanner. The T1-weighted sagittal images were obtained with an eight channel head coil using a 3D fast field echo sequence with the following parameters: field of view, 24 cm; slice thickness, 1 mm with no gap; in-plane resolution, 1.0 x 1.0 mm; sense factor, 1.5. The structural volumes were aligned parallel to the anterior and posterior commissures and used for MEG coregistration.
MEG Data Acquisition
All recordings were conducted in a one-layer magnetically-shielded room (MSR) with active shielding engaged. With an acquisition bandwidth of 0.1 – 330 Hz, neuromagnetic responses were sampled continuously at 1 kHz using an Elekta Neuromag system with 306 magnetic sensors, including 204 planar gradiometers and 102 magnetometers (Helsinki, Finland). Epochs were defined offline and were of 1 s duration, including a 200 ms pre-stimulus baseline. Using MaxFilter (v2.1.15; Elekta), MEG data from each session were transformed into a standard device-centered head position, individually-corrected for head motion, coregistered to structural MRI, and subjected to noise reduction using the signal space separation method with a temporal extension (Taulu et al., 2005; Taulu and Simola, 2006).
Prior to MEG measurement, four coils were attached to the subject’s head and the locations of these coils, together with the three fiducial points and scalp surface, were determined with a 3-D digitizer (Fastrak 3SF0002, Polhemus Navigator Sciences, Colchester, VT, USA). Once the subject was positioned inside the MSR, an electric current with a unique frequency label (e.g., 322 Hz) was fed to each of the coils. This induced a measurable magnetic field and allowed each coil to be localized in reference to the sensors throughout the data acquisition session. Since coil locations were also known in head coordinates, all MEG measurements could be transformed into a common coordinate system. With this coordinate system (including the scalp surface points), each participant’s MEG data was co-registered with their structural T1-weighted MRI data prior to source analyses.
MEG Source Analyses
Artifact rejection was based on a fixed threshold method supplemented with visual inspection. Artifact-free epochs from each session were time-domain averaged with respect to stimulus onset and then digitally (forward/backward Butterworth) bandpass filtered 35-45 Hz to emphasize the 40 Hz steady-state response (Ross et al., 2000, 2002, 2005). For each participant, bilateral neural generators of the 40 Hz response were modeled using the subset of sensors that covered both magnetic flux extrema. Subsequently, these sources were combined into a single two-source model for each participant, which was used to calculate an inverse spatial filter for deriving source waveforms per session of stimulation. This operation, termed source space projection (Tesche et al., 1995; Ross et al., 2000, 2005), exploits the distinct sensitivity profile possessed by each element of the 306-sensor array to activity in a pre-specified cortical patch (determined from the above described localizations), and thereby utilizes the field strength measurements across the array to calculate single waveforms per source indicating the current strength (in nAm) time series within the specific tissue volume. In the current application, this procedure generated two source waveforms (i.e., left/right auditory cortices) for each MEG session per participant. Previous investigations using beamformer approaches, which do not require a priori assumptions regarding source configurations, have demonstrated single-dipoles to be sufficient for modeling the activation of auditory cortices (e.g., Herdman et al., 2003) and thus support the validity of the present analysis. The use of source waveforms confers a significant advantage in signal processing, as averaging across independent sensors reduces intrinsic sensor noise resulting in waveforms with higher signal-to-noise ratios relative to the magnetic field waveforms (Ross et al., 2000).
These 40 Hz current-amplitude (nAm) waveforms per participant, hemisphere, and session were normalized using the respective mean amplitudes of the 200 ms pre-stimulus baseline periods. Normalized means were then derived for an early 50-200 ms post-stimulus period, and the standard 200-500 ms post-stimulus time window. This “standard” 200-500 ms window is commonly utilized to focus analyses on steady-state responses rather than transient evoked components (Ross et al., 2000, 2002, 2005). Finally, since our hypotheses focused on group and treatments effects and not response laterality (nor interactions with laterality), data from each time window was collapsed (averaged) across the left and right auditory cortices. These normalized current-amplitude values were examined using a mixed-model 2 x 2 x 2 ANOVA with time window (i.e., early and standard) and session (i.e., first session/pre-drug and second session/post-drug) as within-subject factors, and group (with and without ADHD) as a between-subjects factor. Significant effects were followed-up with appropriate t-tests, which were always two-tailed and conducted in SPSS for Windows. All MEG pre-processing and source analyses used the Elekta Neuromag base software implemented in Linux CentOS.
Results
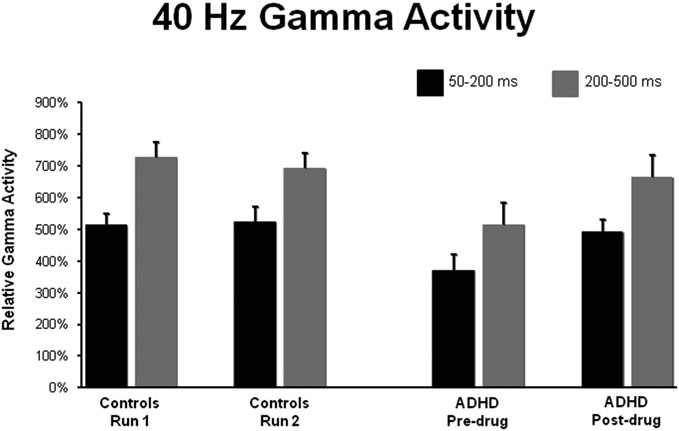
All participants showed robust gamma activity bilaterally during each MEG session. These responses were strong enough to be easily discerned in the sensor-level (fT) magnetic data, and were readily localized to expected areas of Heschl’s gyri and/or along the superior bank of the superior temporal gyri (see Figure 1). In regards to the amplitude of 40 Hz neuronal activity in participants with and without ADHD, our primary 2 x 2 x 2 mixed ANOVA with time window and session as within-subjects factors, and group (with/without ADHD) as a between-subjects factor indicated a main effect of time window F(1,23) = 32.72 (p < 0.001), a main effect of session F(1,23) = 5.99 (p = 0.022), and a session-by-group interaction effect F(1,23) = 8.77 (p < 0.01; see Figure 2). The other three interaction effects were not significant (all p’s > 0.3), but the group main effect approached significance F(1,23) = 3.09 (p = 0.09). The time window main effect indicated that across groups and sessions, 40 Hz gamma activity was significantly stronger during the standard (200-500 ms) window relative to the early time bin, whereas the main effect of session indicated that gamma responses (collapsed across groups and time windows) were stronger during the second/post-drug MEG session. Post-hoc follow up tests were conducted to evaluate the session-by-group interaction effect. These tests revealed that adults with ADHD had significantly less overall gamma activity during session one (pre-drug) t(23) = 2.62 (p = 0.01), but not during session two (p > 0.6). Furthermore, overall gamma activity was increased in session two (post-drug) compared with session one (pre-drug) in the ADHD group t(12) = 2.98 (p = 0.012), but this did not differ in the healthy control group (p =0.46; see Table 1 and Figure 2). The effect size for the session one group difference was d = 1.097, whereas that for the pre-/post-drug difference in patients was d = 0.744 (Cohen, 1988).
Figure 1.
Representative Subject and Synopsis of Data Processing Procedure. For each participant, 40 Hz generators were localized using data averaged across both sessions after head movement correction and head position standardization. Essentially, the subset sensors covering the magnetic flux extremas were used to localize single-ECDs per hemisphere and subsequently these were combined into a single two-source model per participant. Source space projection was then applied to this model so that the 306-sensor magnetic time series, corresponding to each session separately, was collapsed into two waveforms reflecting the current-strength of each cortical patch (left/right auditory cortex) per recording session. The data depicted above is from a representative adult with ADHD. The 40 Hz auditory generators and their source time series (nAm) from each session (pre-drug and post-drug) have been overlaid onto the subject’s 3D rendition. In each plot, the vertical black line represents stimulus onset (0 ms = first pulse in train), each gray line represents 50 ms (−200 ms → 800 ms), and the current amplitude (nAm) is on the ordinate. As shown, the 40 Hz response clearly emerges before ~200 ms, but reaches its peak and is maintained relatively high until it begins to dissipate at ~500 ms post-stimulus. In addition, one can discern that this patient clearly exhibited stronger 40 Hz responses bilaterally in the second session (after stimulant administration). See text for further description of methodological procedures.
Figure 2.
Group Means of Gamma Activity. Un-medicated adults with ADHD exhibited significantly less gamma activity relative to their healthy peers during the standard 200-500 ms time window (grey), and during an earlier window from 50-200 ms post-stimulus (black). On the y-axis, gamma activity is shown in normalized units relative to a −200 to 0 ms pre-stimulus period. The administration of amphetamine (MAS-XR) significantly increased gamma activity in participants with ADHD during both time windows, and the magnitude of this increase eliminated group statistical differences in Run 2. Control subjects showed no significant effects from Run 1 to Run 2. These data indicate that stimulant medications may modulate cortical gamma activation in adults with ADHD. Error bars indicate one standard error of the mean.
Table 1.
Auditory Gamma Frequency Activity
| Gamma-band Activity | Group | Mean | SD | 95% Confidence Interval |
|---|---|---|---|---|
| Session 1, 50-200 ms | Controls | 513.9 | 125.2 | 434.4 – 593.4 |
| ADHD | 371.6 | 175.5 | 265.5 – 477.7 | |
| Session 2, 50-200 ms | Controls | 523.8 | 158.2 | 423.4 – 624.3 |
| ADHD | 492.0 | 143.4 | 405.3 – 578.6 | |
| Session 1, 200-500 ms | Controls | 728.8 | 155.4 | 630.0 – 827.5 |
| ADHD | 515.6 | 243.2 | 368.6 – 662.5 | |
| Session 2, 200-500 ms | Controls | 693.2 | 161.1 | 590.9 – 795.6 |
| ADHD | 664.6 | 245.1 | 516.5 – 812.8 |
All values are expressed in normalized units (% increase) using the baseline period (−200 to 0 ms)
Discussion
We examined whether adults with ADHD have reduced capacity to generate and/or maintain high-frequency cortical responses compared to their non-ADHD peers, and whether stimulant medications modulate this capacity by utilizing an auditory 40 Hz stimulation paradigm that is well-recognized for eliciting robust gamma activity. First, our results showed that 40 Hz gamma neuronal activity was stronger across both groups during the 200-500 ms time window relative to an earlier 50-200 ms window, which is consistent with previous data showing that this response peaks after 200 ms post-stimulus (Ross et al., 2000, 2005). Importantly, we also observed that gamma activity was significantly weaker in the adults with ADHD across both the early (50-200 ms) and standard (200-500 ms) time windows, which suggests that these participants have a reduced capacity to generate high-frequency neural responses and supports the primary hypothesis. Finally, these results demonstrated that stimulant medications (MAS-XR) increase the capacity of the ADHD brain to generate gamma-frequency activity in response to a strongly eliciting stimulus.
The significant reduction in gamma activity seen in the off-medication state, and the overall enhancement of gamma-band neuronal activity in the on-medication state are clearly our most interesting and novel findings. In contrast to a plethora of early investigations that focused exclusively on low-frequency activity in children with ADHD (for review, see Barry et al., 2003), recent electrophysiological studies have started to more fully evaluate higher frequency brain responses (i.e., beta-band and above). Although limited in number, these reports have consistently shown that gamma activity is reduced in children with ADHD (Barry et al., 2009, 2010) and, unlike age-matched controls, is not correlated with real time cognitive performance (Lenz et al., 2008, 2010). The studies to date reveal no regional specificity in regards to gamma abnormalities, but due to the electrical conductivity properties of the skull EEG measures are somewhat limited in this regard. As for medication effects, several EEG studies of children with ADHD have shown that stimulant medications decrease activity in the theta-band (4-7.5 Hz; Clarke et al., 2002, 2003, 2007), and one MEG study reported the opposite effect of an increase in frontal theta following stimulant administration in an ADHD group that included children and adolescents (Wienbruch et al., 2005). However, none of these studies evaluated gamma-frequency responses or any neural activity faster than 24 Hz, which is surprising given the central role of gamma activity in disorders of attention (Uhlhaas and Singer, 2006). One recent EEG study of the non-stimulant atomoxetine did examine higher-frequency responses in children with ADHD (Barry et al., 2009). They found increased theta and globally reduced gamma activity in medication-naive children compared with age-matched controls. The children with ADHD then repeated a resting-state measurement one hour after their initial dose of atomoxetine (20 mg). These children showed reduced theta activity in the on-medication state, but no differences in gamma (Barry et al., 2009). The reduction in gamma-frequency responses in medication-naïve children with ADHD is consistent with our observation of weaker gamma activity in the pre-medication state, and thus extends this finding to adults with ADHD. However, on the contrary, the current data indicated that MAS-XR treatment strongly increased gamma activity in adults with ADHD. This disagreement may reflect that atomoxetine and stimulant-based medications have somewhat distinct mechanisms of action, or the different sensitivities of EEG and MEG, or simply developmental differences in how these drugs affect CNS function. It is also possible that these discrepancies reflect that some of their participants may not have benefitted from the atomoxetine, given that 20 mg is clearly a sub-therapeutic dose, and that typical response rates (with standard dosing) are under 50% (Newcorn et al, 2008).
Analogous to the electrophysiology, previous pharmacological fMRI studies have focused on children and not directly addressed gamma activity (i.e., fMRI cannot distinguish gamma from low-frequency activity). Nonetheless, these data are still informative. Peterson et al. (2009) recently showed that stimulant medications were associated with task-related deactivations being more prominent in the ventral anterior and posterior cingulate cortices of children with ADHD. Essentially, during task performance, these areas of the default mode network were significantly less active in healthy controls compared with ADHD children in the off-medication state, but this difference dissipated when the on-medication data was used in the same comparisons. Moreover, the functional connectivity between lateral prefrontal and anterior cingulate cortices increased in children with ADHD after stimulant administration to levels similar to those observed in controls (Peterson et al., 2009). Such findings suggest that stimulants may act to normalize communication between prefrontal and posterior regions as well as neural activity within crucial nodes of the default-mode network, which is known to be closely linked to performance on attention demanding tasks (e.g., see Spreng et al., 2010). Another recent fMRI study has also indicated that stimulant medications modify activity in brain regions serving attentional networks and their interactions during performance of a demanding cognitive task (Rubia et al., 2009). This study utilized the CPT task in children with and without ADHD and demonstrated that a large number of brain regions showed reduced functional connectivity in children with ADHD before medication, but that almost all of these interactivity differences dissipated following stimulant administration. In fact, of the 19 node-pairs that showed significantly reduced communication in the off-medication state, only two showed such abnormalities after stimulant ingestion and both involved the right cerebellum and inferior parietal cortices (Rubia et al., 2009). Finally, further evidence that stimulants modulate activity in anterior cingulate and lateral prefrontal cortices in ADHD is available in a study of adults (Bush et al., 2008); although these authors did not evaluate changes in functional connectivity.
In conclusion, our primary findings indicate that gamma activity is reduced in adults with ADHD compared to their non-ADHD peers, and that stimulant drugs increase gamma-frequency neuronal activity in persons with ADHD. Although speculative, abnormalities in GABAergic transmission or functional synapses may be the neural mechanism underlying this impairment, and stimulant drugs may modulate (directly or indirectly) such local GABAergic networks. Overall, these findings augment current hemodynamic studies of dysfunctional connectivity in ADHD by supplying evidence of neurophysiological aberrations, and inform structural imaging studies that have shown excessive pruning (i.e., decreased gray matter) in those not receiving stimulants, or at least efficacious treatment. To close, it is important to recognize limitations of this work including the modest sample sizes, relatively older samples (patients and controls) for adult ADHD research that included both sexes, the distinct medication histories of participants with ADHD (e.g., duration of stimulant treatment), limited information about subtype history (i.e., stable or changing) in the patients with ADHD and outright limited understanding of common subtype trajectories, lack of control for temporal order effects in regards to the medication manipulation (i.e., pre-drug scans were always before post-drug scans), and the absence of a placebo-control group. To date only one neuroimaging study (Rubia et al., 2009) has utilized both a healthy control group and a placebo-treatment group, and this study utilized the same 13 children (at two different time points) to form the placebo and treatment groups. Finally, it should be noted that we do not know whether patient-control differences in gamma activity represent causal processes, processes correlated with etiological risks (genetic or environmental) and non-causal, or result from the psychopathological processes associated with ADHD in adults. Future work will need to examine larger and more homogeneous patients groups, control for temporal order effects, utilize placebo-treatment and healthy control comparison groups, as well as extend the current observations to younger persons with ADHD.
Acknowledgements:
Funding for the Center for Magnetoencephalography has been generously provided by an anonymous private donor.
References
- Barry RJ, Clarke AR, Hajos M, McCarthy R, Selikowitz M, Bruggemann JM (2009) Acute atomoxetine effects on the EEG of children with attention-deficit/hyperactivity disorder. Neuropharmacology 57: 702–707. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Hajos M, McCarthy R, Selikowitz M, Dupuy FE (2010) Resting-state EEG gamma activity in children with Attention-Deficit/Hyperactivity Disorder. Clin Neurophysiol 121(11): 1871–1877. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Johnstone SJ (2003) A review of electrophysiology in Attention-Deficit/Hyperactivity Disorder: I. Qualitative and quantitative electroencephalography. Clin Neurophysiol 114: 171–183. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P (2007) Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 8: 45–56. [DOI] [PubMed] [Google Scholar]
- Biederman J, Quinn D, Weiss M, Markabi S, Weidenman M, Edson K, et al. (2003) Efficacy and safety of Ritalin LA, a new, once daily, extended-release dosage form of methylphenidate, in children with attention deficit hyperactivity disorder. Paediatr Drugs 5: 833–841. [DOI] [PubMed] [Google Scholar]
- Bush G, Spencer TJ, Holmes J, Shin LM, Valera EM, Seidman LJ, et al. (2008) Functional magnetic resonance imaging of Methylphenidate and placebo in attention-deficit/hyperactivity disorder during the multi-source interference task. Arch Gen Psychiatry 65: 102–114. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention (2005) Mental health in the United States: Prevalence of diagnosis and medication treatment for attention-deficit/hyperactivity disorder – United States, 2003. MMWR 54(34): 842–847. [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, Bond D, McCarthy R, Selikowitz M (2002) Effects of stimulant medications on the EEG of children with attention-deficit/hyperactivity disorder. Psychopharmacology (Berl) 164(3): 277–284. [DOI] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, McCarthy R, Selikowitz M, Brown CR, Croft RJ (2003) Effects of stimulant medications on the EEG of children with Attention-Deficit/Hyperactivity Disorder Predominantly Inattentive type. Int J Psychophysiol 47: 129–137. [DOI] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, McCarthy R, Selikowitz M, Johnstone SJ (2007) Effects of stimulant medications on the EEG of girls with Attention-Deficit/Hyperactivity Disorder. Clin Neurophysiol 118: 2700–2708. [DOI] [PubMed] [Google Scholar]
- Coghill D, Seth S (2006) Osmotic, controlled-release methylphenidate for the treatment of ADHD. Expert Opin Pharmacother 7: 2119–2138. [DOI] [PubMed] [Google Scholar]
- Cohen J (1988) Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Dockstader C, Gaetz W, Cheyne D, Tannock R (2009) Abnormal neural reactivity to unpredictable sensory events in attention-deficit/hyperactivity disorder. Biol Psychiatry 66: 376–383. [DOI] [PubMed] [Google Scholar]
- Dodds CM, Müller U, Clark L, van Loon A, Cools R, Robbins TW (2008) Methylphenidate has differential effects on blood oxygenation level-dependent signal related to cognitive subprocesses of reversal learning. J Neurosci 28: 5976–5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Glatt SJ (2010) A comparison of the efficacy of medications for adult attention-deficit/hyperactivity disorder using meta-analysis of effect sizes. J Clin Psychiatry 71: 754–763. [DOI] [PubMed] [Google Scholar]
- Fries P (2009) Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci 32: 209–224. [DOI] [PubMed] [Google Scholar]
- Fries P, Nikolic D, Singer W (2007) The gamma cycle. Trends Neurosci 30: 309–316. [DOI] [PubMed] [Google Scholar]
- Herdman AT, Wollbrink A, Chau W, Ishii R, Ross B, Pantev C (2003) Determination of activation areas in the human auditory cortex by means of synthetic aperture magnetometry. Neuroimage 20: 995–1005. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Ames M, Demler O, Faraone S, Hiripi E, et al. (2005) The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med 35: 245–256. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, et al. (2006) The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry 163: 716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Green JG, Adler LA, Barkley RA, Chatterji S, Faraone SV, et al. (2010) Structure and diagnosis of adult attention-deficit/hyperactivity disorder: analysis of expanded symptom criteria from the Adult ADHD Clinical Diagnostic Scale. Arch Gen Psychiatry 67: 1168–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krain AL, Castellanos F (2006) Brain development and ADHD. Clin Psychol Rev 26: 433–444. [DOI] [PubMed] [Google Scholar]
- Lenz D, Krauel K, Flechtner HH, Schadow J, Hinrichs H, Herrmann CS (2010) Altered evoked gamma-band responses reveal impaired early visual processing in ADHD children. Neuropsychologia 48: 1985–1993. [DOI] [PubMed] [Google Scholar]
- Lenz D, Krauel K, Schadow J, Baving L, Duzel E, Herrmann CS (2008) Enhanced gamma-band activity in ADHD patients lacks correlation with memory performance found in healthy children. Brain Res 1235: 117–132. [DOI] [PubMed] [Google Scholar]
- Newcorn JH, Kratochvil CJ, Allen AJ, Casat CD, Ruff DD, Moore RJ, et al. (2008) Atomoxetine and Osmotically Released Methylphenidate for the Treatment of Attention Deficit Hyperactivity Disorder: Acute Comparison and Differential Response. Am J Psychiatry 165: 721–730. [DOI] [PubMed] [Google Scholar]
- Paloyelis Y, Mehta MA, Kuntsi J, Asherson P (2007) Functional MRI in ADHD: A systematic literature review. Expert Rev Neurotherapeutics 7(10): 1337–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Potenza MN, Wang Z, Zhu H, Martin A, Marsh R, et al. (2009) An FMRI study of the effects of psychostimulants on default-mode processing during Stroop task performance in youths with ADHD. Am J Psychiatry 166: 1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross B, Borgmann C, Draganova R, Roberts LE, Pantev C (2000) A high-precision magnetoencephalographic study of human auditory steady-state responses to amplitude-modulated tones. J Acoust Soc Am 108: 679–691. [DOI] [PubMed] [Google Scholar]
- Ross B, Herdman AT, Pantev C (2005) Right hemispheric laterality of human 40 Hz auditory steady-state responses. Cereb Cortex 15: 2029–2039. [DOI] [PubMed] [Google Scholar]
- Ross B, Picton TW, Pantev C (2002) Temporal integration in the human auditory cortex as represented by the development of the steady-state magnetic field. Hear Res 165: 68–84. [DOI] [PubMed] [Google Scholar]
- Rubia K, Halari R, Cubillo A, Mohammad AM, Brammer M, Taylor E (2009) Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naïve children with ADHD during a rewarded continuous performance task. Neuropharmacology 57: 640–652. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Doyle A, Fried R, Valera E, Crum K, Matthews L (2004a) Neuropsychological function in adults with attention-deficit/hyperactivity disorder. Psychiatr Clin North Am 27: 261–282. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Bush G (2004b) Brain function and structure in adults with attention-deficit/hyperactivity disorder. Psychiatr Clin North Am 27: 323–347. [DOI] [PubMed] [Google Scholar]
- Shaw P, Sharp WS, Morrison M, Eckstrand K, Greenstein DK, Clasen LS, et al. (2009) Psychostimulant treatment and the developing cortex in attention deficit hyperactivity disorder. Am J Psychiatry 166: 58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W (1999) Neuronal synchrony: a versatile code for the definition of relations? Neuron 24: 49–65, 111–125. [DOI] [PubMed] [Google Scholar]
- Sobel LJ, Bansal R, Maia TV, Sanchez J, Mazzone L, Durkin K, et al. (2010) Basal Ganglia surface morphology and the effects of stimulant medications in youth with attention deficit hyperactivity disorder. Am J Psychiatry 167: 977–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL (2010) Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage 53: 303–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taulu S, Simola J (2006) Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol 51: 1759–1768. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J, Kajola M (2005) Applications of the signal space separation method (SSS). IEEE Trans Signal Process 53: 3359–3372. [Google Scholar]
- Tesche CD, Uusitalo MA, Ilmoniemi RJ, Huotilainen M, Kajola M, Salonen O (1995) Signal-space projections of MEG data characterize both distributed and well-localized neuronal sources. Electroencephalogr Clin Neurophysiol 95: 189–200. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Pipa G, Lima B, Melloni L, Neuenschwander S, Nikolić D, et al. (2009) Singer W. Neural synchrony in cortical networks: history, concept and current status. Front Integr Neurosci 3: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W (2006) Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron 52: 155–168. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Stollstorff M (2008) Cognitive neuroscience of attention deficit hyperactivity disorder: Current status and working hypotheses. Dev Disabil Res Rev 14: 261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienbruch C, Paul I, Bauer S, Kivelitz H (2005) The influence of methylphenidate on the power spectrum of ADHD children - an MEG study. BMC Psychiatry 5: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]