Abstract
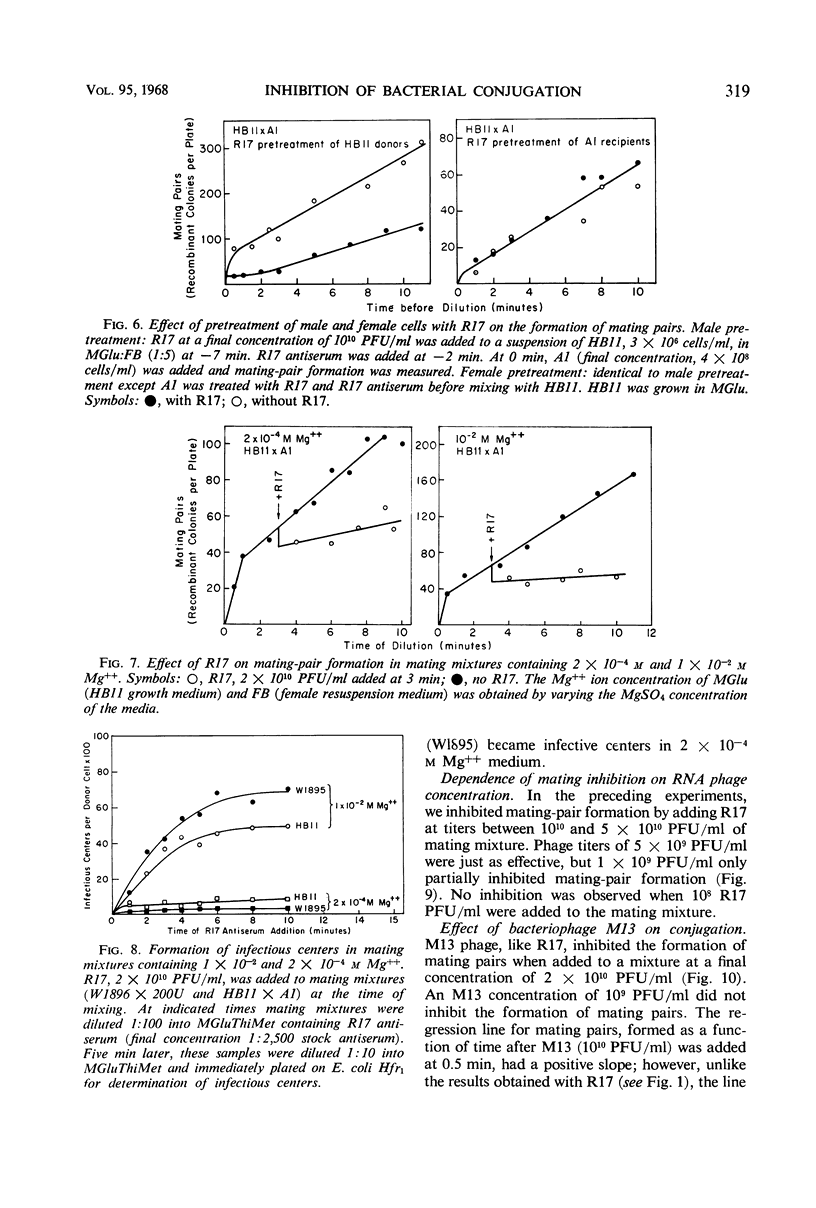
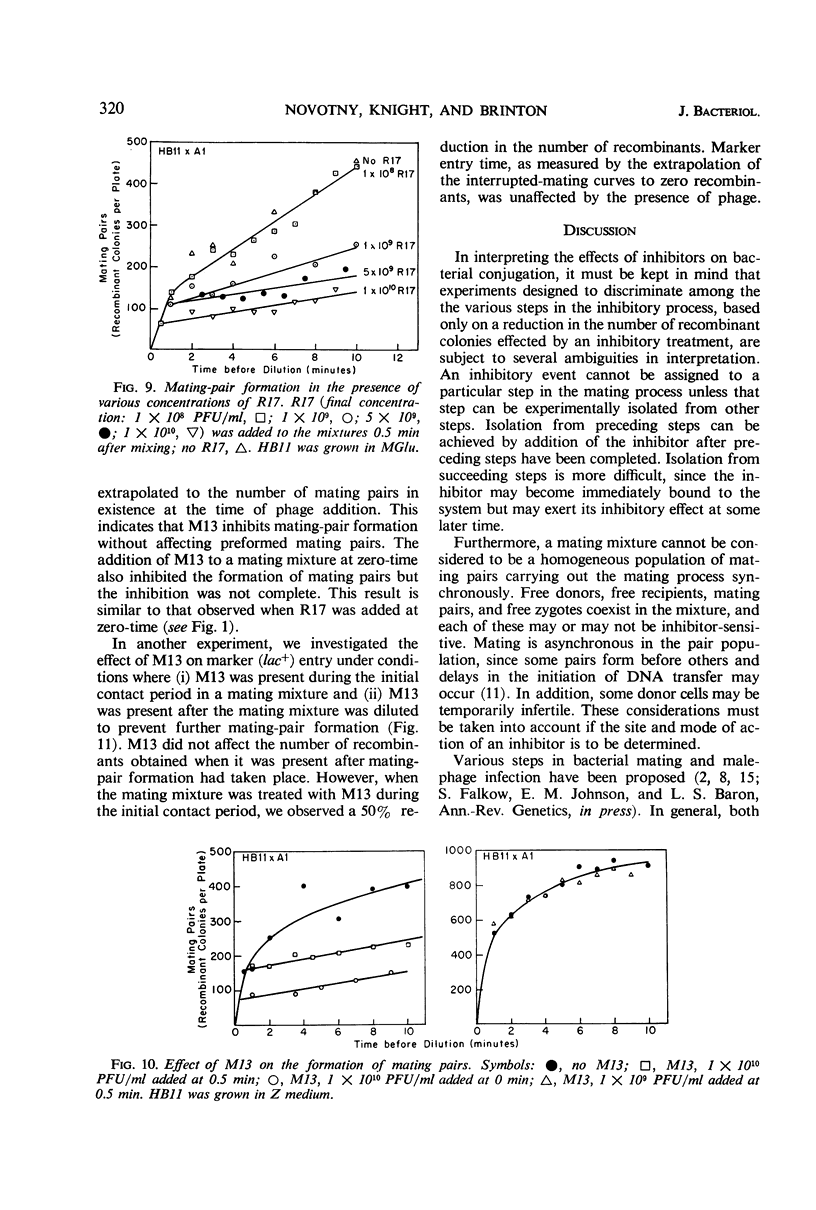
Both deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) male-specific phages, with an F-specific host range, inhibited the bacterial mating process of Escherichia coli. DNA phages prevented the formation of mating pairs but had no effect on mating pairs once they were formed. A step in RNA phage infection, prior to RNA penetration, prevented the formation of mating pairs and, in addition, prevented a fraction of existing mating pairs from completing the mating process. These findings are compatible with the hypothesis that donor cells have a single surface structure involved in both conjugation and male-phage adsorption and that this element is the F pilus.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AKIBA T., KOYAMA K., ISHIKI Y., KIMURA S., FUKUSHIMA T. On the mechanism of the development of multiple-drug-resistant clones of Shigella. Jpn J Microbiol. 1960 Apr;4:219–227. doi: 10.1111/j.1348-0421.1960.tb00170.x. [DOI] [PubMed] [Google Scholar]
- BRINTON C. C., Jr, GEMSKI P., Jr, CARNAHAN J. A NEW TYPE OF BACTERIAL PILUS GENETICALLY CONTROLLED BY THE FERTILITY FACTOR OF E. COLI K 12 AND ITS ROLE IN CHROMOSOME TRANSFER. Proc Natl Acad Sci U S A. 1964 Sep;52:776–783. doi: 10.1073/pnas.52.3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- CAVALLI L. L., LEDERBERG J., LEDERBERG E. M. An infective factor controlling sex compatibility in Bacterium coli. J Gen Microbiol. 1953 Feb;8(1):89–103. doi: 10.1099/00221287-8-1-89. [DOI] [PubMed] [Google Scholar]
- CLARK A. J., ADELBERG E. A. Bacterial conjugation. Annu Rev Microbiol. 1962;16:289–319. doi: 10.1146/annurev.mi.16.100162.001445. [DOI] [PubMed] [Google Scholar]
- Caro L. G., Schnös M. The attachment of the male-specific bacteriophage F1 to sensitive strains of Escherichia coli. Proc Natl Acad Sci U S A. 1966 Jul;56(1):126–132. doi: 10.1073/pnas.56.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER K. W. The nature of the endergonic processes in conjugation in Escherichia coli K-12. J Gen Microbiol. 1957 Feb;16(1):136–145. doi: 10.1099/00221287-16-1-136. [DOI] [PubMed] [Google Scholar]
- FREDERICQ P. Colicins. Annu Rev Microbiol. 1957;11:7–22. doi: 10.1146/annurev.mi.11.100157.000255. [DOI] [PubMed] [Google Scholar]
- Ganesan A. K., Rotman B. Transfer and incorporation of genes controlling beta-D-galactosidase synthesis from Hfr and F' donors of Escherichia coli. J Bacteriol. 1966 Nov;92(5):1378–1382. doi: 10.1128/jb.92.5.1378-1382.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. D., Caro L. Genetic transfer in bacterial mating. Science. 1965 Dec 24;150(3704):1679–1684. doi: 10.1126/science.150.3704.1679. [DOI] [PubMed] [Google Scholar]
- HAYES W. [Observations on a transmissible agent determining sexual differentiation in Bacterium coli]. J Gen Microbiol. 1953 Feb;8(1):72–88. doi: 10.1099/00221287-8-1-72. [DOI] [PubMed] [Google Scholar]
- HOFSCHNEIDER P. H., PREUSS A. M 13 BACTERIOPHAGE LIBERATION FROM INTACT BACTERIA AS REVEALED BY ELECTRON MICROSCOPY. J Mol Biol. 1963 Oct;7:450–451. doi: 10.1016/s0022-2836(63)80038-8. [DOI] [PubMed] [Google Scholar]
- Ippen K. A., Valentine R. C. The sex hair of E. coli as sensory fiber, conjugation tube, or mating arm? Biochem Biophys Res Commun. 1967 Jun 23;27(6):674–680. doi: 10.1016/s0006-291x(67)80088-3. [DOI] [PubMed] [Google Scholar]
- Knolle P. Evidence for the identity of the mating-specific site of male cells of Escherichia coli with the receptor site of an RNA phage. Biochem Biophys Res Commun. 1967 Apr 7;27(1):81–87. doi: 10.1016/s0006-291x(67)80043-3. [DOI] [PubMed] [Google Scholar]
- LOEB T. Isolation of a bacteriophage specific for the F plus and Hfr mating types of Escherichia coli K-12. Science. 1960 Mar 25;131(3404):932–933. doi: 10.1126/science.131.3404.932. [DOI] [PubMed] [Google Scholar]
- LOEB T., ZINDER N. D. A bacteriophage containing RNA. Proc Natl Acad Sci U S A. 1961 Mar 15;47:282–289. doi: 10.1073/pnas.47.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg J, Cavalli L L, Lederberg E M. Sex Compatibility in Escherichia Coli. Genetics. 1952 Nov;37(6):720–730. doi: 10.1093/genetics/37.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell E., Datta N. Functional homology of the sex-factor and resistance transfer factors. Nature. 1965 Aug 21;207(999):884–885. doi: 10.1038/207884a0. [DOI] [PubMed] [Google Scholar]
- Meynell E., Datta N. Mutant drug resistant factors of high transmissibility. Nature. 1967 May 27;214(5091):885–887. doi: 10.1038/214885a0. [DOI] [PubMed] [Google Scholar]
- Meynell E., Datta N. The nature and incidence of conjugation factors in Escherichia coli. Genet Res. 1966 Feb;7(1):141–148. doi: 10.1017/s001667230000954x. [DOI] [PubMed] [Google Scholar]
- Meynell E., Datta N. The relation of resistance transfer factors to the F-factor (sex-factor) of Escherichia coli K12. Genet Res. 1966 Feb;7(1):134–140. doi: 10.1017/s0016672300009538. [DOI] [PubMed] [Google Scholar]
- Meynell G. G., Lawn A. M. Sex pili and common pili in the conjugational transfer of colicin factor Ib by Salmonella typhimurium. Genet Res. 1967 Jun;9(3):359–367. doi: 10.1017/s0016672300010636. [DOI] [PubMed] [Google Scholar]
- Paranchych W. Stages in phage R17 infection: the role of divalent cations. Virology. 1966 Jan;28(1):90–99. doi: 10.1016/0042-6822(66)90309-6. [DOI] [PubMed] [Google Scholar]
- TZAGOLOFF H., PRATT D. THE INITIAL STEPS IN INFECTION WITH COLIPHAGE M13. Virology. 1964 Nov;24:372–380. doi: 10.1016/0042-6822(64)90174-6. [DOI] [PubMed] [Google Scholar]
- Tatum E. L., Lederberg J. Gene Recombination in the Bacterium Escherichia coli. J Bacteriol. 1947 Jun;53(6):673–684. doi: 10.1128/jb.53.6.673-684.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T. Evolutionary relationships of R factors with other episomes and plasmids. Fed Proc. 1967 Jan-Feb;26(1):23–28. [PubMed] [Google Scholar]


