Abstract
Background and Purpose
Post-mortem studies of advanced Parkinson’s disease (PD) have revealed disease-related pathology in the thalamus with an apparent predilection for specific thalamic nuclei. In the present study, we used diffusion tensor imaging (DTI) to investigate in vivo the microstructural integrity of six thalamic regions in de novo PD patients relative to healthy controls.
Materials and Methods
Forty subjects (20 with early-stage, untreated PD and 20 age- and sex-matched controls) were studied with a high-resolution DTI protocol at 3 Tesla to investigate the integrity of thalamic nuclei projection fibers. Two blinded, independent raters drew regions of interest (ROIs) in the following six thalamic regions: anterior nucleus (AN), ventral anterior nucleus (VA), ventral lateral nucleus (VL), dorsomedial nucleus (DM), ventral posterior lateral nucleus (VPL)/ventral posterior medial nucleus (VPM), and pulvinar (PU). Fractional anisotropy (FA) values were then calculated from the projection fibers in each region.
Results
FA values were reduced significantly in the fibers projecting from the AN, VA, and DM, but not the VPL/VPM and PU, in the PD group compared to the control group. In addition, there was a reduction in FA values that approached significance in the VL of PD patients. These findings were consistent across both raters.
Conclusion
The present study provides preliminary in vivo evidence of thalamic projection fiber degeneration in de novo PD and sheds light on the extent of disrupted thalamic circuitry as a result of the disease itself.
Keywords: Parkinson’s disease, thalamus, diffusion tensor imaging, tractography, fractional anisotropy, magnetic resonance imaging
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder with a slow, predictable course through vulnerable regions of subcortical and cortical gray matter (GM)1. The pathological hallmark of PD is the selective and substantial loss of dopaminergic neurons in the substantia nigra (SN) pars compacta1, 2. It is generally accepted that a reduction of dopamine in the SN pars compacta underlies dysfunction within the direct basal ganglia (BG) pathway3, which results in reduced excitation of cortical neurons by the thalamus4. This increased inhibition of cortical-subcortical circuits in PD results in bradykinesia and varying degrees of cognitive impairment5–7. Thus, while dysfunction of subcortical structures such as the BG and thalamus have historically been conceptualized as a consequence of SN pars compacta degeneration, there is also evidence to suggest that the thalamus may also undergo structural changes8, display functional abnormalities9, and be a site of direct disease pathology in PD1, 10, 11. Given that symptom presentation in PD only occurs after significant dopamine loss, it is important to examine the integrity of structures further into the direct BG pathway, such as the thalamus, particularly early in the disease.
While conventional magnetic resonance imaging (MRI) has limited utility as an investigative and diagnostic tool in PD, recent advances in neuroimaging techniques have allowed for the non-invasive, in vivo assessment of PD-related pathology12, 13. For example, diffusion tensor imaging (DTI) measures within the SN have shown excellent sensitivity and specificity in differentiating de novo PD patients from healthy controls14. Recent studies support the reduction in fractional anisotropy (FA) values within the SN of PD patients15–17 and also suggest that microstructural changes occur in areas beyond the SN, including the thalamus16. As a technique, DTI is based upon the diffusivity of water molecules, which is variably restricted by different tissues. FA values, which are calculated from the diffusion tensor images, range from 0 to 1, with 0 representing isotropic diffusion and 1 anisotropic diffusion. In white matter (WM), where the tissue is highly organized, the free diffusion of water is limited, resulting in FA values near 1. FA values calculated in WM reflect a combination of several factors, including axonal density, degree of myelination, and orientational coherence18, 19. The direction of diffusion is less restricted in GM, resulting in FA values near 0. It is unclear exactly what FA values represent in GM structures, but one hypothesis is that they reflect neuronal density, with lower FA values representing a decrease in density20.
Post-mortem studies demonstrate that PD differentially affects specific thalamic nuclei10, 11, 21–23, indicating that there could be degeneration or disruption of the microstructural integrity of the thalamus. To date, most studies utilizing DTI to investigate the thalamus have focused on the thalamus as a single entity, and have not evaluated the integrity of specific nuclei or the critical cortical-subcortical fibers that project from the thalamus16, 24–26. Further, these studies examined relatively advanced PD patients who had been taking antiparkinsonian medication16, 24–26, thus making it unclear whether differences between the patients and controls were caused by the disease, medication, or a combination of both.
In the current study, we conducted a detailed examination of the projection fibers from specific thalamic nuclei in de novo PD patients, allowing investigation of the disease without concern for the potential effects of antiparkinsonian medication. Using a high-resolution DTI sequence at 3 Tesla (T), we placed small regions of interest (ROIs) in six thalamic regions and used the voxels within the ROIs as seeds for fiber tracking. Similar methods have been applied previously with high inter-rater reliability in a study of traumatic brain injury27. Our hypothesis was that there would be degeneration of thalamic projection fibers in early-stage, untreated PD, and that fibers from specific nuclei may be preferentially affected.
Materials and Methods
Subjects
Forty individuals participated in this study: 20 patients with de novo PD (10 men, 10 women) and 20 age- and sex-matched healthy volunteers. There was no significant difference in age between the groups (PD: 57.9 ± 8.9 years versus control: 58.2 ± 9.6 years; P = .7). All patients were diagnosed with PD by a movement disorders neurologist at Rush University Medical Center and met the United Kingdom PD Society Brain Bank diagnostic criteria28, 29. For the PD group, the mean (SD) Unified Parkinson’s Disease Rating Scale (UPDRS) Part III motor score was 16.7 (7.6), indicating mild disease, and the mean (SD) disease duration in months was 12.8 (10.7). None of the controls reported a history of neuropsychiatric or neurological problems. All subjects provided written informed consent for the procedures in this study, which were approved by the Institutional Review Boards at Rush University Medical Center and the University of Illinois at Chicago.
Image Acquisition
In order to reliably perform FA analysis and fiber tracking in the thalamus, we used a high-resolution DTI protocol that was designed to minimize eddy current induced distortion.30 All images were collected on a GE 3 T Signal HDx (General Electric Healthcare, Waukesha, WI) using an 8-channel phased-array head coil, together with parallel imaging. The DTI data acquisition parameters were as follows: TR = 4,500 ms, TE = 82 ms, flip angle = 90°, b values = 0, 1000 s/mm2, diffusion directions = 27, FOV = 200 mm2, image matrix size = 256 × 256, number of slices = 15, slice thickness = 4 mm, slice gap = 1 mm, number of excitations (NEX) = 4, and acceleration factor = 2. Thus, the voxel size was 0.78 × 0.78 × 4 mm. The images were collected in the axial orientation, with the top slice placed approximately 4 mm above the corpus callosum. The total acquisition time of the DTI sequence was 8 minutes 33 seconds.
To visualize the thalamus and differentiate it from surrounding structures, a set of T2-weighted images was acquired using a fast spin echo pulse sequence in the axial plane (TR = 5,000 ms, TE = 97 ms, flip angle = 90°, FOV = 200 mm2, image matrix size = 512 × 512, number of slices = 15, slice thickness = 4 mm, slice gap = 1 mm, NEX = 2).
To help visualize the DM, we collected axial images using a T1-weighted 3D inversion recovery prepared fast spoiled gradient recalled echo pulse sequence (TR = 25 ms, TE = 3 ms, FA = 40°, FOV = 240 mm2, image matrix size = 256 × 256, number of slices = 120, slice thickness = 1.5 mm, slice gap = 0 mm, NEX = 1).
Image Analysis
DtiStudio31 was used to reconstruct the images and calculate FA values. For all subjects, each of the 15 diffusion tensor images was examined visually for artifacts related to eddy currents and motion, namely spatial blurring, scaling, and shearing. Head motion was further assessed using AFNI32 software and required to be within 1 mm. A background noise level of 125 (MR units) was applied prior to calculation of the pixel-wise FA maps.
Fiber Tracking
Fiber tracking in DtiStudio31 was used to assess projection fibers from the six thalamic regions. Following the calculation of pixel-wise FA and eigenvector maps, fiber tracking parameters were set to exclude tracking fibers with FA values < .20 or a turning angle > 70°. For each subject, two circular ROIs (3 mm in diameter) were placed in the AN, VA, VL, VPL/VPM, and PU. Given its larger size, three ROIs of the same diameter were placed in the DM. ROIs were drawn independently by two raters who were blinded to patient or control status. Figure 1A shows the regional boundaries for placing the ROIs, which were based on methods described in Little and colleagues27, Appendix e-1. VPL and VPM were considered as one region due to the difficulty visualizing their boundaries. For each thalamic region and subject, mean FA values were calculated across the entire extent of the fibers identified by the ROI seed voxels. ROIs were drawn separately for the right and left hemispheres.
Fig 1.
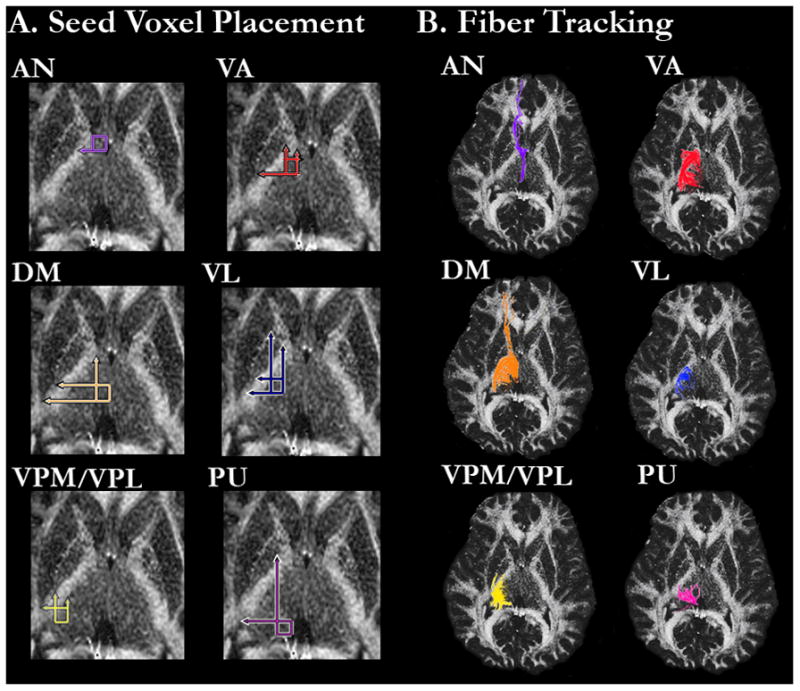
FA image from a representative healthy control subject showing A. Regional boundaries of the six thalamic regions in which the circular ROIs were placed and B. Fiber tracking from the ROIs in each thalamic region.
Placement of ROIs in Thalamic Regions
Anterior Nucleus
The AN ROIs were defined laterally by the internal capsule, medially by the edge of the thalamus, and anteriorly by the posterior edge of the caudate head and lateral ventricle. The posterior boundary for the ROIs was defined by a horizontal line extending from the posterior edge of the anterior limb of the internal capsule. Any fiber that extended to the putamen or globus pallidus was excluded. Furthermore, any fibers that were also identified from ROIs placed in the VA, VPL/VPM, PU or DM were excluded.
Ventral Anterior Nucleus
The anterior edge of the VA ROIs were defined by a horizontal line extending from the anterior edge of the internal capsule and extended medially the width of the internal capsule. The VA ROIs extended posterior to a horizontal line drawn from the posterior boundary of the putamen. Any fibers that were also identified by the AN ROIs were excluded.
Ventral Lateral Nucleus
The VL ROIs were defined medially by a vertical line drawn down from the medial edge of the bend of the internal capsule (the intersection between the anterior limb of the internal capsule and the body of the internal capsule), laterally by the edge of the internal capsule, anteriorly by a horizontal line extending from the posterior edge of the anterior limb of the internal capsule, and posteriorly by a horizontal line extending from the posterior boundary of the putamen. Fibers that were previously identified by either the AN or VA ROIs were excluded.
Dorsal Medial Nucleus
The DM ROIs were defined medially by the edge of the thalamic body, laterally by a vertical line extending from the posterior edge of the anterior limb of the medial edge of the internal capsule, posteriorly by a horizontal line extending from the posterior edge of the posterior limb of the internal capsule and anteriorly by a horizontal line extending from the posterior edge of the putamen. Fibers that were previously identified by the AN, VA, or VL ROIs were excluded.
Ventral Posterior Lateral and Ventral Posterior Medial Nuclei
The VPL/VPM ROI was defined anteriorly by a horizontal line extending from the posterior edge of the putamen, laterally by a vertical line extending from the posterior intersection of the internal capsule and putamen, medially by a vertical line extending the intersection of the anterior limb of the internal capsule with the anterior edge of the putamen and posteriorly by the posterior edge of the thalamus. Fibers that were also identified by the AN, VA, VL, or DM ROIs were excluded.
Pulvinar
The PU ROIs were defined medially and posteriorly by the edge of the thalamus, laterally by a vertical line extending from the medial intersection of the anterior limb of the internal capsule and body of the internal capsule and anteriorly by a horizontal line extending from the intersection of the posterior limb of the internal capsule and putamen. Fibers that were previously identified by the AN, VA, VL, VPL/VPM, or DM ROIs were excluded.
Statistical Analysis
For each thalamic region, FA values within each group were assessed for outliers. No FA value fell more than 3 SD from its respective mean, thus all data were included in the analyses. Next, we performed a two-way mixed design ANOVA on the FA values for each thalamic region, with group (PD, Control) as the between-subjects factor and hemisphere as the within-subjects factor (Right, Left). The assessment of inter-rater reliability for ROI placement showed strong agreement between the two raters, with all interclass correlation coefficients above .82. Because the two-way interaction did not approach significance for any analysis, we collapsed the data across the cerebral hemispheres. For thalamic regions that differed significantly in FA values between groups, we performed a correlation analysis between FA values and UPDRS Part III scores.
Results
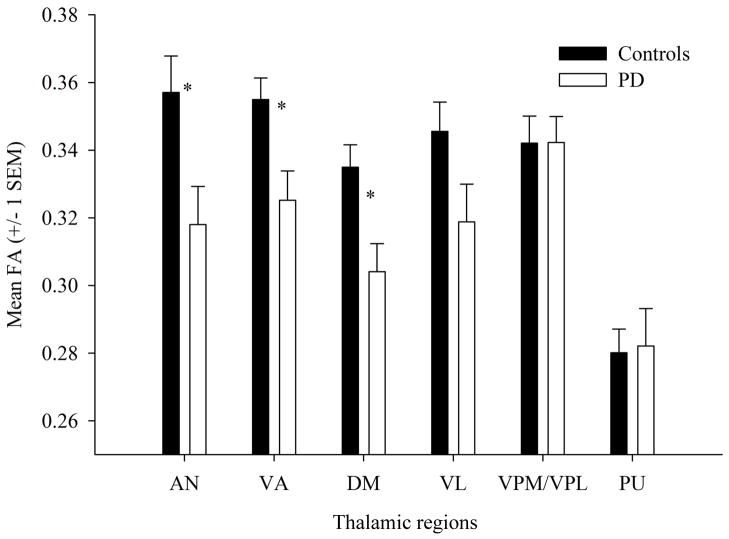
The two-way interaction between group and hemisphere was not significant for any of the six thalamic regions (all Ps > .4). The main effect of hemisphere was only significant for the VL (P = .012), with higher FA values on the left than right side. There was a significant main effect of group for three of the six thalamic regions. As presented in Figure 2, FA values were reduced in the PD compared to the control group for fibers projecting from the AN (P = .016), VA (P = .009), and DM (P = .006). In addition, the reduction in FA values from the VL approached significance for the PD compared to the control group (P = .065). The FA values in the VPL/VPM and PU projection fibers did not differ significantly between the groups (both Ps > .4).
Fig 2.
Mean (± SEM) FA values from each of the six thalamic regions for the control and PD groups. Note that the PD group has significantly reduced of FA values compared to the control group in the AN, VA, and DM, and shows a marginally significant reduction in the VL.
For the three thalamic regions that differed significantly between the groups, we performed a correlation analysis to investigate the relationship between UPDRS Part III scores and FA values in PD. There was a significant correlation between UPDRS Part III scores and FA values in the AN (r = −.487, P = .029), indicating a moderate negative association; as UPDRS Part III scores (i.e., motor impairment) increased, FA values decreased. The correlations between UPDRS Part III scores and FA values in the DM, VA, and VL were not significant (all Ps > .2).
Discussion
The thalamus plays an important role in the output pathway of the BG circuitry. Not only does it transmit nearly all cortical inputs, including cognitive, sensory, and motor information, but it also modulates and integrates this information. Further, cerebellar output influences the cortical areas involved in motor processing via the thalamus. The thalamus is organized into distinct nuclear regions, from which diffuse and specific efferent projections are sent to cortical, cerebellar, and subcortical regions3.
The present study was the first to examine the integrity of thalamic nuclei projections in de novo PD using in vivo DTI tractography. We showed that there were reduced FA values in the fibers projecting from the AN, VA, and DM, but not the VPL/VPM or PU, in PD patients compared to controls. Further, the reduction in FA values approached significance in the VL of the PD patients. Although the pathological cause of these reduced FA values is not known, it may reflect demyelination, neuronal loss, or gliosis.20, 33–36 We also showed that there was a significant negative correlation between FA values in the AN and UPDRS Part III scores. This relationship should be investigated further in future work. Collectively, our results provide preliminary evidence of thalamic fiber degeneration in early-stage PD and shed light on the extent of disrupted thalamic circuitry as a result of the disease.
Our DTI results are largely consistent with post-mortem studies of the thalamus in PD. Rub et al11 reported evidence of mild inclusion pathology in the AN, VA, VL, and DM of patients with mild to severe PD. Further, there was no evidence of pathology in the VPM and VPL, and relatively little pathology in the PU of these patients. However, other studies have reported that the volume and number of neurons in the AN and DM are not reduced significantly in PD21–23.
The AN, VA, and DM are considered specific nuclei, in that they relay information to functionally distinct cortical regions. The AN is an associative nucleus that is part of the major limbic pathway, and primarily projects information related to emotion and memory to the cingulate gyrus37. Similarly, the DM is involved in emotion38 and cognition and is connected to the amygdala39 and the anterior cingulate and prefrontal cortices37, 40, 41. The VA, which in combination with the VL is known as the “motor thalamus,” primarily relays somatomotor information to the supplementary motor area from the BG. In functional imaging studies of PD, the supplementary motor area is often hypoactive during movement tasks compared to controls42, 43. Taken together, damage to these different thalamic projection fibers may result in widespread impairments39, 44 and may contribute to symptom presentation in PD.
Although ex vivo studies remain the gold standard for pathological confirmation of PD, it is important to study the brain in vivo to gain insight into the structures affected early in the disease and to evaluate differences across disease stages. As a technique, DTI has proven useful in identifying structural abnormalities in the SN of PD patients in vivo14, 17, 24, 26, 45. However, DTI studies comparing the thalamus between PD patients and controls have been less conclusive. In a large cohort, Chan et al 46 did not observe differences in FA values at 1.5 T between mild to severe PD patients on antiparkinsonian medication and controls in an ROI that covered a single slice of the thalamus. Similarly, there were no differences in MD values (FA values were not reported) at 1.5 T in a circular ROI placed on one slice of the thalamus between early-stage, medicated PD patients and controls47. However, in another large cohort, Péran et al 26 reported reduced FA and MD values at 3 T in the whole thalamus of early-stage, but medicated, PD patients and controls. Most recently, a DTI study that examined the thalamus using both voxel-wise and ROI-based approaches reported reduced FA values at 4 T in mild to severe, medicated PD patients versus controls16. Collectively, these studies suggest that magnet field strength and/or ROI size and placement may affect our ability to detect microstructural changes in the thalamus.
As discussed earlier, the nuclei of the thalamus have specialized connectivity and functions, and thus may be differentially affected in disease states, as supported by post-mortem research. By studying the thalamus as a single entity, it is unclear whether significant effects in large ROIs or the whole thalamus are driven by degeneration in a few select nuclei and non-significant effects are the result of placing small ROIs on relatively spared nuclei. The present study addressed these concerns by using high-resolution fiber tracking at 3 T, allowing for specific thalamic nuclei to be studied with great sensitivity. Indeed, we showed that FA values were reduced in the AN, VA, and DM in the PD patients compared to controls. There was also a marginally significant reduction in FA values from the VL projections of the PD patients.
Although DTI has proven useful in studying sub-regions of the SN in PD14, relatively few studies have used this technique to examine individual thalamic nuclei. In fact, to date, only the DM and VL have been examined in PD. Consistent with the role of the DM in affect and cognition, Li et al 25 reported that PD patients with depression had reduced FA values compared to non-depressed PD patients, and that these FA values correlated negatively with depression severity. Given that depression is prevalent in PD48, it is possible that the patients in our study had greater affective disturbances than the controls. The VL segment of the motor thalamus receives input from the cerebellum49 and BG and projects primarily to the pre- and primary motor cortices, retaining its topographic organization throughout. Two previous MRI studies at 1.5 T have examined the VL in PD, neither of which showed differences in DTI measures between the PD and control groups45, 50. In the present study, the difference in FA values between the two groups approached significance in the VL. Using a high field strength scanner to test larger samples may increase the ability to detect microstructural changes in the VL.
There were two thalamic regions whose FA values did not differ significantly between the groups, namely the VPL/VPM and PU, both of which are sensory nuclei. Human DTI tractography has shown that the PU is connected ipsilaterally to several cortical and subcortical areas, particularly those in the visual pathways51. Although its function is not completely understood, the PU is thought to play an important role in higher-order visual processing and integration, including visual attention and oculomotor behavior52. The VPL and VPM relay touch and proprioceptive information from the contralateral body and head, respectively, to the primary and secondary somatosensory cortices41. The fact that we did not show a significant group difference in the PU or VPL/VPM suggests that visual and somatosensory processing should be relatively intact in early-stage PD.
There are a few limitations to the present study. First, a detailed neuropsychological examination was not performed on the PD patients. Given that cognitive and affective problems occur frequently in PD48 and we found differences in thalamic nuclei known to be involved in emotion and cognitive processing, it is important to collect these data in the future to better understand the relationship between thalamic degeneration and these non-motor functions in PD. Second, the reduction of FA values in the VL of PD patients did not quite reach significance, suggesting that larger sample sizes or more impaired patients may be necessary to detect a difference in this nucleus. Third, intralaminar and midline nuclei (e.g., centromedian-parafasicular complex) were not examined because their boundaries could not be distinguished on the MRI scans with acceptable inter-rater reliability. These nuclei, which are involved in arousal and awareness53, have shown severe pathology in post-mortem studies of PD11, 21–23. Fourth, data in superior cortical areas were not collected due to limitations in the number of slices that can be collected within a reasonable acquisition time. This limited quantification of the tract projections.
Conclusion
The present study provides the first in vivo evidence that there is a disruption of the projection fibers in certain thalamic nuclei, namely the AN, VA, and DM, in de novo PD patients. These results suggest that structural degeneration occurs beyond the SN in early PD and thus help further our understanding of the neural structures affected by the disease. Specifically, we showed that nuclei involved in motor, cognitive, and affective processes are disrupted, whereas those involved in sensory processes are relatively spared. In addition, this study showed that the current methodology not only has utility in examining the microstructural integrity of thalamic nuclei in traumatic brain injury, 27 but also in PD. Future research should use these methods longitudinally to determine whether thalamic fiber integrity changes with disease progression and whether or not it is affected by antiparkinsonian medication. Further, a detailed neuropsychological examination should be conducted along with DTI of thalamic regions to better understand the impact of fiber degeneration on cognitive, affective, and behavioral functioning in PD.
Acknowledgments
Funding
This work was supported in part by grants from the Department of Defense/Congressionally Directed Medical Research Program (PT 075675 to DML), Michael J. Fox Foundation (DEV), and National Institutes of Health (R01-NS-52318 and R01-NS-58487 to DEV).
Abbreviation key
- AN
anterior nucleus
- BG
basal ganglia
- DM
dorsomedial nucleus
- FA
fractional anisotropy
- GM
gray matter
- MD
mean diffusivity
- NEX
number of excitations
- PD
Parkinson’s disease
- PU
pulvinar
- SN
substantia nigra
- T
Tesla
- UPDRS
Unified Parkinson’s Disease Rating Scale
- VA
ventral anterior nucleus
- VL
ventral lateral nucleus
- VPL
ventral posterior lateral nucleus
- VPM
ventral posterior medial nucleus
References
- 1.Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology Of Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 2.Hodaie M, Neimat JS, Lozano AM. The dopaminergic nigrostriatal system and Parkinson’s disease: Molecular events in development, disease, and cell death, and new therapeutic strategies. Neurosurgery. 2007;60:17–28. doi: 10.1227/01.NEU.0000249209.11967.CB. [DOI] [PubMed] [Google Scholar]
- 3.Blumenfeld H. Neuroanatomy through clinical cases. Sunderland, MA: Sinauer Associates; 2002. [Google Scholar]
- 4.Owen AM, Doyon J, Dagher A, et al. Abnormal basal ganglia outflow in Parkinson’s disease identified with PET - Implications for higher cortical functions. Brain. 1998;121:949–965. doi: 10.1093/brain/121.5.949. [DOI] [PubMed] [Google Scholar]
- 5.Braak H, Braak E. Pathoanatomy of Parkinson’s disease. J Neurol. 2000;247:3–10. doi: 10.1007/PL00007758. [DOI] [PubMed] [Google Scholar]
- 6.Emre M. What causes mental dysfunction in Parkinson’s disease? Mov Disord. 2003;18:S63–S71. doi: 10.1002/mds.10565. [DOI] [PubMed] [Google Scholar]
- 7.Nobili F, Campus C, Arnaldi D, et al. Cognitive-Nigrostriatal Relationships in De Novo, Drug-Naive Parkinson’s Disease Patients: A [I-123]FP-CIT SPECT Study. Mov Disord. 2010;25:35–43. doi: 10.1002/mds.22899. [DOI] [PubMed] [Google Scholar]
- 8.McKeown MJ, Uthama A, Abugharbieh R, et al. Shape (but not volume) changes in the thalami in Parkinson disease. BMC Neurol. 2008:8. doi: 10.1186/1471-2377-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prodoehl J, Spraker M, Corcos D, et al. Blood Oxygenation Level-Dependent Activation in Basal Ganglia Nuclei Relates to Specific Symptoms in De Novo Parkinson’s Disease. Mov Disord. 2010;25:2035–2043. doi: 10.1002/mds.23360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halliday GM. Thalamic changes in Parkinson’s disease. Parkinsonism & Related Disorders. 2009;15:S152. doi: 10.1016/S1353-8020(09)70804-1. [DOI] [PubMed] [Google Scholar]
- 11.Rub U, Del Tredici K, Schultz C, et al. Parkinson’s disease: the thalamic components of the limbic loop are severely impaired by a-synuclein immunopositive inclusion body pathology. Neurobiol Aging. 2002;23:245–254. doi: 10.1016/s0197-4580(01)00269-x. [DOI] [PubMed] [Google Scholar]
- 12.Planetta PJ, Prodoehl J, Corcos DM, et al. Use of MRI to monitor Parkinson’s disease. Neurodegenerative Disease Management. 2011;1:67–77. [Google Scholar]
- 13.Mahlknecht P, Hotter A, Hussl A, et al. Significance of MRI in diagnosis and differential diagnosis of Parkinson’s disease. Neurodegener Dis. 2010;7:300–318. doi: 10.1159/000314495. [DOI] [PubMed] [Google Scholar]
- 14.Vaillancourt D, Spraker M, Prodoehl J, et al. High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology. 2009;72:1378–1384. doi: 10.1212/01.wnl.0000340982.01727.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peran P, Cherubini A, Assogna F, et al. Magnetic resonance imaging markers of Parkinson’s disease nigrostriatal signature. Brain. 2010;133:3423–3433. doi: 10.1093/brain/awq212. [DOI] [PubMed] [Google Scholar]
- 16.Zhan W, Kang GA, Glass GA, et al. Regional alterations of brain microstructure in parkinson’s disease using diffusion tensor imaging. Mov Disord. 2011 doi: 10.1002/mds.23917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du G, Lewis MM, Styner M, et al. Combined r2* and diffusion tensor imaging changes in the substantia nigra in Parkinson’s disease. Mov Disord. 2011;26:1627–1632. doi: 10.1002/mds.23643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basser PJ, Mattiello J, Lebihan D. MR Diffusion Tensor Spectroscopy And Imaging. Biophysical Journal. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minati L, Banasik T, Brzezinski J, et al. Elevating tensor rank increases anisotropy in brain areas associated with intra-voxel orientational heterogeneity (IVOH): a generalised DTI (GDTI) study. NMR Biomed. 2008;21:2–14. doi: 10.1002/nbm.1143. [DOI] [PubMed] [Google Scholar]
- 20.Boska MD, Hasan KM, Kibuule D, et al. Quantitative diffusion tensor imaging detects dopaminergic neuronal degeneration in a murine model of Parkinson’s disease. Neurobiology Of Disease. 2007;26:590–596. doi: 10.1016/j.nbd.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brooks D, Halliday GM. Intralaminar nuclei of the thalamus in Lewy body diseases. Brain Res Bull. 2009;78:97–104. doi: 10.1016/j.brainresbull.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Henderson JM, Carpenter K, Cartwright H, et al. Degeneration of the centre median-parafascicular complex in Parkinson’s disease. Annals Of Neurology. 2000;47:345–352. [PubMed] [Google Scholar]
- 23.Henderson JM, Carpenter K, Cartwright H, et al. Loss of thalamic intralaminar nuclei in progressive supranuclear palsy and Parkinson’s disease: clinical and therapeutic implications. Brain. 2000;123:1410–1421. doi: 10.1093/brain/123.7.1410. [DOI] [PubMed] [Google Scholar]
- 24.Chan LL, Rumpel H, Yap K, et al. Case control study of diffusion tensor imaging in Parkinson’s disease. Journal Of Neurology Neurosurgery And Psychiatry. 2007;78:1383–1386. doi: 10.1136/jnnp.2007.121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li W, Liu J, Skidmore F, et al. White Matter Microstructure Changes in the Thalamus in Parkinson Disease with Depression: A Diffusion Tensor MR Imaging Study. American Journal Of Neuroradiology. 2010;31:1861–1866. doi: 10.3174/ajnr.A2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Péran P, Cherubini A, Assogna F, et al. Magnetic resonance imaging markers of Parkinson’s disease nigrostriatal signature. Brain. 2010 doi: 10.1093/brain/awq212. [DOI] [PubMed] [Google Scholar]
- 27.Little DM, Kraus MF, Joseph J, et al. Thalamic integrity underlies executive dysfunction in traumatic brain injury. Neurology. 2010;74:558–564. doi: 10.1212/WNL.0b013e3181cff5d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughes A, Daniel S, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes AJ, Ben-Shlomo Y, Daniel SE, et al. What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study. 1992. Neurology. 2001;57:S34–38. [PubMed] [Google Scholar]
- 30.Zhou X, Naier J, Reynolds H. Method to reduce eddy current effects in diffusion-weighted echo planar imaging. United States Patent, 5. 1999;864:233. [Google Scholar]
- 31.Jiang H, van Zijl PC, Kim J, et al. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006;81:106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 33.DeBoy CA, Zhang J, Dike S, et al. High resolution diffusion tensor imaging of axonal damage in focal inflammatory and demyelinating lesions in rat spinal cord. Brain. 2007;130:2199–2210. doi: 10.1093/brain/awm122. [DOI] [PubMed] [Google Scholar]
- 34.Budde MD, Janes L, Gold E, et al. The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections. Brain. 2011;134:2248–2260. doi: 10.1093/brain/awr161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wimberger DM, Roberts TP, Barkovich AJ, et al. Identification of “premyelination” by diffusion-weighted MRI. J Comput Assist Tomogr. 1995;19:28–33. doi: 10.1097/00004728-199501000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Klingberg T, Vaidya CJ, Gabrieli JD, et al. Myelination and organization of the frontal white matter in children: a diffusion tensor MRI study. Neuroreport. 1999;10:2817–2821. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- 37.Zhang D, Snyder AZ, Shimony JS, et al. Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cereb Cortex. 2010;20:1187–1194. doi: 10.1093/cercor/bhp182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Metzger CD, Eckert U, Steiner J, et al. High field FMRI reveals thalamocortical integration of segregated cognitive and emotional processing in mediodorsal and intralaminar thalamic nuclei. Front Neuroanat. 2010;4:138. doi: 10.3389/fnana.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmahmann JD. Vascular syndromes of the thalamus. Stroke. 2003;34:2264–2278. doi: 10.1161/01.STR.0000087786.38997.9E. [DOI] [PubMed] [Google Scholar]
- 40.Klein JC, Rushworth MF, Behrens TE, et al. Topography of connections between human prefrontal cortex and mediodorsal thalamus studied with diffusion tractography. Neuroimage. 2010;51:555–564. doi: 10.1016/j.neuroimage.2010.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Behrens TE, Johansen-Berg H, Woolrich MW, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- 42.Jahanshahi M, Jenkins IH, Brown RG, et al. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects. Brain. 1995;118 ( Pt 4):913–933. doi: 10.1093/brain/118.4.913. [DOI] [PubMed] [Google Scholar]
- 43.Spraker M, Prodoehl J, Corcos D, et al. Basal ganglia hypoactivity during grip force in drug naïve Parkinson’s disease. Hum Brain Mapp. 2010 doi: 10.1002/hbm.20987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carrera E, Bogousslavsky J. The thalamus and behavior - Effects of anatomically distinct strokes. Neurology. 2006;66:1817–1823. doi: 10.1212/01.wnl.0000219679.95223.4c. [DOI] [PubMed] [Google Scholar]
- 45.Yoshikawa K, Nakata Y, Yamada K, et al. Early pathological changes in the parkinsonian brain demonstrated by diffusion tensor MRI. J Neurol Neurosurg Psychiatry. 2004;75:481–484. doi: 10.1136/jnnp.2003.021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan L, Rumpel H, Yap K, et al. Case control study of diffusion tensor imaging in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78:1383–1386. doi: 10.1136/jnnp.2007.121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gattellaro G, Minati L, Grisoli M, et al. White matter involvement in idiopathic Parkinson disease: a diffusion tensor imaging study. AJNR Am J Neuroradiol. 2009;30:1222–1226. doi: 10.3174/ajnr.A1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chaudhuri KR, Healy DG, Schapira AH, et al. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006;5:235–245. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- 49.Kwon HG, Hong JH, Hong CP, et al. Dentatorubrothalamic tract in human brain: diffusion tensor tractography study. Neuroradiology. 2011 doi: 10.1007/s00234-011-0878-7. [DOI] [PubMed] [Google Scholar]
- 50.Nicoletti G, Manners D, Novellino F, et al. Diffusion tensor MRI changes in cerebellar structures of patients with familial essential tremor. Neurology. 2010;74:988–994. doi: 10.1212/WNL.0b013e3181d5a460. [DOI] [PubMed] [Google Scholar]
- 51.Leh SE, Chakravarty MM, Ptito A. The connectivity of the human pulvinar: a diffusion tensor imaging tractography study. Int J Biomed Imaging. 2008;2008:789539. doi: 10.1155/2008/789539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arend I, Machado L, Ward R, et al. The role of the human pulvinar in visual attention and action: evidence from temporal-order judgment, saccade decision, and antisaccade tasks. Prog Brain Res. 2008;171:475–483. doi: 10.1016/S0079-6123(08)00669-9. [DOI] [PubMed] [Google Scholar]
- 53.Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]