Abstract
In the biomedical sciences, particularly in wound healing, tissue engineering, and regenerative medicine, the development of natural-based biomaterials as a carrier has revealed a wide range of advantages. Tissue engineering is one of the therapeutic approaches used to replace damaged tissue. Polymers have received a lot of attention for their beneficial interactions with cells, but they have some drawbacks, such as poor mechanical properties. Due to their relatively large surface area, nanoparticles can cause significant changes in polymers and improve their mechanical properties. The nanoparticles incorporated into biomaterial scaffolds have been associated with positive effects on cell adhesion, viability, proliferation, and migration in the majority of studies. This review paper discusses recent applications of polymer-nanoparticle composites in the development of tissue engineering scaffolds, as well as the effects of these nanomaterials in the fields of cardiovascular, neural, bone, and skin tissue engineering.
Keywords: Nanoparticle, Tissue engineering, Polymer, Scaffold, Cardiac, Nerve, Bone, Skin
Nanoparticle; Tissue engineering; Polymer; Scaffold, Cardiac; Nerve, Bone; Skin.
1. Introduction
When one of the body's organs or tissues loses function due to disease, severe shock, or biological ageing, the person suffers from degeneration and the inability to carry out daily activities (Eivazzadeh-Keihan et al., 2020). Traditional methods for treating and replacing damaged tissues include tissue grafting in the form of autograft or allograft, each of which has advantages and disadvantages. Infection and hematoma in the transplanted area, as well as exclusion of the transplant by the host immune system, have all contributed to the failure of these methods. On the other hand, the challenges of high demand for organ transplants and donor shortages have forced scientists to consider alternatives to organ transplants (Bashiri et al., 2022; Khosrowpour et al., 2022). One of these alternative therapies that has become very attractive to researchers in this field today is the use of tissue engineering (Gholipourmalekabadi et al., 2016). Tissue engineering, treats damaged tissue with inspired by extracellular matrix of the body and simulates (Kankala et al., 2018). The extracellular matrix, or ECM, is a network of glycoproteins, cells, and interstitial fluid that plays a key role in the development of tissues and organs (Mehrasa et al., 2015). Biomaterial scaffolds play a pivotal role in tissue engineering and the production of alternative functional organs. They promote cell growth and proliferation by creating a platform for adhesion and cellular interactions and by using interactions such as biochemical, bioelectrical, or biomechanical signals (Mehdizadeh et al., 2015; Guo and Ma 2018). Among the materials used to make scaffolding are polymers that have different physical and chemical properties (Azadbakht et al., 2022). One of the advantages of polymers is that they change their properties by functionalizing. In this way, they can be optimized for specific applications (Balint et al., 2014). In addition to the type of polymers used, the scaffolding synthesis method also plays a key role in the success of this method (Ghasemi Hamidabadi, Rezvani et al., 2017). The use of single polymer scaffolds cannot provide all the requirements for cellular interactions. Therefore, the use of multi-polymer scaffolds can provide a significant strategy to support cell growth and proliferation and the development of innovative methods (Armentano et al., 2010). However, tissue engineering also has drawbacks, including the lack of suitable biomaterials to produce scaffolds and their inability to mimic the characteristics of tissues in vivo, lack of precise control of the physiological structure of scaffolds and cell function, as well as insufficient cellular communication (Hasan et al., 2018). Aging is a term used to describe the effects of plasma treatment on polymer surfaces, which not only results in surface modifications during the plasma exposure but also leaves active sites at the surfaces that are susceptible to post-reactions. There have been numerous attempts to use plasma treatments to develop monofunctional surfaces on polymers (Hegemann et al., 2003). The frequently employed plasma treatment (with oxygen) yields a wide range of functionalities, more often with a low stability. It is therefore preferable to reduce or, if possible, completely avoid these effects. Two general approaches are used: first, minimising the applied energy (for example, by using low power or pulsed powered plasmas) and minimising the kind and density of harmful particles during treatment in the plasma; and second, separating substrate functionalization from plasma in space (downstream) or in time (grafting). Panwar and coworkers developed elastic hydrogels with excellent physical properties that are functionalized with ureidopyrimidinone (Upy) (Panwar et al., 2022).
The use of nanotechnology in tissue engineering in order to create distinct conditions to achieve appropriate mechanical and biological performance as well as recent advances in the production of various nanoparticles have had a significant impact on their use in tissue engineering and regenerative medicine. Nanoparticles in nanoscale dimensions with high surface area, adjustable surface properties, increasing the mechanical strength of scaffolds and their antibacterial and antiseptic properties, have the potential in research to improve engineered tissues and overcome tissue engineering barriers (Hasan et al., 2018; Fathi-Achachelouei et al., 2019). These nanoparticles are a wide range of materials that are divided into different types based on morphology, size and chemical properties (Khan et al., 2019). To date, many nanoparticles have been used to modify synthesized scaffolds and improve tissue engineering performance in combination with polymers, including gold nanoparticles, ceramic nanoparticles, silver nanoparticles, magnetic nanoparticles and polymer nanoparticles (Fathi-Achachelouei et al., 2019). It should be noted that scaffold surface topography and chemistry (wettability, softness and stiffness, roughness); microstructure (porosity, pore size, pore shape, interconnectivity, specific surface area) and mechanical properties have been shown to significantly influence cell behaviors such as adhesion, growth and differentiation, and to affect the bioactivity of scaffolds used for in vivo regeneration applications of various tissues, such as cartilage, skin and peripheral nerves. More hydrophilic surface of material results more cell adhesion on the surface. It has been already reported that as the contact angle of surface increases then cell adhesion decreases. Additionally, the rate of cell spreading and differentiation is correlated with surface hydrophobicity. Cells displayed good spreading, proliferation, and differentiation on surfaces that were hydrophilic in most cases. Hydrophobic surfaces adsorb more proteins, whereas hydrophilic surfaces resist protein adsorption. Surface charge, like surface hydrophobicity, has recently received a lot of attention in the cell attachment phenomenon. As the amount of surface charges of polymers increases, more cell adhesion and proliferation are observed. It has been reported that using positive and negative ions improves biocompatibility, cell affinity, and cell differentiation on implanted surfaces. Positively charged HEMA hydrogels, for example, supported significantly more cell attachment and spreading of osteoblasts and fibroblasts than negative or neutral charges. In comparison to neutral or positively charged hydrogel scaffolds, negatively charged oligo (poly (ethylene glycol) fumarate) hydrogels increased the extent of chondrocyte differentiation, such as collagen and glycosaminoglycan expression. Through the chemical functionalities of polymer materials, surface charges can be used to modify cell behavior. Polar and positively charged surfaces (amine group-grafted PE) showed the best cell adhesion, growth, and spreading rate, whereas the negatively charged surface (carboxylic acid group-grafted PE) still had lesser growth. Another significant factor affecting cell adhesion and behavior is roughness of material surface (Chang and Wang 2011).
Piezoelectric materials can produce electrical signals in response to the applied stress, which can be imposed even by cell attachment and migration or physical movement. There are several scaffolds of polymeric materials with different cell attachments such as polylactic acids fibers for nerve stem cells, poly (3-hydroxybutyrate-co-3-hydroxyvalerate) for human mesenchymal stem cell, collagen for Schwann cells, poly (vinylidene fluoride-co-trifluoroethylene) for preosteoblasts and osteoblasts SaOS-2 cells, poly (3,4-ethylenedioxythiophene) for neural stem cells and brain neuroglioma cells etc (Surmeneva et al., 2017; Shkarina et al., 2018; Chernozem et al., 2019; Surmenev et al., 2019; Chernozem et al., 2021). For applications in tissue engineering, there are numerous magnetoactive composites that have been developed and investigated to date. For instance, hybrid scaffolds of PHBV and cobalt ferrite nanoparticles after the degradation of the polymer due to the exposure of the CoFe2O4 revealed suitability for magnetoactive tissue engineering applications. The magnetic cobalt ferrite nanoparticles required for magnetoactive applications, however, might be released and have cytotoxic effects. Therefore, it is important to look into replacing cobalt ferrite with biocompatible ferrites, such as Fe3O4, MnFe2O4. Additionally, the FDA has only approved Fe3O4 nanoparticles as the only metal oxide nanoparticles for clinical use (Kopyl et al., 2021).
A significant challenge is the fabrication of biopolymeric scaffolds that mimic the complex architecture of native tissues while having an appropriate porous structure for cell growth guidance in three dimensions. Several methods starting with polymer solutions or polymer melts have been proposed to process synthetic and natural biomaterials into porous scaffolds. It is possible to avoid using organic solvents that might have cytotoxic effects on the finished structure with melt-based technologies. However, melt-based techniques typically result in constructions with porosity levels that are lower than those suggested in the literature for tissue engineering applications. Various solution-based fabrication techniques, such as freeze-drying, thermally or diffusion-induced phase separation, electrospinning, or a useful combination of them, could be used depending on the expected structural and mechanical performance for tissue replacement. Polymer-filler scaffolds with different properties are tabulated in Table 1.
Table 1.
Polymer-filler scaffolds for tissue engineering applications.
| Polymer based composite | Properties | Application | Reference |
|---|---|---|---|
| PHB and PHBV | Biodegradable and Piezoelectric response | Tissue engineering applications | (Chernozem et al., 2022) |
| PLA/rGO composite | Biodegradable and Piezoelectric response | Tissue engineering applications. | (Pariy et al., 2022) |
| PHBV with filler (nanocellulose, walnut shell flour, eggshell flour, and tuff) | Excellent mechanical properties | Food packaging properties | (Kuciel et al., 2019) |
| PBS and PHBV with natural fillers talc and starch | Oxygen and water vapor permeabilities | Sustainable packaging | (Rodriguez-Uribe et al., 2021) |
| PHBV with agricultural fibers | Mechanical properties (tensile, flexural, and impact) | Tissue engineering applications | (Mazur et al., 2022) |
| PHBV scaffold with barium titanate | Compressive strength and elastic modulus | Tissue engineering applications | (Jiao et al., 2020) |
Poly(3-hydroxybutyrate): PHB; poly(3-hydroxybutyrate-co-3-hydroxyvalerate): PHBV; reduced graphene oxide: rGO; poly(l-lactic acid): PLA; poly(butylene succinate); PBS.
In this paper, we reviewed recent advances in tissue engineering and regenerative medicine using scaffolds decorated with nanoparticles and highlight the possibility of using these systems for tissue engineering.
2. Cardiovascular tissue engineering
Cardiac tissue engineering is the process of recovering, preserving, and improving the function of damaged heart tissue through the development of biological alternatives and their deployment in the body, as well as assisting in the development of in vitro models to better and more accurately study heart function (Hirt et al., 2014). Cardiac tissue engineering has the potential to significantly improve the quality of life of people suffering from heart disease. Cardiac tissue engineering reconstructs and repairs heart defects by combining three components: scaffolding, cells, and growth factors. A good scaffold should be mechanically similar to natural heart tissue and have a stable function. Furthermore, one of the critical tasks of these scaffolds is the propagation of electrical signals to generate mechanical contractions (Vunjak-Novakovic et al., 2010).
Biodegradable polyurethane nanocomposite scaffolds based on castor oil and combined with gold nanotubes/nanowires were invented by M. Janmohammadi et al. A total of 106 H9C2 cardiomyocytes were implanted on a cylindrical scaffold 10 mm in diameter and 4 mm in thickness. To improve cell communication and interaction, cells were stimulated by a function generator with a square pulse of 1V/mm amplitude, pulse duration of 2 ms, and frequency of 1 Hz. This procedure was carried out for 15 min on three consecutive days. Cell confluency on scaffolds PU-50 and PU-100 increased by 39% and 14%, respectively, after cell stimulation by the generator. Cells seeded on scaffolding that did not contain gold PU-0 had no effect on increasing cell confluency. The expression of genes H9C2 cardiomyocyte-related gene expression analysis revealed increased levels of Nkx2.5, atrial natriuretic peptide (ANF), and natriuretic peptide precursor B (NPPB) expression in scaffolds integrated with gold nanotubes/nanowires compared to no gold (Ganji et al., 2016).
Because of their ability to absorb water and resemble extracellular matrices, hydrogels are widely used in tissue engineering. A thermosensitive conductive hydrogel was developed using chitosan and gold nanoparticles, with spherical gold nanoparticles with an average particle size of 7.24 nm uniformly distributed throughout the hydrogel network. The sample was highly porous, with pores about 25 m in diameter that were joined uniformly across the network. The CS and CSGNP solutions were then combined with 107 mesenchymal stem cells (MSCs) and incubated at 37 °C for subsequent gelation. The results showed that using gold nanoparticles in the hydrogel network increased the network's electrical conductivity, which resulted in more cell differentiation into cardiac lineages. The viability, migration, and proliferation of MSCs in chitosan/gold nanoparticle hybrid scaffolds were also taken into account (Baei et al., 2016). To investigate the suppression of oxidative stress damage and repair of cardiac tissue in this study, an antioxidant hydrogel of fullerenol/alginate was prepared by adding fullerenol to a % wt solution of alginate. The cells BADSC were then mixed with the above solution, and calcium gluconate solution was added. Incubation took place. The number of cells seeded in fullerenol/alginate hydrogels increased significantly on day 7 compared to day 3, indicating low fullerenol cytotoxicity and excellent cell viability (Figure 1). At day 7, the cells increased in fullerenol/alginate hydrogel when compared to pure alginate hydrogel. The effect of fullerenol/alginate hydrogel on oxidative stress damage in BADSC revealed that the hydrogel activated the ERK and p38 pathways while inhibiting the JNK pathway. This, in turn, reduces oxidative stress damage to BADSCs and increases their ability to survive in a ROS-rich environment. The effect of fullerenol/alginate hydrogel on MI microenvironment in Dawley rat body was then investigated. The developed fullerenol/alginate hydrogel, in combination with BADSC, was injected into the body of a rat with MI. The findings suggest that BADSC implanted in fullerenol/alginate hydrogel have a higher retention and survival rate than BADSC injected directly because antioxidant activity reduces ROS levels and promotes blood supply by increasing angiogenesis (Hao et al., 2017).
Figure 1.
Cytocompatibility of fullerenol/alginate hydrogel. (A) Acridine orange/Propidium iodide (AO/PI) staining images of BADSCs at day 3, 7 and 14. (B) Proliferation behaviors of BADSCs within 14 days. (C) After 14 days of culture, 3D live cell pictures in fullerenol/alginate hydrogel. Reprinted with permission from ref. Hao et al., (2017).
The use of PCL polymer in clinical applications may be promising due to its desired mechanical properties, such as high strength and similarity to extracellular matrix. By solvent casting and freeze-drying, nanocomposite scaffolds containing PCL/PEG, PCL/PEG/MWCNT, and PCL/PEG/MWCNT with FG coating compounds, with concentrations of 0.5 and 1 (wt %) MWCNTs were prepared (Figure 1). According to the findings, the scaffolds have a porous structure with a porosity of approximately 50%, which is beneficial for cellular metabolism and the exchange of nutrients and waste cells. Furthermore, FG coated scaffolds demonstrated higher tensile strength than other scaffolds. The addition of MWCNT increased the elastic modulus, electrical conductivity, and wettability. The presence of MWCNT caused cells to adhere to the scaffold pore wall and to orient toward the nanotubes (Mehdikhani and Ghaziof 2018).
GelMA is a biocompatible and biodegradable hydrogel that has piqued the interest of researchers in the field of tissue engineering due to its excellent mechanical and biological properties. Due to their smaller diameter and higher aspect ratio than spherical gold nanoparticles, gold nanowires (AuNWs) can cause cardiac cells to respond to electrical stimulation more quickly. GelMA solution was mixed with various concentrations of AuNWs and exposed to UV light to develop the scaffold. Cardiomyocytes were cultured on a hydrogel scaffold after being extracted from neonatal rat ventricles.
High concentrations of AuNWs improved the electrical conductivity of hydrogels and facilitated signal propagation between cardiomyocytes and their coupling, according to hydrogel electrical conductivity measurements. Furthermore, the addition of AuNWs to GelMA increases the Young's modulus of scaffolds, improving mechanical properties such as more coherent pore distribution and scaffold stability. Cytotoxicity experiments revealed that incorporating AuNWs into hydrogels had no significant cytotoxicity on cardiomyocyte cells while improving cell adhesion, cellular density, and homogeneous distribution of cells on hybrid scaffolds compared to the control group. Electrophysiological examinations of cardiomyocytes seeded on GelMA hydrogels revealed that cells beat randomly and unsynchronizedly, whereas cardiomyocytes in the AuNWs hybrid hydrogel group beat spontaneously and synchronously (Li et al., 2020).
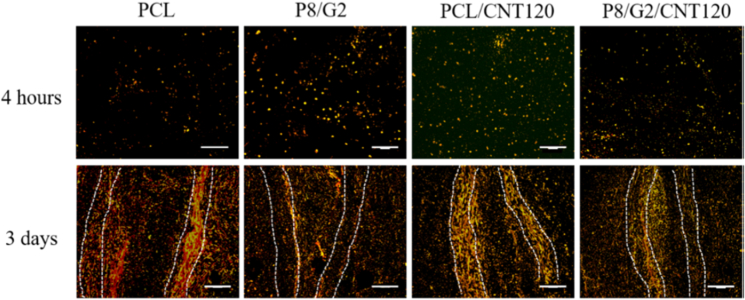
CNT was used to fabricate electrospun scaffolds in the form of ducts for vascular tissue engineering in a study conducted in 2021. PCL solution and gelatin solution were combined with different weight percentages for wet electrospinning and combined with CNT in the device's nozzle. The mechanical properties of wet electrospinning yarns were affected by CNT concentration, so that in a limited weight fraction of CNTs, the tensile strength and modulus of the composites increased. ECS cells were seeded onto the scaffold for biological studies. After two days, measurements were taken to determine cell viability, elongation, and alignment. The results showed that CNTs have concentration-dependent cytotoxicity, with cell viability significantly decreasing at a concentration of 180 mg/L due to CNT agglomeration on the surface of wet electrospun yarns, while increasing the carbon concentration from 0 to 180 mg/L increases the aspect ratios of cells grown on PCL yarns. Cell alignment measurements revealed that in the presence of CNT, approximately 72 % and 80 % of cells are aligned, whereas in the absence of CNT, this ratio is around 50%. Furthermore, proper scaffold biocompatibility promotes cellular interactions such as cell attachment and proliferation (Figure 2 and Table 2) (Jiang et al., 2021).
Figure 2.
Tracking ECS cells (red) on scaffolds fabricated by different yarns.
Table 2.
Modified composites for cardiac tissue engineering.
| Scaffold type | Cell type | Results | Reference |
|---|---|---|---|
| PU-GNT/NW composite scaffolds | H9C2 rat cardiomyocytes | More native morphology, increased proliferation and increased expression level of genes related to cardiac differentiation | (Ganji et al., 2016) |
| AuNPS–Cs thermosensitive hydrogels | MSCs | Survival and metabolism of mesenchymal stem cells, differentiation into cardiac lineage | (Baei et al., 2016) |
| Fullerenol/alginate hydrogel | BADSCs | Reducing the amount of oxidative stress, increasing the amount of angiogenesis and improving heart function | (Hao et al., 2017) |
| PCL/PEG/MWCNTs with FG-coating nanocomposite scaffolds | Mouse myoblasts | Increase in electrical conductivity, increase in wettability, distribution and proper development of myoblasts | (Mehdikhani and Ghaziof 2018) |
| GelMA-AuNWs hybrid hydrogels | Neonatal rat cardiomyocytes | Better biological activity, synchronous activity and faster spontaneous rate of cardiomyocytes | (Li et al., 2020) |
| PCL/Gt/CNT | ECS cells | Increasing biocompatibility, increasing the length of cells and an excellent candidate for making vessels | (Jiang et al., 2021) |
Graphene oxide: GO; Carbon nanotubes: CNT, Gold nanoparticles: AuNPs; Gelatin: Gt; polycaprolactone: PCL; polyethylene glycol: PEG; Chitosan: CS; mesenchymal stem cells: MSCs; Polyurethane: PU; Gold nanotube: GNT; MWCNTs: multi wall carbon nanotubes; Brown adipose-derived stem cells: BADSCs.
3. Neural tissue engineering or nerve regeneration
Nerve regeneration has always received a lot of attention from researchers because it directly affects the quality of life (Zare et al., 2020). The primary cause of neurological defects is axonal disruption and association degeneration (Kaplan and Levenberg 2022). To repair injured nerves, several conventional and novel therapies have been developed. Treatments for nerve injuries have recently centred on mimicking the extracellular microenvironment via the development of functional scaffolds. Many aspects of neural development are regulated by the extracellular matrix (ECM), which has long been studied. This includes neuronal cell proliferation, differentiation, morphology, axonal and dendritic elongation, migration, and connectivity (Long and Huttner 2021). Because the ECM follows the rules at the nanoscales (Yoshida and Kisley 2021), there has been a significant effort to develop scaffolds with nanoscale features that are designed for neural repair. A variety of materials, including polymers, nanoparticles, and biomolecules, have been used in various ways for this purpose. Neural tissue, for example, is electrically sensitive. We need some strategies to stimulate electrical signalling because electrical conductivity is critical for nervous tissue regeneration (Zare et al., 2020; Li et al., 2021; Magaz et al., 2021). Since a few years, the development and application of electroactive materials has been a thriving field of study in neural tissue engineering. Eftekhari and coworkers have fabricated polyaniline and chitosan based conductive hydrogel to induce PC12 cell surface topography using cell imprinting technique for nerve tissue engineering (Eftekhari et al., 2021). This assessment summarizes the progress of electroactive nanomaterials for neural regeneration and highlights the possibility of using those structures to regenerate neural tissue (Figure 3).
Figure 3.
Illustration of crucial factors for nerve tissue engineering.
3.1. Carbon based nanomaterials
Carbon nanotubes (CNTs) have been widely used by researchers in imaging, regenerative medicine, and pharmaceutical applications such as drug and gene delivery (Ahadian et al., 2016). The cytotoxicity of carbon nanotubes is undeniable. However, surface modification of CNTs has significantly reduced this toxicity (Smart et al., 2006). CNTs are promising neural tissue engineering booster substances due to their biocompatibility, physical properties, and conductivity (Mooney et al., 2012; Shin et al., 2013). Kabiri and colleagues demonstrated in 2015 that single walled carbon nanotubes (SWCNT) decorated poly (L-Lactic acid) (PLLA) nanofibers intended for nerve tissue engineering can support olfactory ensheathing glial cell (OEC) adhesion, growth, survival, and proliferation. They concluded that SWCNTs not only make the scaffold semiconducting, but can also reduce fibre diameter due to increased electrostatic forces (Kabiri et al., 2015; Ning et al., 2021; Sheng et al., 2021). Poly-lactide co glycolide/carbon nanotube (PLGA/CNT) modified by Laminin was introduced by Nazeri et al. Laminin has previously been identified as a protein that induces neurites. The presence of laminin not only improved neural cell proliferation but also resulted in extensive neuronal growth (Zhang et al., 2020; Nazeri et al., 2021; Han et al., 2022). A conductive composite based on poly (p-dioxanone) (PPDO) and carbon nanotubes was electrospun in a recent study. They demonstrated that a high concentration of CNTs had no negative effects on the cellular process, but it did better preserve the phenotype of rabbit Schwann cells (rSCs). Human adipose-derived mesenchymal stem cells (hADMSC) were significantly differentiated into SC-like cells after electrical stimulation (SCLC). Up-regulation of schwann cell myelination associated genes, in particular, confirmed maturation of hADMSC-SCLCs (Wu et al., 2022). Shrestha et al. created polyurethane/silk-functionalized multiwall carbon nanotubes (MWCNTs) ranging in size from 550nm to 867nm. MWCNT functionalization increased conductivity and made extracellular matrix absorption possible (ECM). Schwann cells and PC12 cells were stimulated to grow and reproduce as a result of the scaffold that was created. With successful axonal outgrowth, the expression of neural markers (III-tubulin, MAP2) was also increased (Figure 4) (Shrestha et al., 2019). Another study found that using MWCNTs in a chitosan/polyethylene glycol polymeric structure improved cellular adhesion, growth, proliferation, and neuronal differentiation. The physiochemical properties of the composite scaffold were evaluated, and it was discovered that the presence of MWCNTs resulted in a highly connected porous structure with a high conductivity rate (Sang et al., 2022). The incorporation of carbon nanotubes (CNTs) into polymeric structures has resulted in neural cell growth and axonal regeneration. In 2018, scientists combined carbon nanotubes (CNTs) with polycaprolactone fumarate (PCLF) to develop a conductive scaffold. Electrical stimulation promoted not only PC-12 cell proliferation and neurite extension, but also cell migration and intracellular connections (Zhou et al., 2018). CNTs have also been used in hydrogels (Park and Lakes 2007). Shafiee and colleagues loaded CNT-containing poly (lactic-co-glycolic acid) (PLGA) microspheres into a hydrogel. The presence of CNTs increased the porosity and conductivity of the polymeric structure, which stimulated the adhesion, proliferation, and differentiation of MSC-derived neural stem cells. CNTs are essential for enhancing synaptogenesis and neurite outgrowth. The presence of CNTs in the polymeric structure increased the expression of neuronal factors (Sox2-SYP and -Tubulin III) (Shafiee et al., 2021).
Figure 4.
(A) SEM Images and (B) Confocal fluorescence micrographs of PC12 cells cultured on aligned nanofibers (a and d) Pristine PU, (b and e) PU/Silk fibroin, and (c and d) PU/Silk fMWCNTs respectively. Nuclei and actin filaments were stained by DAPI (blue) and Actin green respectively in fluorescence images (Shrestha et al., 2019).
Blending CNTs with other substances, such as chitin hydrogel, significantly improved the hydrogel structure's tensile strength and elongation at break. In comparison to pure chitin hydrogel, the swelling ratio and biodegradability were reduced. Hemocompatibility, cell viability, proliferation, and neurite outgrowth were all observed with the hybrid hydrogel (Wu et al., 2017). Furthermore, Lijun Ye et al. reported long viability term, neural cell differentiation, and synaptic signaling. They also created a hydrogel composite out of poly (ethylene glycol) (PEG) and carbon nanotubes (CNTs) (Ye et al., 2021). The scaffolds' high positively charged surfaces and electrical amplifiers improved nerve cell differentiation. Yuan Sun et al. used graphene and CNTs embedded in poly (caprolactone fumarate) (PCLF) with the positively charged functional group [2-(methacryloyloxy)ethyl]trimethylammonium chloride for this purpose (MTAC) (Sun et al., 2021). A novel study in the field of retinal nerve regeneration using a conductive poly (lactic-co-glycolic acid) (PLGA)/CNTs scaffold. The biodegradability, biocompatibility, and electrical conductivity of scaffold containing CNTs are significantly higher than those of neat PLGA, which resulted in the growth and differentiation of human induced pluripotent stem cells (hiPSCs) into retinal ganglion cells (RGC) (Yang et al., 2021). Hong-Sun et al. designed an in-vivo study in 2015 that interfaced CNTs in phosphate glass microfibers (PGFs) before placing them in three-dimensional poly (L/D-lactic acid) (PLDLA). Electrical stimulation of CNTs was thought to be a major cause of the regeneration process. Under in vitro conditions, CNTs-PGFs outgrowth activity and neurite length were increased. Axon regeneration and motor function restoration were also reported sixteen weeks after scaffold implantation within a 10 mm gap in a transected rat sciatic nerve (Ahn et al., 2015). In addition, Xiaolin et al. developed a conductive nerve conduit out of carbon nanotubes (CNT) and Sericin with the goal of causing peripheral nerve injury (PNI). The nerve conduit was used in a 10mm lesion injury to the dissected rat sciatic nerve. External electrical stimulation was also used concurrently with placement. By the end of 12 weeks, motor function and nerve structure had recovered (Li et al., 2020).
Graphene is an aromatic hexagonal structure with carbon-carbon covalent bonds. Because of its hydrophilicity, low stress induction, high electrical conductivity, and surface area, it is ideal for use in scaffolds (Chong et al., 2015; Ahadian et al., 2017). Zhu et al. discovered stem cell differentiation and neurite elongation into neural cells by combining graphene nanoplatelets with gelatin methacrylamide hydrogel (Wei et al., 2016). Guo et al. investigated mesenchymal stem cells (MSC) on the scaffold by incorporating reduced graphene oxide (rGO) into poly (3,4-ethylenedioxythiophene) (PEDOT) microfibers. The incorporation of rGO increased electrical conductivity and cytocompatibility significantly. Furthermore, the use of a hybrid scaffold accelerated neural differentiation (Guo et al., 2016). Heidari et al. doped rGO into PCL/GELATIN mats in another study. The use of rGO significantly improved antibacterial properties, drug release, hydrophilicity, and conductivity (Heidari et al., 2019). Grafting rGO with poly (trimethylene carbonate) (PTMC) increased cell viability compared to neat PTMC fibers (Guo et al., 2019). Additionally, using rGO decorated GELMA/PCL scaffold improved electrical conductivity and biocompatibility. The recovery of injured rats' proliferation, differentiation, and sensory/motor function was then improved (Fang et al., 2020). Magaz et al. investigated the incorporation of rGO into silk fibers, which enhanced electrical conductivity, cell proliferation, and metabolic activity, making it ideal for PNIs regeneration (Magaz et al., 2021). Furthermore, the incorporation of rGO modifies the physicochemical properties of scaffolds, which can significantly improve the viability, proliferation, and differentiation of neural progenitor cells (Girão et al., 2020). GO was functionalized by grafting raffinose and incorporated into silk fibroin in a recent study. Raf-GO/silk exhibits improved electrical conductivity and hydrophilicity. When GO/silk and raf-GO/silk mats were compared to neat silk mats, overexpression of neural specific proteins and genes was observed (Jafari et al., 2021). Table 3 provides a summary of carbon-based nanomaterials used in neural tissue engineering.
Table 3.
Carbon based nanomaterials for nerve regeneration.
| Scaffold type | Application | References |
|---|---|---|
| Conductive nanofibrous CNT/poly (L-Lactic acid) composite scaffold | Scaffold to support olfactory ensheathing glial cell (OEC) adhesion, growth, survival, and proliferation | (Kabiri et al., 2015) |
| Poly-lactide-co-glycolide/carbon nanotube nanofibrous scaffolds by laminin protein | Scaffold to induce proliferation of neural cells and extensive neuronal growth | (Nazeri et al., 2021) |
| PPDO + CNTs nanofibers | Scaffold to accelerate mesenchymal stem cells differentiation and maturation into Schwann cell-like cells | (Wu et al., 2022) |
| Polyurethane/silk-functionalized MWCNTs | To induce neuroregeneration | (Shrestha et al., 2019) |
| Cs/PEG/MWCNTs composite scaffolds | Scaffold to increase cellular adhesion, growth, proliferation and neuronal differentiation | (Sang et al., 2022) |
| CNT incorporated in polycaprolactone fumarate (PCLF) | Scaffold to increase PC-12 cell proliferation, migration and neurite extension | (Zhou et al., 2018) |
| In situ hydrogel-forming scaffold loaded by PLGA microspheres containing carbon nanotube | Scaffold to stimulate adhesion, proliferation and differentiation of neural stem cells (NSCs) derived from MSC | (Shafiee et al., 2021) |
| Chitin/carbon nanotubes composite hydrogel | Scaffold to enhance hemo-compatibility, cell viability, proliferation and neurite outgrowth | (Wu et al., 2017) |
| PEG and CNTs hydrogel | Scaffold to facilitate Neuronal Differentiation | (Ye et al., 2021) |
| Embedded graphene and CNTs into poly(caprolactone fumarate) (PCLF)+MTAC | Scaffold to improve nerve cells differentiation | (Sun et al., 2021) |
| Poly(lactic-co-glycolic acid) (PLGA)/CNTs | Scaffold for BV2 and RGCs cells growth and differentiation of human induced pluripotent stem cells (hiPSCs) into retinal ganglion Cell (RGC) | (Yang et al., 2021) |
| PLDLA + CNT + PGFs | Scaffold for regeneration of transected sciatic nerve | (Ahn et al., 2015) |
| Nerve conduit based on CNT and Sericin | Scaffold for peripheral nerve injury | (Li et al., 2020) |
| Gelatin methacrylamide hydrogel with graphene nanoplatelets | differentiation and elongation neurite of stem cells into neural cells for nerve tissue engineering | (Wei et al., 2016) |
| Incorporated rGO into PEDOT microfibers | Scaffold to Enhance Neural Differentiation of Mesenchymal Stem Cells | (Guo et al., 2016) |
| Smart electrospun nanofibers containing PCL/Gt/GO | Scaffold to improve Antibacterial properties, sustainable release of drug, hydrophilicity and conductivity intended nerve tissue engineering | (Heidari et al., 2019) |
| Poly (trimethylene carbonate)/reduced graphene oxide-graft-poly (trimethylene carbonate) | Scaffold to enhance cell viability for nerve regeneration | (Guo et al., 2019) |
| rGO-GelMA-PCL hybrid nanofibers | Scaffold to improve electrical conductivity, biocompatibility, proliferation, differentiation and sensory/motor function recovery of injured rats | (Fang et al., 2020) |
| GO and electroactive reduced graphene oxide | Scaffold for cells proliferation and metabolic activity intended peripheral nerve injury regeneration | (Magaz et al., 2021) |
| Raffinose-grafted GO in silk fibroin-based scaffold | Scaffold to enhance viability, proliferation and differentiation of neural progenitor cells | (Jafari et al., 2021) |
3.2. Metallic and magnetic nanoparticles
Because of their conductivity, biocompatibility, modification capability, nanoscale topography, and optic properties, gold nanoparticles (AuNPs) have been used in biological applications (Chen et al., 2005; Dykman and Khlebtsov 2012). Pooshidani et al. developed a conductive nanofibrous scaffold by synthesizing Polycaprolactone/chitosan/gold nanoparticles (PCL/Chit/AuNPs) in situ. The analysis revealed that scaffold conductivity and wettability were improved, resulting in increased schwann cell viability and neurite (Pooshidani et al., 2021). In another study, Dana et al. combined gold nanorods with silk fibroins to produce a conductive nanocomposite for peripheral nerve regeneration. When compared to pure silk, the porosity and electrical conductivity of the nanocomposite improved. As a result, PC12 cell attachment, growth, proliferation, and overexpression of neural specific proteins on the silk/GNRs performed better than neat silk (Figure 5) (Afjeh-Dana et al., 2019). The use of 3D porous cobalt-doped alginate/water borne polyurethane has been shown to improve neurite growth and reduce inflammatory response (Chen et al., 2021). In the field of magnetic nanomaterials, Liu et al. used nanohydroyapatite coated iron oxide nanoparticles. While using n-HA, neural outgrowth was increased. Thus, the (Fe3O41n-HA) scaffold improved cellular viability and activated Netrin-1 signaling (Liu et al., 2015). Wang et al. used Ni nanoparticles doped with electrospun polyacrylonitrile/polyaniline (PAN/PANI) nanofibers. They combined this scaffold with external electrostimulation. Electrical stimulation accelerated the growth and proliferation of Schwann cells compared to control (Wang et al., 2021). Different scaffolds based on polymer and metal oxide are tabulated in Table 4.
Figure 5.
Micrographs of PC12 cells in SF scaffolds and SF/GNRs nanocomposites. DAPI staining of PC12 cells on (a) SF scaffolds and (b) SF/GNRs nanocomposites (scale bar: 200 μm). Confocal micrograph of PC12 cells on (c) SF scaffold and (d) SF/GNRs nanocomposites (scale bar: 200 μm). Morphology of PC12 cells on (e) SF scaffold and (f) SF/GNRs nanocomposites (scale bar: 10 μm). SEM micrograph of PC12 cells on (g) SF scaffold and (h) SF/GNRs nanocomposites (Afjeh-Dana et al., 2019).
Table 4.
Magnetic and metal nanoparticle for nerve regeneration.
| Scaffold type | Application | Reference |
|---|---|---|
| Polycaprolactone/Chitosan/Gold nanoparticles (PCL/Chit/AuNPs) | Scaffold to enhance schwann cells viability and neurite | (Pooshidani et al., 2021) |
| Integrated gold nanorods into silk fibroins | Scaffold for PC12 cells attachment, growth, proliferation and overexpression of neural specific proteins | (Afjeh-Dana et al., 2019) |
| Bioactive 3D porous cobalt-doped alginate/waterborne polyurethane | Scaffold for Neurite growth enhancement and inflammatory response reduction | (Chen et al., 2021) |
| Nano-hydroxyapatite-coated magnetic nanoparticles | Scaffold for axonal guidance growth of rat dorsal root ganglion neurons | (Liu et al., 2015) |
| Polyacrylonitrile/polyaniline with nickel nanoparticles | Scaffold to accelerate Schwann cells growth and proliferation upon electrical stimulation | (Wang et al., 2021) |
3.3. Electroactive polymers
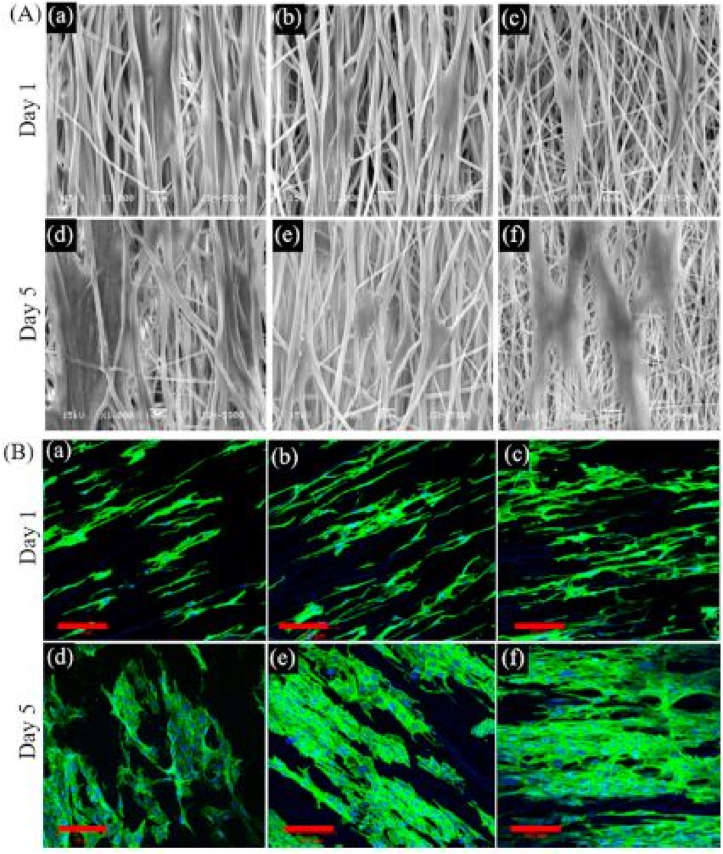
Electroactive polymers are widely used in the synthesis of conductive scaffolds for muscle, nerve, and cardiac tissue reconstruction (Balint et al., 2014). PEDOT (poly (3,4-ethylenedioxythiophene)) is a well-known conductive polymer with no cytotoxicity. Wang et al. assembled this polymeric nanoparticle on a chitosan/gelatin (Cs/Gel) composite for use in nerve tissue engineering in 2017. This scaffold also passes physiochemical features testes and enhanced PC12 cell adhesion, proliferation, and neurite growth (Wang et al., 2017). Researchers have been drawn to the appropriate properties of polypyrrole (PPy), which include ease of synthesis, biocompatibility, tunable conductivity, suitable mechanical features, and good stability. Tavakoli et al. conducted research to assess the potential of using Nano-chitosan incorporated polypyrrole/alginate scaffold in nerve engineering. The findings revealed high electrical conductivity, biodegradability, and porosity. Furthermore, cellular analysis confirmed the attachment, viability, and proliferation of OLN-93 neural and fibroblast cells (Figure 6) (Manzari-Tavakoli et al., 2020). Electroactive polymer scaffolds for nerve regeneration are tabulated in Table 5.
Figure 6.
(a) The attachment of OLN-93 neural cells on nanochitosan/PPy–Alg scaffold. (b) High magnitude illustration of the cell which shows by blue arrow (Manzari-Tavakoli et al., 2020).
Table 5.
Electroactive polymers for nerve regeneration.
| Scaffold type | Application | Reference |
|---|---|---|
| Chitosan/gelatin (Cs/Gel) composite | Scaffold to enhance PC12 cells adhesion, proliferation and neurite growth | (Wang et al., 2017) |
| Nanochitosan incorporated polypyrrole/alginate | Scaffold for attachment, viability and proliferation of OLN-93 neural and fibroblast cells | (Manzari-Tavakoli et al., 2020) |
4. Bone tissue engineering
Bone is one of the most important tissues in the body, acting as a lever for soft tissue structural strength. This vital tissue is also the foundation for muscle movement and contraction. Bones also have other functions, such as producing blood cells and storing minerals like calcium and phosphate. Bone injuries can occur due to a number of factors, including ageing. Infection, injury, or tumor. Because bone tissue can repair defects on a small scale, it is classified as a dynamic tissue. While larger-scale defect repair necessitates clinical and surgical interventions. Today, bone graft treatment and the use of implants are common. It is a common bone with limitations. Researchers in this field have focused a lot of attention on bone tissue engineering with the goal of repairing and repairing damaged tissue and restoring the tissue to its previous function (Gholipourmalekabadi et al., 2015; Taghvaei et al., 2020; Collins et al., 2021; Manzini et al., 2021). In 2015, researchers synthesized three-dimensional homogeneous hydrogels consisting of graphene oxide nanosheets and hydroxyapatite (G/HA) nanoparticles for bone tissue engineering using colloidal chemistry. The nanocomposites that were produced were porous and biocompatible, with excellent conductivity and mechanical properties. Cell viability tests on G/HA hydrogel revealed that hydroxyapatite nanoparticles improved mesenchymal stem cell adhesion. Although the distribution of cells on the rGO hydrogel was uniform, the morphology of the cells in the G/HA hydrogel was more elongated (Xie et al., 2015).
Another study was conducted the same year to evaluate the effect of reduced graphene oxide (rGO) and hydroxyapatite (HAp) nanocomposites (rGO/HAp NCs) on osteogenesis and the production of new bone tissue. Increased differentiation of MC3T3-E1 cells in the presence of rGO/HAp nanocomposite confirms NCs' stimulatory role in cell differentiation via intracellular signalling promotion. As a result, the use of graphene-based composites in bone grafting and dental implants will be feasible in the not-too-distant future (Lee et al., 2015).
Graphene oxide has also been used to modify other scaffolds like poly (L-lactic-co-glycolic acid) and tussah silk fibroin. The scaffold, which was developed via electrospinning, had a high Young's modulus and tensile strength, which increased the osteogenic potential of mouse mesenchymal stem cells. The scaffold's hydrophilicity and protein adsorptivity increased the proliferation and adhesion of mouse mesenchymal stem cells (Shao et al., 2016).
Zheng et al. repaired femoral bone fractures with a combination of collagen, silver nanoparticles, and mMSCs. The use of collagen reduced the toxicity of nanoparticles. At the fracture site, scaffold grafting containing silver nanoparticles completely closed the fracture with new callus and cartilage matrix. Silver nanoparticles induced the proliferation and differentiation of mMSCs into osteoblasts (Zhang et al., 2015).
In addition, multifunctional zirconium oxide (ZrO2) doped nanocomposites containing chitosan, organically modified montmorillonite (OMMT), and nano-hydroxyapatite (nHAP) were used to engineer bone tissue. The presence of ZrO2 nanoparticles in the nanocomposite produced strong antibacterial effects. This raises the possibility of using this nanocomposite to prevent infection in orthopedic implants. Furthermore, the scaffold's compatibility with human body pH, cell compatibility, and low toxicity led to adequate proliferation of MG-63 cells (Bhowmick et al., 2016).
Ceramic nanoparticles are now used in tissue engineering due to their ability to increase cell adhesion. The researchers used metal nanoparticles of copper (Cu) and zinc (Zn) to modify the chitosan/gelatin/nanohydroxyapatite (Ch/G/nHAp) nanocomposite scaffolds and cultured mouse embryonic fibroblasts (MEFs) on it. Proper cell growth and proliferation revealed that the synthesized scaffold has good porosity and pore size, and the presence of copper and zinc nanoparticles (nCuZn) due to the roughness of the scaffold surface causes more cell adhesion and facilitates cell migration. Biocompatibility induced by nanoparticles (nHAp/nCuZn) infiltrated new cells and tissues into the matrix and formed granulation tissue at the edges, contradicting observations on the scaffold surface without nanoparticles (Forero et al., 2017).
After incorporation in gelatin coated scaffolds, Cu-containing bioactive glass nanoparticles (Cu-BGN) increased the bioactivity of the scaffold. MC3T3-E1 cells' increased alkaline phosphatase activity, hydrox-yapatite formation, and bone formation indicate appropriate scaffold morphology and low toxicity (Figure 7) (Zheng et al., 2018).
Figure 7.
CLSM images of MC3T3-E1 cells in culture with the coated scaffolds for 24 h: (a) 20Cu-BGS; (b) 5Cu-BGS; and (c) 0Cu-BGS. The cytoskeleton was stained with FITC-Phalloidin (red) and nuclei were stained with DAPI (blue).
20Cu-BGS; (b) 5Cu-BGS; and (c) 0Cu-BGS. The cytoskeleton was stained with FITC-Phalloidin (red) and nuclei were stained with DAPI (blue).
To regenerate bone tissue, Das et al. combined superparamagnetic iron oxide nanoparticles (SPIONs) and quantum carbon dots (CDs) with gelatin. These nanoparticles were found to be capable of scavenging free radicals. Mesenchymal stem cells (MSCs) derived from Wharton's jelly were cultured on the scaffold with and without magnetic stimulation before being implanted subcutaneously in a rodent model. Histological observations revealed that, unlike culture samples without magnetic stimulation, in which iron particles were deposited, iron particles were distributed throughout the sample in samples cultured with magnetic stimulation, and these samples formed osteoblast-like nested island structures after subcutaneous culture. As a result, the thick calcium deposition around the nests confirms bone differentiation (Figure 8) (Das et al., 2019).
Figure 8.
Fluorescence microscopic images after incubation with FeCDs: (a) green filter; (b) red filter; (c) merged images (d) time dependent MTT assay; (e) superoxide degradation assay; (f) hydroxyl radical activity assay.
In another study, the use of silver nanoparticles and bioactive glass in gelatin nanocomposites resulted in antibacterial properties and, as a result, increased cell proliferation in electrospun scaffolds. Furthermore, the addition of silver nanoparticles to the nanocomposite decreased the diameter of the fibers and their proximity to the extracellular matrix of bone, resulting in increased success for use in bone tissue engineering (Akturk et al., 2020).
Due to its size similarity to bone tissue apatite, nanohydroxyapatite has been widely used in tissue engineering. In one study, polylactic acid (PLA) scaffolding was used to repair bone defects because of its good results, but nanohydroxyapatite (n-HA) was used to modify the composite because of its poor performance in osteogenic induction. The presence of inflammation and necrosis, as well as the growth and proliferation of bone marrow mesenchymal stem cells (BMSCS), indicate that the scaffold is biocompatible and n-HA is non-toxic (Wang et al., 2021).
Another study used nano-hydroxyapatite (nHAP) and Fe3O4 nanoparticles to change the composition of chitosan/collagen (CS/Col). The scaffold was biocompatible and cytoactive. There was clearly increased interaction between the scaffold and the MC3T3-E1 cells cultured on the scaffold. Cell viability was higher in CS/Col/Fe3O4/nHAP scaffolds than in CS/Col/nHAP scaffolds. It was also observed that calvarial defect restoration by scaffold grafting at the defect site was destroyed over time as new bone tissue was formed (Zhao et al., 2019).
Dandan et al. created a composite scaffold with antibacterial and osteogenic properties using MgSrFe-LDH/CS and silver nanoparticles in 2017. Cell growth and proliferation were stimulated by the scaffold's porosity. The incorporation of nanoparticles into the scaffold composition, as well as their uniform distribution throughout the scaffold, resulted in improved adhesion, increased alkaline phosphatase activity, and further differentiation of human bone marrow-derived mesenchymal stem cells (hBMSCs), indicating that nanoparticles are non-toxic to cells (Cao et al., 2018). Various polymer based composites for tissue engineering applications are tabulated in Table 6.
Table 6.
Modified composites for bone tissue engineering.
| Scaffold | Cell type | Results | Reference |
|---|---|---|---|
| G/HA hydrogels | Mouse multipotent MSCs | Increasing mechanical properties, electrical conductivity, porosity and high biocompatibility | (Xie et al., 2015) |
| rGO/HAp Nanocomposites | MC3T3-E1 cells | Increasing the expression level of osteopontin and osteocalcin and repairing skull defects with appropriate thickness and without inflammatory responses | (Lee et al., 2015) |
| PLGA-tussah silk fibroin/GO scaffolds | mMSC | Increasing Young's modulus, increasing adhesion and osteogenesis | (Shao et al., 2016) |
| Col/AgNPs | mMSC | Increasing proliferation and bone differentiation of mesenchymal stem cells and early fracture repair | (Zhang et al., 2015) |
| CS-OMMT-HAP-ZrO2 nanocomposites | Human osteoblastic MG-63 cells | Increasing thermal stability, tensile strength and antibacterial properties, increasing biocompatibility and cell proliferation | (Bhowmick et al., 2016) |
| CS/Gt/nHAp/nCuZn nanocomposites scaffold | Mouse Embryonic Fibroblasts (MEFs) | Improving mechanical properties and increasing porosity, proper cell growth | (Forero et al., 2017) |
| Cu-BGN/Gt coated 45S5 BG scaffold | MC3T3-E1 | Increasing bioactivity and osteogenic potential | (Zheng et al., 2018) |
| FeCD-Gt nanocomposite scaffolds | Wharton's jelly derived mesenchymal stem cells (MSCs) | Increasing cellular compatibility and reducing the amount of free radicals | (Das et al., 2019) |
| Gt/AgNPs/BG nanocomposite | L929 fibroblast | Suitable for bone tissue engineering due to its antibacterial and cell proliferation potential | (Akturk et al., 2020) |
| PLA/n-HA composite scaffolds | Bone marrow mesenchymal stem cells (BMSCS) | Increased biocompatibility and bone induction properties | (Wang et al., 2021) |
| CS/Col/Fe3O4/nHAP scaffolds | MC3T3-E1 cells | Creating superior structural and mechanical properties for adhesion, proliferation and differentiation of osteogenesis | (Zhao et al., 2019) |
| Ag–Mg–Sr–Fe/CS composite scaffolds | Human bone marrow-derived mesenchymal stem cells (hBMSCs) | Improving cell adhesion, growth and proliferation. Inducing bacterial properties | (Cao et al., 2018) |
| PLMA-NF composite scaffolds | - | Increased the biocompatibility of NF coated scaffolds | (Mozafari et al., 2015) |
| Oc-HA-Gt nanocomposite scaffold | MSCs | Enhanced biocompatibility and osteoinductivity | (Samadikuchaksaraei et al., 2016) |
Graphene: G; Reduced graphene oxide: rGO; Silver nanoparticles: AgNPs; Gelatin: Gt; Bioactive glasses: BG; Chitosan: CS; poly(lactic acid): PLA; Nano-hydroxyapatite: n-HA; Organo-modified montmoillonite: OMMT; Collegen: CO; mesenchymal stem cells: MSCs; Mouse mesenchymal stem cells: mMSC; poly(L-lactide-co-β-malic acid): PLMA; nanocrystalline forsterite: NF; Gelatin: Gt; Osteoblast: Oc.
5. Skin tissue engineering
Skin tissue engineering (STE) has the potential to improve skin care quality, particularly in the elderly population, due to its unique capabilities (Gholipourmalekabadi et al., 2019; Gholipourmalekabadi et al., 2020; Ahovan et al., 2022; Chizari et al., 2022). Polymers and nanomaterials have recently been highlighted as a new medical challenge in STE and wound healing (Fang et al., 2022). Bioactive and electroactive polymers could be used individually or in combination. Polymers have frequently been mixed with various nanomaterials in recent years. Polymers and nanomaterials can accelerate wound healing, induce antimicrobial activity, improve skin absorption, and stimulate electrical conductivity. Nanomaterials are important in skin regeneration. They influence angiogenesis, for example, by stimulating basic factors (Figure 9). This review focuses on the use of polymers and nanomaterials as a composite in skin tissue engineering (Sahana and Rekha 2018; Talikowska et al., 2019; Cui et al., 2020; Zhang et al., 2022).
Figure 9.
Illustration of skin tissue engineering approach.
Nyambat et al. (2018) used graphene oxide (GO) in ASD-derived ECM that was cross-linked with Ginipin. The goal of their research was to develop a biodegradable, biocompatible scaffold with lower immunogenicity than other medical approaches such as skin grafts. Proper porosity and degradability were found to be significantly improved in an in vitro study. Similarly, they discovered material feasibility, biodegradability, and insignificant immunogenic reaction after 4 weeks of subcutaneous implantation of composite (Nyambat et al., 2018). Sadeghianmaryan and colleagues used an electrospinning method to combine GO with PCL/PU nanofibers. The presence of GO improved scaffold wettability. Furthermore, they reported that the use of GO in composite did not cause toxicity in human fibroblast cells (Sadeghianmaryan et al., 2020). Polymeric nanomaterials have also been used to treat wounds. Alamsian et al. developed PU/Pluronic F127 nanofibers containing a hydro-alcoholic extract of Mentha piperita. They improved extract release by crosslinking it with gelatin nanoparticles. At day 14, this study demonstrated wound healing with significant neovascularization and insignificant inflammatory response (Figures 10 and 11) (Almasian et al., 2021). In another study, collagen was grafted onto PCL nanofibers to create a 3D scaffold. In comparison to neat PCL and Collagen, the combination of PCL/Collagen resulted in higher hydrophilicity with proper mechanical cues. Furthermore, this study revealed significant attachment and proliferation of human endometrial stem cells (hEnSCs) on the composite (Sharif et al., 2018). Because of their mechanical properties, cellulose fibrils and silk fibroin have been used for wound healing and bleeding homeostasis. Afrin Shefa et al. created a bioactive scaffold out of TEMPO-oxidized cellulose nanofiber (TOCN) and silk fibroin. The scaffold aided the growth of L929 primary fibroblast cells. Furthermore, histological examination revealed that the TOCN-silk fibroin scaffold overexpressed wound healing markers (Shefa et al., 2017). Chitosan's antimicrobial property is one of its maternal properties (CS). Furthermore, Chitosan has the potential to be a wound healing accelerator. In 2019, researcher developed a chitosan-based scaffold using chitosan, polyethylene oxide, and berberine (CS/PEO/BBR). They studied wound healing in BALB/c mice with Leishmania major wound ulcers. The findings revealed reductions in lesion diameter and parasite load (Seyyed Tabaei, Rahimi et al., 2020). Sanhueza et al. used electrospinning to combine PHB with a natural polymer. They chose gelatin (GE) as the main component of collagen. Fibroblast cells adhered to and proliferated on the scaffold. After 21 days, the use of scaffold for diabetic ulcer demonstrated the potential for treatment (Sanhueza et al., 2021). Metal-based nanoparticles have been widely used in biological applications (Himiniuc et al., 2022). Silicon dioxide (SiO2), an inorganic material, is essential for cell metabolism. Polyvinylpyrrolidone (PVP) and SIO2 were used to develop a wound dressing by Ori et al. Wound contraction and re-epithelialization were observed as a result of wound dressing in an animal model (Öri et al., 2017). In another study, silica nanoparticles (SiNP) were mixed into chitosan hydrogel. The adhesion of hydrogel with skin tissue in an animal model demonstrated that it is appropriate for biological tissues. Meanwhile, they proposed that encapsulating fibroblast cells in hydrogel in situ could result in skin regeneration (Zhu et al., 2017). Mebert et al. used collagen hydrogel and silica core-shell nanoparticles to sustain the release of gentamicin and rifampin to prevent skin ulcer dressing contamination. Antimicrobial activity was found to be excellent in vitro and in vivo (Mebert et al., 2018). Zinc oxide (ZnO) is an inorganic nanoparticle type. It is frequently used in cosmetic applications due to its photocatalytic properties (Himiniuc et al., 2022). The freeze-dry method was used to develop alginate hydrogel decorated ZnO in 2015. Porosity, wettability, degradability, and antimicrobial cues are all present in the nanocomposite. The nanocomposite also demonstrated human dermal fibroblast cell viability and ex-vivo re-epithelialization (Mohandas et al., 2015). To prevent wound microbial contamination, Zhai et al. produced a wound dressing with keratin-chitosan and ZnO nanoparticles. They reported that the fabricated nanocomposite not only has appropriate physiochemical and antimicrobial properties, but it can also lead to wound treatment acceleration (Zhai et al., 2018). Because of its photosensitizer property, nano-Titania (TiO2) has recently been used in medical approaches such as hyperthermia therapy. Wang et al. used a thermogel made of Chitosan and TiO2 to treat skin cancer and regenerate skin tissue. The developed thermogel is used not only to limit skin tumor cells but also to accelerate skin cell regeneration (Wang et al., 2019). Based on previous studies, cerium dioxide is an antioxidant that can adjust the PH of the wound environment. In 2021, Russian researchers used a polymeric nanocomposite made of nano-cerium dioxide and mesenchymal stem cells (MSC) to heal wounds. The use of smart scaffolding results in anti-inflammation, cell proliferation, and re-epithelialization (Silina et al., 2021). Janani et al. used polycaprolactone nanofiber decorated with molybdenum oxide to treat skin cancer. Molybdenum oxide is a metal element that can cause electrical conductivity. They demonstrated a significant reduction in cancer cell viability and progression (Janani et al., 2018). AuNRs (gold nanorods) are another metallic nanoparticle with distinct optical and electronic properties. In 2019, researchers used a thermogel made of poly ethylene glycol (PEG) and cationic poly allyl amine hydrochloride (PAH) as the scaffold's base. In animal models, the incorporation of AuNRs into thermosensitive hydrogel can result in anti-inflammatory effects, antimicrobial activity, signalling, reepithelization enhancement, and eventually wound healing (Mahmoud et al., 2019). Because of their native antimicrobial, anti-inflammatory, and wound healing properties, silver nanoparticles (AgNPs) have recently opened a new avenue in skin care research. In a burned animal model, the use of hydrogels containing AgNPs can have a significant effect on wound healing (Xi et al., 2018). In another study, a wound dressing nanocomposite hydrogel composed of guar gum-grafted-polyacrylamidoglycolic acid (GG-g-PAGA) and AgNPs was developed. The in vitro results revealed antibacterial, as well as low cytotoxicity in skin fibroblast cells (Palem et al., 2019). Polyvinyl Alcohol/Polyethylene Glycol/Chitosan/AgNPs Hydrogels were tested for antimicrobial activity by Li et al. They discovered that low concentrations of AgNPs demonstrated proper bacteriostatic and cell viability rates (Li et al., 2019). To reduce toxicity, AgNPs were synthesized in 2020 using a green synthesis method that included a hydroalcoholic extract of Brazilian pepper. The developed nanocomposite hydrogel demonstrated good antimicrobial activity and excellent viability of L929 fibroblast cells (de Oliveira et al., 2021). Recently, nanocomposite hydrogels have received attention in the field of drug delivery systems for the treatment of chronic diseases on the skin. This method was successful due to appropriate results, such as bactericidal effect against wound with multidrug resistance pathogens. In addition, the rate of skin regeneration and angiogenesis in the animal model could be accelerated (Ragothaman et al., 2021). In 2022, Farshi and coworkers have developed blends based on carboxymethyl cellulose and gelatin with different concentrations of glycerol and modified the film using silk fibroin layer using electrospinning technique (Farshi et al., 2022). Various polymer based nanomaterials for skin tissue engineering applications are tabulated in Table 7.
Figure 10.
H&E and MT stained microscopic sections of healed incisions in treatment groups, Black thick arrows, crusty scab; white thick arrow, epidermal layer; red thick arrows, rejuvenation of skin appendages (Almasian et al., 2021).
Figure 11.
Representative wounds on an animal on days 0, 3, 7, 14, and 21 after treatment for (a) conventional gauze bandage, (b) PU/F, (c) PU/F/15, and (d) PU/F/15/10 (Almasian et al., 2021).
Table 7.
Polymer based nanomaterial in skin tissue engineering application.
| Scaffold type | Application | Reference |
|---|---|---|
| GO in ASC derived ECM that crosslinked with Ginipin | Scaffold for skin tissue engineering | (Nyambat et al., 2018) |
| PCL/PU composite with GO | Scaffold for human fibroblast cell | (Sadeghianmaryan et al., 2020) |
| PU/Pluronic F127 Nanofibers containing peppermint extract loaded gelatin nanoparticles | Scaffold for diabetic wounds healing | (Almasian et al., 2021) |
| Collagen-coated nano-electrospun PCL | Scaffold for attachment and proliferation of human endometrial stem cells (hEnSCs) on the composite | (Sharif et al., 2018) |
| TEMPO-oxidized cellulose nanofiber-silk fibroin | overexpression of wound healing markers | (Shefa et al., 2017) |
| Chitosan-based nano-scaffolds | Scaffold for antileishmanial wound dressing in BALB/c mice | (Seyyed Tabaei, Rahimi et al., 2020) |
| Gelatin/poly-3-hydroxybutyrate nano/microfibers | Scaffold for diabetic ulcer dressing | (Sanhueza et al., 2021) |
| Silicon dioxide- PVP | Scaffold for wound contraction and reepithelialization | (Öri et al., 2017) |
| Chitosan hydrogel with silica nanoparticle | Scaffold for regeneration of injured skin | (Zhu et al., 2017) |
| Collagen hydrogel and silica core-shell nanoparticle | Scaffold for sustain release of gentamicin and rifampin to prevent skin ulcers dressing contamination | (Mebert et al., 2018) |
| Alginate hydrogel/nano zinc oxide composite | Scaffold for for infected wounds | (Mohandas et al., 2015) |
| Keratin-chitosan/n-ZnO nanocomposite hydrogel | Scaffold to accelerate of wound treatment | (Zhai et al., 2018) |
| chitosan and TiO2 | Scaffold for skin tumor cure and regeneration of skin tissue | (Wang et al., 2019) |
| Nano-cerium dioxide and MSC | Scaffold for wound healing | (Silina et al., 2021) |
| Molybdenum oxide-PCL nanofiber | Scaffold to reduce viability of cancer cells and cancer progression | (Janani et al., 2018) |
| Gold nanoparticles loaded into PEG and cationic PAH | Scaffold for wound healing in animal model | (Mahmoud et al., 2019) |
| nano-silver hydrogel coating film | Scaffold for burned animal model | (Xi et al., 2018) |
| GG-g-PAGA hydrogel and AgNPs | Scaffold for wound dressing | (Palem et al., 2019) |
| PVA/PEG/Chitosan Hydrogels with AgNPs | Scaffold to increase bacteriostatic and cell viability rate | (Li et al., 2019) |
| Hydrogel contained AgNPs obtained from brazilian pepper extracts | Antimicrobial effect and excellent L929 fibroblast cells viability | (de Oliveira et al., 2021) |
| Collagen-capped AgNPs and melatonin PCL-Gt electrosun scaffold |
Scaffold to accelerate skin tissue regeneration Biocompatible with improved angiogenic Property |
(Ragothaman et al., 2021) (Fabin Cheng et al., 2022) |
Graphene oxide: GO; Adipose stem cell: ASC; Polycaprolactone: PCL; Polyurethane: PU; Polyvinylpyrrolidone: PVP; Mesenchymal stem cells: MSC; poly allyl amine hydrochloride:PAH; poly ethylene glycol:PEG; Guar gum-grafted-polyacrylamidoglycolic acid:GG-g-PAGA; Polyvinyl alcohol: PVA; Polyethylene glycol: PEG; Silver nanoparticles: AgNPs.
6. Conclusion
The goal of tissue engineering is to develop functional tissue substitutes and/or encourage endogenous regeneration by combining the principles of engineering, cell transplantation, and biomaterials. Tissue engineering uses naturally derived biopolymers extensively because of their benefits. They facilitate cell migration, adhesion, proliferation, and differentiation, all of which are essential steps in the repair process. Nanomaterials were incorporated into bio-polymeric constructs to provide multifunctionality and improved properties, which opened up new possibilities for tissue engineering. Materials like polymers, metals, and carbon-based structures are all used in tissue engineering.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
No data was used for the research described in the article.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Narendra Pal Singh Chauhan, Email: narendrapalsingh14@gmail.com.
Ghasem Sargazi, Email: g.sargazi@gmail.com.
References
- Afjeh-Dana E., et al. Gold nanorods reinforced silk fibroin nanocomposite for peripheral nerve tissue engineering applications. Int. J. Biol. Macromol. 2019;129:1034–1039. doi: 10.1016/j.ijbiomac.2019.02.050. [DOI] [PubMed] [Google Scholar]
- Ahadian S., et al. Fabrication of poly(ethylene glycol) hydrogels containing vertically and horizontally aligned graphene using dielectrophoresis: an experimental and modeling study. Carbon. 2017;123:460–470. [Google Scholar]
- Ahadian S., et al. Carbon nanotubes and graphene-based nanomaterials for stem cell differentiation and tissue regeneration. J. Nanosci. Nanotechnol. 2016;16(9):8862–8880. [Google Scholar]
- Ahn H.S., et al. Carbon-nanotube-interfaced glass fiber scaffold for regeneration of transected sciatic nerve. Acta Biomater. 2015;13:324–334. doi: 10.1016/j.actbio.2014.11.026. [DOI] [PubMed] [Google Scholar]
- Ahovan Z.A., et al. Antibacterial smart hydrogels: new hope for infectious wound management. Mater. Today Biol. 2022:100499. doi: 10.1016/j.mtbio.2022.100499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akturk A., et al. Optimization of the electrospinning process variables for gelatin/silver nanoparticles/bioactive glass nanocomposites for bone tissue engineering. Polym. Compos. 2020;41(6):2411–2425. [Google Scholar]
- Almasian A., et al. Preparation of polyurethane/pluronic F127 nanofibers containing peppermint extract loaded gelatin nanoparticles for diabetic wounds healing: characterization, in vitro, and in vivo studies. Evid. Based Compl. Alternat. Med. 2021;2021 doi: 10.1155/2021/6646702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armentano I., et al. Biodegradable polymer matrix nanocomposites for tissue engineering: a review. Polym. Degrad. Stabil. 2010;95(11):2126–2146. [Google Scholar]
- Azadbakht A., et al. Macromolecular Bioscience; 2022. Chitosan-placental ECM Composite Thermos-responsive Hydrogel as a Biomimetic Wound Dressing with Angiogenic Property. [DOI] [PubMed] [Google Scholar]
- Baei P., et al. Electrically conductive gold nanoparticle-chitosan thermosensitive hydrogels for cardiac tissue engineering. Mater. Sci. Eng. C. 2016;63:131–141. doi: 10.1016/j.msec.2016.02.056. [DOI] [PubMed] [Google Scholar]
- Balint R., et al. Conductive polymers: towards a smart biomaterial for tissue engineering. Acta Biomater. 2014;10(6):2341–2353. doi: 10.1016/j.actbio.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Bashiri Z., et al. In vitro production of mouse morphological sperm in artificial testis bioengineered by 3D printing of extracellular matrix. Int. J. Biol. Macromol. 2022;217:824–841. doi: 10.1016/j.ijbiomac.2022.07.127. [DOI] [PubMed] [Google Scholar]
- Bhowmick A., et al. Multifunctional zirconium oxide doped chitosan based hybrid nanocomposites as bone tissue engineering materials. Carbohydr. Polym. 2016;151:879–888. doi: 10.1016/j.carbpol.2016.06.034. [DOI] [PubMed] [Google Scholar]
- Cao D., et al. Ag-loaded MgSrFe-layered double hydroxide/chitosan composite scaffold with enhanced osteogenic and antibacterial property for bone engineering tissue. J. Biomed. Mater. Res. B Appl. Biomater. 2018;106(2):863–873. doi: 10.1002/jbm.b.33900. [DOI] [PubMed] [Google Scholar]
- Chang H.-I., Wang Y. Regenerative medicine and tissue engineering-cells and biomaterials. InTechOpen; 2011. Cell responses to surface and architecture of tissue engineering scaffolds. [Google Scholar]
- Chen J., et al. Gold nanocages: engineering their structure for biomedical applications. Adv. Mater. 2005;17(18):2255–2261. [Google Scholar]
- Chen Y., et al. Bioactive 3D porous cobalt-doped alginate/waterborne polyurethane scaffolds with a coral reef-like rough surface for nerve tissue engineering application. J. Mater. Chem. B. 2021;9(2):322–335. doi: 10.1039/d0tb02347g. [DOI] [PubMed] [Google Scholar]
- Chernozem R., et al. Piezoelectric 3-D fibrous poly (3-hydroxybutyrate)-based scaffolds ultrasound-mineralized with calcium carbonate for bone tissue engineering: inorganic phase formation, osteoblast cell adhesion, and proliferation. ACS Appl. Mater. Interfaces. 2019;11(21):19522–19533. doi: 10.1021/acsami.9b04936. [DOI] [PubMed] [Google Scholar]
- Chernozem R.V., et al. A comprehensive study of the structure and piezoelectric response of biodegradable polyhydroxybutyrate-based films for tissue engineering applications. Polym. J. 2022:1–12. [Google Scholar]
- Chernozem R.V., et al. Enhanced piezoresponse and surface electric potential of hybrid biodegradable polyhydroxybutyrate scaffolds functionalized with reduced graphene oxide for tissue engineering. Nano Energy. 2021;89 [Google Scholar]
- Chizari M., et al. Fabrication of an antimicrobial peptide-loaded silk fibroin/gelatin bilayer sponge to apply as a wound dressing; an in vitro study. Int. J. Pept. Res. Therapeut. 2022;28(1):1–13. [Google Scholar]
- Chong Y., et al. Reduced cytotoxicity of graphene nanosheets mediated by blood-protein coating. ACS Nano. 2015;9(6):5713–5724. doi: 10.1021/nn5066606. [DOI] [PubMed] [Google Scholar]
- Collins M.N., et al. Scaffold fabrication technologies and structure/function properties in bone tissue engineering. Adv. Funct. Mater. 2021;31(21) [Google Scholar]
- Cui L., et al. Nanomaterials for angiogenesis in skin tissue engineering. Tissue Eng. B Rev. 2020;26(3):203–216. doi: 10.1089/ten.TEB.2019.0337. [DOI] [PubMed] [Google Scholar]
- Das B., et al. Carbon nanodots doped super-paramagnetic iron oxide nanoparticles for multimodal bioimaging and osteochondral tissue regeneration via external magnetic actuation. ACS Biomater. Sci. Eng. 2019;5(7):3549–3560. doi: 10.1021/acsbiomaterials.9b00571. [DOI] [PubMed] [Google Scholar]
- de Oliveira D.M., et al. Silver nanoparticles obtained from Brazilian pepper extracts with synergistic anti-microbial effect: production, characterization, hydrogel formulation, cell viability, and in vitro efficacy. Pharmaceut. Dev. Technol. 2021;26(5):539–548. doi: 10.1080/10837450.2021.1898634. [DOI] [PubMed] [Google Scholar]
- Dykman L., Khlebtsov N. Gold nanoparticles in biomedical applications: recent advances and perspectives. Chem. Soc. Rev. 2012;41(6):2256–2282. doi: 10.1039/c1cs15166e. [DOI] [PubMed] [Google Scholar]
- Eftekhari B.S., et al. Conductive chitosan/polyaniline hydrogel with cell-imprinted topography as a potential substrate for neural priming of adipose derived stem cells. RSC Adv. 2021;11(26):15795–15807. doi: 10.1039/d1ra00413a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eivazzadeh-Keihan R., et al. Metal-based nanoparticles for bone tissue engineering. J. Tissue Eng. Regen. Med. 2020;14(12):1687–1714. doi: 10.1002/term.3131. [DOI] [PubMed] [Google Scholar]
- Fabin Cheng, Huanqing Wang, Liang Zhang, A. AHMAD. Ning Xu. Decentralized adaptive neural two-bit-triggered control for nonstrict-feedback nonlinear systems with actuator failures, Neurocomputing, 500:856-867, 2022.
- Fang X., et al. Reduced graphene oxide-GelMA-PCL hybrid nanofibers for peripheral nerve regeneration. J. Mater. Chem. B. 2020;8(46):10593–10601. doi: 10.1039/d0tb00779j. [DOI] [PubMed] [Google Scholar]
- Farshi P., et al. Design, preparation, and characterization of silk fibroin/carboxymethyl cellulose wound dressing for skin tissue regeneration applications. Polym. Eng. Sci. 2022 [Google Scholar]
- Fathi-Achachelouei M., et al. Use of nanoparticles in tissue engineering and regenerative medicine. Front. Bioeng. Biotechnol. 2019;7:113. doi: 10.3389/fbioe.2019.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forero J.C., et al. Development of useful biomaterial for bone tissue engineering by incorporating nano-copper-zinc alloy (nCuZn) in chitosan/gelatin/nano-hydroxyapatite (Ch/G/nHAp) scaffold. Materials. 2017;10(10):1177. doi: 10.3390/ma10101177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganji Y., et al. Cardiomyocyte behavior on biodegradable polyurethane/gold nanocomposite scaffolds under electrical stimulation. Mater. Sci. Eng. C. 2016;59:10–18. doi: 10.1016/j.msec.2015.09.074. [DOI] [PubMed] [Google Scholar]
- Ghasemi Hamidabadi H., et al. Chitosan-intercalated montmorillonite/poly (vinyl alcohol) nanofibers as a platform to guide neuronlike differentiation of human dental pulp stem cells. ACS Appl. Mater. Interfaces. 2017;9(13):11392–11404. doi: 10.1021/acsami.6b14283. [DOI] [PubMed] [Google Scholar]
- Gholipourmalekabadi M., et al. Modulation of hypertrophic scar formation using amniotic membrane/electrospun silk fibroin bilayer membrane in a rabbit ear model. ACS Biomater. Sci. Eng. 2019;5(3):1487–1496. doi: 10.1021/acsbiomaterials.8b01521. [DOI] [PubMed] [Google Scholar]
- Gholipourmalekabadi M., et al. Development of a cost-effective and simple protocol for decellularization and preservation of human amniotic membrane as a soft tissue replacement and delivery system for bone marrow stromal cells. Adv. Healthc. Mater. 2015;4(6):918–926. doi: 10.1002/adhm.201400704. [DOI] [PubMed] [Google Scholar]
- Gholipourmalekabadi M., et al. Silk fibroin for skin injury repair: where do things stand? Adv. Drug Deliv. Rev. 2020;153:28–53. doi: 10.1016/j.addr.2019.09.003. [DOI] [PubMed] [Google Scholar]
- Gholipourmalekabadi M., et al. Oxygen-generating biomaterials: a new, viable paradigm for tissue engineering? Trends Biotechnol. 2016;34(12):1010–1021. doi: 10.1016/j.tibtech.2016.05.012. [DOI] [PubMed] [Google Scholar]
- Girão A.F., et al. 3D reduced graphene oxide scaffolds with a combinatorial fibrous-porous architecture for neural tissue engineering. ACS Appl. Mater. Interfaces. 2020;12(35):38962–38975. doi: 10.1021/acsami.0c10599. [DOI] [PubMed] [Google Scholar]
- Guo B., Ma P.X. Conducting polymers for tissue engineering. Biomacromolecules. 2018;19(6):1764–1782. doi: 10.1021/acs.biomac.8b00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., et al. Self-powered electrical stimulation for enhancing neural differentiation of mesenchymal stem cells on graphene-poly(3,4-ethylenedioxythiophene) hybrid microfibers. ACS Nano. 2016;10(5):5086–5095. doi: 10.1021/acsnano.6b00200. [DOI] [PubMed] [Google Scholar]
- Guo Z., et al. Fabrication of poly (trimethylene carbonate)/reduced graphene oxide-graft-poly (trimethylene carbonate) composite scaffolds for nerve regeneration. Biomed. Mater. 2019;14(2) doi: 10.1088/1748-605X/ab0053. [DOI] [PubMed] [Google Scholar]
- Han M.-C., et al. Single-side superhydrophobicity in Si3N4-doped and SiO2-treated polypropylene nonwoven webs with antibacterial activity. Polymers. 2022;14(14):2952. doi: 10.3390/polym14142952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao T., et al. Injectable fullerenol/alginate hydrogel for suppression of oxidative stress damage in brown adipose-derived stem cells and cardiac repair. ACS Nano. 2017;11(6):5474–5488. doi: 10.1021/acsnano.7b00221. [DOI] [PubMed] [Google Scholar]
- Hasan A., et al. Nanoparticles in tissue engineering: applications, challenges and prospects. Int. J. Nanomed. 2018;13:5637. doi: 10.2147/IJN.S153758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegemann D., et al. Plasma treatment of polymers for surface and adhesion improvement. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 2003;208:281–286. [Google Scholar]
- Heidari M., et al. Smart electrospun nanofibers containing PCL/gelatin/graphene oxide for application in nerve tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;103 doi: 10.1016/j.msec.2019.109768. [DOI] [PubMed] [Google Scholar]
- Himiniuc L.M., et al. Update on the use of nanocarriers and drug delivery systems and future directions in cervical cancer. J. Immunol. Res. 2022;2022:1636908. doi: 10.1155/2022/1636908. 1636908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt M.N., et al. Cardiac tissue engineering: state of the art. Circ. Res. 2014;114(2):354–367. doi: 10.1161/CIRCRESAHA.114.300522. [DOI] [PubMed] [Google Scholar]
- Jafari A., et al. PC12 cells proliferation and morphological aspects: inquiry into raffinose-grafted graphene oxide in silk fibroin-based scaffold. Mater. Sci. Eng. C Mater. Biol. Appl. 2021;121 doi: 10.1016/j.msec.2020.111810. [DOI] [PubMed] [Google Scholar]
- Janani I., et al. Selectivity and sensitivity of molybdenum oxide-polycaprolactone nanofiber composites on skin cancer: preliminary in-vitro and in-vivo implications. J. Trace Elem. Med. Biol. 2018;49:60–71. doi: 10.1016/j.jtemb.2018.04.033. [DOI] [PubMed] [Google Scholar]
- Jiang C., et al. Using wet electrospun PCL/gelatin/CNT yarns to fabricate textile-based scaffolds for vascular tissue engineering. ACS Biomater. Sci. Eng. 2021;7(6):2627–2637. doi: 10.1021/acsbiomaterials.1c00097. [DOI] [PubMed] [Google Scholar]
- Jiao H., et al. Synthesis and properties of porous piezoelectric BT/PHBV composite scaffold. J. Biomater. Sci. Polym. Ed. 2020;31(12):1552–1565. doi: 10.1080/09205063.2020.1764164. [DOI] [PubMed] [Google Scholar]
- Kabiri M., et al. Cytocompatibility of a conductive nanofibrous carbon nanotube/poly (L-Lactic acid) composite scaffold intended for nerve tissue engineering. Excli J. 2015;14:851–860. doi: 10.17179/excli2015-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankala R.K., et al. Cardiac tissue engineering on the nanoscale. ACS Biomater. Sci. Eng. 2018;4(3):800–818. doi: 10.1021/acsbiomaterials.7b00913. [DOI] [PubMed] [Google Scholar]
- Kaplan B., Levenberg S. The role of biomaterials in peripheral nerve and spinal cord injury: a review. Int. J. Mol. Sci. 2022;23(3) doi: 10.3390/ijms23031244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I., et al. Nanoparticles: properties, applications and toxicities. Arab. J. Chem. 2019;12(7):908–931. [Google Scholar]
- Khosrowpour Z., et al. Coculture of adipose-derived mesenchymal stem cells/macrophages on decellularized placental sponge promotes differentiation into the osteogenic lineage. Artif. Organs. 2022 doi: 10.1111/aor.14394. [DOI] [PubMed] [Google Scholar]
- Kopyl S., et al. Magnetoelectric effect: principles and applications in biology and medicine–a review. Mater. Today Biol. 2021;12 doi: 10.1016/j.mtbio.2021.100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuciel S., et al. Novel biorenewable composites based on poly (3-hydroxybutyrate-co-3-hydroxyvalerate) with natural fillers. J. Polym. Environ. 2019;27(4):803–815. [Google Scholar]
- Lee J.H., et al. Enhanced osteogenesis by reduced graphene oxide/hydroxyapatite nanocomposites. Sci. Rep. 2015;5(1):1–13. doi: 10.1038/srep18833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., et al. Preparation and performance of antibacterial Polyvinyl alcohol/polyethylene glycol/chitosan hydrogels containing silver chloride nanoparticles via one-step method. Nanomaterials. 2019;9(7) doi: 10.3390/nano9070972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.-P., et al. High-aspect-ratio water-dispersed gold nanowires incorporated within gelatin methacrylate hydrogels for constructing cardiac tissues in vitro. J. Mater. Chem. B. 2020;8(32):7213–7224. doi: 10.1039/d0tb00768d. [DOI] [PubMed] [Google Scholar]
- Li X., et al. CNT/Sericin conductive nerve guidance conduit promotes functional recovery of transected peripheral nerve injury in a rat model. ACS Appl. Mater. Interfaces. 2020;12(33):36860–36872. doi: 10.1021/acsami.0c08457. [DOI] [PubMed] [Google Scholar]
- Li Y., et al. Tissue engineering strategies for peripheral nerve regeneration. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.768267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., et al. Effect of nano-hydroxyapatite-coated magnetic nanoparticles on axonal guidance growth of rat dorsal root ganglion neurons. J. Biomed. Mater. Res. 2015;103(9):3066–3071. doi: 10.1002/jbm.a.35426. [DOI] [PubMed] [Google Scholar]
- Long K.R., Huttner W.B. The role of the extracellular matrix in neural progenitor cell proliferation and cortical folding during human neocortex development. Front. Cell. Neurosci. 2021;15 doi: 10.3389/fncel.2021.804649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magaz A., et al. Graphene oxide and electroactive reduced graphene oxide-based composite fibrous scaffolds for engineering excitable nerve tissue. Mater. Sci. Eng. C Mater. Biol. Appl. 2021;119 doi: 10.1016/j.msec.2020.111632. [DOI] [PubMed] [Google Scholar]
- Mahmoud N.N., et al. Gold nanoparticles loaded into polymeric hydrogel for wound healing in rats: effect of nanoparticles' shape and surface modification. Int. J. Pharm. 2019;565:174–186. doi: 10.1016/j.ijpharm.2019.04.079. [DOI] [PubMed] [Google Scholar]
- Manzari-Tavakoli A., et al. Fabrication of nanochitosan incorporated polypyrrole/alginate conducting scaffold for neural tissue engineering. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-78650-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzini B.M., et al. Advances in Bone tissue engineering: a fundamental review. J. Biosci. 2021;46(1):1–18. [PubMed] [Google Scholar]
- Mazur K.E., et al. Mechanical, thermal and hydrodegradation behavior of poly (3-hydroxybutyrate-co-3-hydroxyvalerate)(PHBV) composites with agricultural fibers as reinforcing fillers. Sustain. Mater. Technol. 2022;31 [Google Scholar]
- Mebert A.M., et al. Collagen-silica nanocomposites as dermal dressings preventing infection in vivo. Mater. Sci. Eng. C Mater. Biol. Appl. 2018;93:170–177. doi: 10.1016/j.msec.2018.07.078. [DOI] [PubMed] [Google Scholar]
- Mehdikhani M., Ghaziof S. Electrically conductive poly-$${∖upepsilon} $$ ε-caprolactone/polyethylene glycol/multi-wall carbon nanotube nanocomposite scaffolds coated with fibrin glue for myocardial tissue engineering. Appl. Phys. A. 2018;124(1):1–15. [Google Scholar]
- Mehdizadeh H., et al. Design of polymer scaffolds for tissue engineering applications. Ind. Eng. Chem. Res. 2015;54(8):2317–2328. [Google Scholar]
- Mehrasa M., et al. Electrospun aligned PLGA and PLGA/gelatin nanofibers embedded with silica nanoparticles for tissue engineering. Int. J. Biol. Macromol. 2015;79:687–695. doi: 10.1016/j.ijbiomac.2015.05.050. [DOI] [PubMed] [Google Scholar]
- Mohandas A., et al. Exploration of alginate hydrogel/nano zinc oxide composite bandages for infected wounds. Int. J. Nanomed. 2015;10(Suppl 1):53–66. doi: 10.2147/IJN.S79981. (Suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney E., et al. The electrical stimulation of carbon nanotubes to provide a cardiomimetic cue to MSCs. Biomaterials. 2012;33(26):6132–6139. doi: 10.1016/j.biomaterials.2012.05.032. [DOI] [PubMed] [Google Scholar]
- Mozafari M., et al. Synthesis and characterization of nanocrystalline forsterite coated poly (l-lactide-co-β-malic acid) scaffolds for bone tissue engineering applications. Mater. Sci. Eng. C. 2015;50:117–123. doi: 10.1016/j.msec.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Nazeri N., et al. The effect of surface modification of poly-lactide-co-glycolide/carbon nanotube nanofibrous scaffolds by laminin protein on nerve tissue engineering. J. Biomed. Mater. Res. 2021;109(2):159–169. doi: 10.1002/jbm.a.37013. [DOI] [PubMed] [Google Scholar]
- Ning F., et al. Yarn on yarn abrasion performance of high modulus polyethylene fiber improved by graphene/polyurethane composites coating. J. Eng. Fibers Fabr. 2021;16 [Google Scholar]
- Nyambat B., et al. Genipin-crosslinked adipose stem cell derived extracellular matrix-nano graphene oxide composite sponge for skin tissue engineering. J. Mater. Chem. B. 2018;6(6):979–990. doi: 10.1039/c7tb02480k. [DOI] [PubMed] [Google Scholar]
- Öri F., et al. Silicon-dioxide-polyvinylpyrrolidone as a wound dressing for skin defects in a murine model. J. Cranio-Maxillo-Fac. Surg. 2017;45(1):99–107. doi: 10.1016/j.jcms.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Palem R.R., et al. Self-healable and dual-functional guar gum-grafted-polyacrylamidoglycolic acid-based hydrogels with nano-silver for wound dressings. Carbohydr. Polym. 2019;223 doi: 10.1016/j.carbpol.2019.115074. [DOI] [PubMed] [Google Scholar]
- Panwar A., et al. Synthesis and fabrication of gelatin-based elastomeric hydrogels through cosolvent-induced polymer restructuring. RSC Adv. 2022;12(13):7922–7934. doi: 10.1039/d1ra09084d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariy I.O., et al. Hybrid biodegradable electrospun scaffolds based on poly (l-lactic acid) and reduced graphene oxide with improved piezoelectric response. Polym. J. 2022:1–16. [Google Scholar]
- Park J., Lakes R.S. Springer Science & Business Media; 2007. Biomaterials: an Introduction. [Google Scholar]
- Pooshidani Y., et al. Fabrication and evaluation of porous and conductive nanofibrous scaffolds for nerve tissue engineering. J. Mater. Sci. Mater. Med. 2021;32(4):46. doi: 10.1007/s10856-021-06519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Fang, Xiang Liu, Kehan Zeng, Xuedong Zhang, Mozhen Zhou, Jianming Du, Centrifuge modelling of tunnelling below existing twin tunnels with different types of support, Undergr Space, 7, 6, 2022, 1125-1138, ISSN 2467-9674.
- Ragothaman M., et al. Bio-hybrid hydrogel comprising collagen-capped silver nanoparticles and melatonin for accelerated tissue regeneration in skin defects. Mater. Sci. Eng. C Mater. Biol. Appl. 2021;128 doi: 10.1016/j.msec.2021.112328. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Uribe A., et al. Injection moldable hybrid sustainable composites of BioPBS and PHBV reinforced with talc and starch as potential alternatives to single-use plastic packaging. Composites Part C: Open Access. 2021;6 [Google Scholar]
- Sadeghianmaryan A., et al. Electrospinning of scaffolds from the polycaprolactone/polyurethane composite with graphene oxide for skin tissue engineering. Appl. Biochem. Biotechnol. 2020;191(2):567–578. doi: 10.1007/s12010-019-03192-x. [DOI] [PubMed] [Google Scholar]
- Sahana T.G., Rekha P.D. Biopolymers: applications in wound healing and skin tissue engineering. Mol. Biol. Rep. 2018;45(6):2857–2867. doi: 10.1007/s11033-018-4296-3. [DOI] [PubMed] [Google Scholar]
- Samadikuchaksaraei A., et al. Fabrication and in vivo evaluation of an osteoblast-conditioned nano-hydroxyapatite/gelatin composite scaffold for bone tissue regeneration. J. Biomed. Mater. Res. 2016;104(8):2001–2010. doi: 10.1002/jbm.a.35731. [DOI] [PubMed] [Google Scholar]
- Sang S., et al. Biocompatible chitosan/polyethylene glycol/multi-walled carbon nanotube composite scaffolds for neural tissue engineering. J. Zhejiang Univ. - Sci. B. 2022;23(1):58–73. doi: 10.1631/jzus.B2100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanhueza C., et al. One-step electrospun scaffold of dual-sized gelatin/poly-3-hydroxybutyrate nano/microfibers for skin regeneration in diabetic wound. Mater. Sci. Eng. C Mater. Biol. Appl. 2021;119 doi: 10.1016/j.msec.2020.111602. [DOI] [PubMed] [Google Scholar]
- Seyyed Tabaei S.J., et al. Chitosan-based nano-scaffolds as antileishmanial wound dressing in BALB/c mice treatment: characterization and design of tissue regeneration. Iran J. Basic Med. Sci. 2020;23(6):788–799. doi: 10.22038/ijbms.2020.41361.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiee A., et al. An in situ hydrogel-forming scaffold loaded by PLGA microspheres containing carbon nanotube as a suitable niche for neural differentiation. Mater. Sci. Eng. C Mater. Biol. Appl. 2021;120 doi: 10.1016/j.msec.2020.111739. [DOI] [PubMed] [Google Scholar]
- Shao W., et al. Enhanced bone formation in electrospun poly (l-lactic-co-glycolic acid)–tussah silk fibroin ultrafine nanofiber scaffolds incorporated with graphene oxide. Mater. Sci. Eng. C. 2016;62:823–834. doi: 10.1016/j.msec.2016.01.078. [DOI] [PubMed] [Google Scholar]
- Sharif S., et al. Collagen-coated nano-electrospun PCL seeded with human endometrial stem cells for skin tissue engineering applications. J. Biomed. Mater. Res. B Appl. Biomater. 2018;106(4):1578–1586. doi: 10.1002/jbm.b.33966. [DOI] [PubMed] [Google Scholar]
- Shefa A.A., et al. In vitro and in vivo evaluation of effectiveness of a novel TEMPO-oxidized cellulose nanofiber-silk fibroin scaffold in wound healing. Carbohydr. Polym. 2017;177:284–296. doi: 10.1016/j.carbpol.2017.08.130. [DOI] [PubMed] [Google Scholar]
- Sheng C., et al. Yarn on yarn abrasion failure mechanism of ultrahigh molecular weight polyethylene fiber. J. Eng. Fibers Fabr. 2021;16 [Google Scholar]
- Shin S.R., et al. Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS Nano. 2013;7(3):2369–2380. doi: 10.1021/nn305559j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shkarina S., et al. 3D biodegradable scaffolds of polycaprolactone with silicate-containing hydroxyapatite microparticles for bone tissue engineering: high-resolution tomography and in vitro study. Sci. Rep. 2018;8(1):1–13. doi: 10.1038/s41598-018-27097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha S., et al. A conducting neural interface of polyurethane/silk-functionalized multiwall carbon nanotubes with enhanced mechanical strength for neuroregeneration. Mater. Sci. Eng. C. 2019;102:511–523. doi: 10.1016/j.msec.2019.04.053. [DOI] [PubMed] [Google Scholar]
- Silina E.V., et al. Application of polymer drugs with cerium dioxide nanomolecules and mesenchymal stem cells for the treatment of skin wounds in aged rats. Polymers. 2021;13(9) doi: 10.3390/polym13091467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart S.K., et al. The biocompatibility of carbon nanotubes. Carbon. 2006;44(6):1034–1047. [Google Scholar]
- Sun Y., et al. Enhanced nerve cell proliferation and differentiation on electrically conductive scaffolds embedded with graphene and carbon nanotubes. J. Biomed. Mater. Res. 2021;109(2):193–206. doi: 10.1002/jbm.a.37016. [DOI] [PubMed] [Google Scholar]
- Surmenev R.A., et al. Hybrid lead-free polymer-based nanocomposites with improved piezoelectric response for biomedical energy-harvesting applications: a review. Nano Energy. 2019;62:475–506. [Google Scholar]
- Surmeneva M.A., et al. Fabrication of multiple-layered gradient cellular metal scaffold via electron beam melting for segmental bone reconstruction. Mater. Des. 2017;133:195–204. [Google Scholar]
- Taghvaei A.H., et al. Synthesis and characterization of novel mesoporous strontium-modified bioactive glass nanospheres for bone tissue engineering applications. Microporous Mesoporous Mater. 2020;294 [Google Scholar]
- Talikowska M., et al. Application of conducting polymers to wound care and skin tissue engineering: a review. Biosens. Bioelectron. 2019;135:50–63. doi: 10.1016/j.bios.2019.04.001. [DOI] [PubMed] [Google Scholar]
- Vunjak-Novakovic G., et al. Challenges in cardiac tissue engineering. Tissue Eng. B Rev. 2010;16(2):169–187. doi: 10.1089/ten.teb.2009.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., et al. Optimizing the electrical conductivity of polyacrylonitrile/polyaniline with nickel nanoparticles for the enhanced electrostimulation of Schwann cells proliferation. Bioelectrochemistry. 2021;140 doi: 10.1016/j.bioelechem.2021.107750. [DOI] [PubMed] [Google Scholar]
- Wang S., et al. Chitosan/gelatin porous scaffolds assembled with conductive poly(3,4-ethylenedioxythiophene) nanoparticles for neural tissue engineering. J. Mater. Chem. B. 2017;5(24):4774–4788. doi: 10.1039/c7tb00608j. [DOI] [PubMed] [Google Scholar]
- Wang W., et al. 3D printing of PLA/n-HA composite scaffolds with customized mechanical properties and biological functions for bone tissue engineering. Compos. B Eng. 2021;224 [Google Scholar]
- Wang X., et al. Defective Black nano-titania thermogels for cutaneous tumor-induced therapy and healing. Nano Lett. 2019;19(3):2138–2147. doi: 10.1021/acs.nanolett.9b00367. [DOI] [PubMed] [Google Scholar]
- Wei Z., et al. Gelatin methacrylamide hydrogel with graphene nanoplatelets for neural cell-laden 3D bioprinting. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2016:4185–4188. doi: 10.1109/EMBC.2016.7591649. [DOI] [PubMed] [Google Scholar]
- Wu S., et al. Biocompatible chitin/carbon nanotubes composite hydrogels as neuronal growth substrates. Carbohydr. Polym. 2017;174:830–840. doi: 10.1016/j.carbpol.2017.06.101. [DOI] [PubMed] [Google Scholar]
- Wu S., et al. Electrospun conductive nanofiber yarns for accelerating mesenchymal stem cells differentiation and maturation into Schwann cell-like cells under a combination of electrical stimulation and chemical induction. Acta Biomater. 2022;139:91–104. doi: 10.1016/j.actbio.2020.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi P., et al. Effect of nano-silver hydrogel coating film on deep partial thickness scald model of rabbit. Saudi J. Biol. Sci. 2018;25(4):797–800. doi: 10.1016/j.sjbs.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., et al. Graphene and hydroxyapatite self-assemble into homogeneous, free standing nanocomposite hydrogels for bone tissue engineering. Nanoscale. 2015;7(17):7992–8002. doi: 10.1039/c5nr01107h. [DOI] [PubMed] [Google Scholar]
- Yang R., et al. Carbon nanotube polymer scaffolds as a conductive alternative for the construction of retinal sheet tissue. ACS Chem. Neurosci. 2021;12(17):3167–3175. doi: 10.1021/acschemneuro.1c00242. [DOI] [PubMed] [Google Scholar]
- Ye L., et al. Carbon nanotube-hydrogel composites facilitate neuronal differentiation while maintaining homeostasis of network activity. Adv. Mater. 2021;33(41) doi: 10.1002/adma.202102981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Kisley L. Super-resolution fluorescence imaging of extracellular environments. Spectrochim. Acta Mol. Biomol. Spectrosc. 2021;257 doi: 10.1016/j.saa.2021.119767. [DOI] [PubMed] [Google Scholar]
- Zare E.N., et al. Progress in conductive polyaniline-based nanocomposites for biomedical applications: a review. J. Med. Chem. 2020;63(1):1–22. doi: 10.1021/acs.jmedchem.9b00803. [DOI] [PubMed] [Google Scholar]
- Zhai M., et al. Keratin-chitosan/n-ZnO nanocomposite hydrogel for antimicrobial treatment of burn wound healing: characterization and biomedical application. J. Photochem. Photobiol., B. 2018;180:253–258. doi: 10.1016/j.jphotobiol.2018.02.018. [DOI] [PubMed] [Google Scholar]
- Zhang R., et al. Silver nanoparticles promote osteogenesis of mesenchymal stem cells and improve bone fracture healing in osteogenesis mechanism mouse model. Nanomed. Nanotechnol. Biol. Med. 2015;11(8):1949–1959. doi: 10.1016/j.nano.2015.07.016. [DOI] [PubMed] [Google Scholar]
- Zhang X., et al. Preparation of PI porous fiber membrane for recovering oil-paper insulation structure. J. Mater. Sci. Mater. Electron. 2020;31(16):13344–13351. [Google Scholar]
- Zhang Haoyu, Zou Quan, Ju Ying, Song Chenggang. Distance-based Support Vector Machine to Predict DNA N6-methyladine Modification. Curr. Bioinform. 2022;17(5):473–482. [Google Scholar]
- Zhao Y., et al. Magnetic bioinspired micro/nanostructured composite scaffold for bone regeneration. Colloids Surf. B Biointerfaces. 2019;174:70–79. doi: 10.1016/j.colsurfb.2018.11.003. [DOI] [PubMed] [Google Scholar]
- Zheng K., et al. Incorporation of Cu-containing bioactive glass nanoparticles in gelatin-coated scaffolds enhances bioactivity and osteogenic activity. ACS Biomater. Sci. Eng. 2018;4(5):1546–1557. doi: 10.1021/acsbiomaterials.8b00051. [DOI] [PubMed] [Google Scholar]
- Zhou Z., et al. Effective nerve cell modulation by electrical stimulation of carbon nanotube embedded conductive polymeric scaffolds. Biomater. Sci. 2018;6(9):2375–2385. doi: 10.1039/c8bm00553b. [DOI] [PubMed] [Google Scholar]
- Zhu F., et al. Injectable tissue adhesive composite hydrogel with fibroblasts for treating skin defects. J. Mater. Chem. B. 2017;5(13):2416–2424. doi: 10.1039/c7tb00384f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.