Abstract
The interleukin 17 (IL-17) family of cytokines contains 6 structurally related cytokines, IL-17A through IL-17F. IL-17A, the prototypical member of this family, just passed the 25th anniversary of its discovery. While less is known about IL-17B-F, IL-17A (commonly known as IL-17) has received much attention for its pro-inflammatory role in autoimmune disease. Over the past decade, however, it has become clear that the functions of IL-17 are far more nuanced than simply turning on inflammation. Accumulating evidence indicates that IL-17 has important context- and tissue-dependent roles in maintaining health during response to injury, physiological stress and infection. Here, we discuss the functions of the IL-17 family, with a focus on the balance between the pathogenic and protective roles of IL-17 in cancer and autoimmune disease, including results of therapeutic blockade and novel aspects of IL-17 signal transduction regulation.
Highlights/eToc:
The IL-17 cytokine family is relatively poorly understood, apart from the prototypical, founding member, IL-17A, which has achieved notoriety for its role in autoimmunity. In this review, McGeachy, Cua and Gaffen discuss the pathogenic and protective roles of the IL-17 family in health, inflammation, injury, microbial regulation and cancer.
Introduction
Interleukin 17A (IL-17A) was cloned in 1993 (Rouvier et al., 1993; Yao et al., 1995b), and the first IL-17-binding receptor (IL-17RA) was described in 1995 by researchers at Immunex (Gaffen, 2011; Yao et al., 1995a). These molecules were striking in that both the ligand and receptor were distinct in sequence from other known mammalian cytokines. Both were subsequently recognized to be the founding members of a unique and evolutionarily ancient cytokine family that is present in species as early as jawless fish as well as Mollusca and sea urchins (Buckley et al., 2017; Han et al., 2015; Novatchkova et al., 2003). While IL-17 remained obscure throughout the 1990’s and early 2000’s, several early studies hinted that this cytokine was elevated in human inflammatory or autoimmune diseases and was expressed in a non-canonical T helper cell population (Albanesi et al., 1999; Antonysamy et al., 1999; Infante-Duarte et al., 2000; Kostulas et al., 1999; Kotake et al., 1999; Luzza et al., 2000; Shin et al., 1999; Teunissen et al., 1998). The field’s interest in IL-17 function accelerated rapidly with the discovery in 2005 that IL-17 is the signature cytokine of a distinct CD4+ T helper subset, T helper 17 (Th17) cells, which are characterized by expression of the “master” transcription factor RORγt and activated by the IL-12-family cytokine, IL-23. The so-called “IL-23-IL-17 axis” was found to function as a critical driver of autoimmune disease, first in mouse models lacking either IL-23 or IL-17, and later validated in humans by genome wide association studies (GWAS) and eventually by clinical blockade of these cytokines in autoimmunity (Duerr et al., 2006; Harrington et al., 2005; Langrish et al., 2005; McInnes et al., 2017). IL-17 was subsequently shown to be produced by other cell populations, such as CD8+ (Tc17) cells as well as various subsets of innate lymphocytes including γδ T, natural killer T (NKT), group 3 innate lymphoid cells (ILC3) and ‘natural’ Th17 cells (Cua and Tato, 2010). Some reports indicate that myeloid-lineage cells including neutrophils and microglia also produce IL-17, though this process is less understood and remains somewhat controversial (Chen et al., 2016; Tamassia et al., 2018; Taylor et al., 2014; Werner et al., 2011).
The fact that IL-17 has been conserved in evolution demonstrates that its inflammatory activities have, on balance, been beneficial for human survival. Nonetheless, these host-protective attributes can also be problematic, giving rise to immunopathology in autoimmunity, cancer or other inflammatory syndromes. We will briefly summarize the functions of IL-17 family members, but this review will focus on IL-17A as it has been most strongly implicated in human health and disease. We will address our current understanding of the contrasting outcomes of IL-17-driven inflammation, and how the various factors directing IL-17 production or signaling circuitry contribute to maintaining homeostasis mediated by this cytokine system. We will also highlight gaps that remain in our understanding of this topic.
Structure and signaling of IL-17 family cytokines and receptors
Originally named CTLA8, the founding member of the family IL-17A showed unexpected sequence homology with an open reading frame in Herpesvirus saimiri, a T cell tropic γ-herpesvirus, but not with other known cytokine families (Gaffen, 2011; Yao et al., 1995a). The presence of an AU-rich instability sequence in the 3’ UTR and a capacity to induce cytokine secretion in target cells led to its designation as a cytokine. Screens for homologous genes led to the discovery of IL-17B, IL-17C, IL-17D, IL-17E (now known as IL-25) and IL-17F (Figure 1). These cytokines adopt an unusual cysteine-knot fold architecture, analogous to NGF and PDGF but not other immune cytokine subclasses (Hymowitz et al., 2001). Similarly, the IL-17 receptor family comprises a distinct subclass of receptors characterized by a shared cytoplasmic motif termed a “SEFIR”, which has a distant relationship with the TIR domain found in Toll and IL-1 receptors (Novatchkova et al., 2003) (Figure 1). There is evidence for physical and functional interactions with members of the FGFR and EGFR families as well (Chen et al., 2019; Tsang et al., 2002). Nonetheless, there are still orphan ligands and receptors in the IL-17 family, and hence there is still much to learn about this enigmatic cytokine receptor family.
Figure 1: IL-17R family receptors and ligands.
As a distinct cytokine family, IL-17 receptor subunits share a cytoplasmic motif conserved within the IL-17R family called a “SEFIR” (“SEF/IL-17 receptor”) domain, which is analogous to the Toll/Il-1 Receptor (TIR) domain found in TLR and IL-1 receptors (Novatchkova et al., 2003). The initial event in IL-17R signaling is recruitment of Act1 (aka CIKS), a multifunctional signaling protein that also contains a SEFIR domain necessary for IL-17R-Act1 association (Liu et al., 2011a; Qian et al., 2007; Sonder et al., 2011). Although first identified as a negative regulator of B cell activating factor (BAFF) and CD40 signaling, Act1 is best understood as a nonredundant activator of IL-17RA-dependent signals (Chang et al., 2006; Qian et al., 2007; Qian et al., 2004; Sonder et al., 2012). Mice lacking Act1 phenocopy Il17ra−/− mice, and rare humans with null mutations in ACT1 have identical phenotypes with respect to fungal susceptibility as those lacking IL17RA or IL17RC (Conti and Gaffen, 2015; Li et al., 2017).
Act1 has E3 Ubiquitin ligase activity (Liu et al., 2009), and upon IL-17 signaling, Act1 rapidly recruits and ubiquitinates TNF-receptor associated factor (TRAF)6, another E3 Ubiquitin ligase (Figure 2) (Qian et al., 2007; Schwandner et al., 2000). Like other receptors that employ TRAF6, IL-17 triggers activation of the canonical NF-κB cascade through IKK activation and IκBα degradation (Schwandner et al., 2000; Yao et al., 1995a). NF-κB subsequently upregulates expression of IκBζ and Bcl3, noncanonical members of the NF-κB family that in turn promote expression of various IL-17-NF-κB driven pro-inflammatory and anti-microbial genes (Karlsen et al., 2010; Ruddy et al., 2004; Tohyama et al., 2018). IκBζ is implicated in psoriasis both genetically and functionally (Johansen et al., 2015; Tsoi et al., 2015). TRAF6 also promotes activation of MAPK/AP1 pathways and the C/EBPβ and δ transcription factors (Chang et al., 2006; Maitra et al., 2007; Qian et al., 2007; Ruddy et al., 2004). Conversely, IL-17-NF-κB signaling induces several negative feedback circuits that restrain NF-κB activation, including deubiquitinating enzymes or related factors, including A20 (TNFAIP3), Abin-1 (TNIP1) and USP25 (Cruz et al., 2017; Garg et al., 2013; Zhong et al., 2012) (Figure 4).
Figure 2: IL-17 signaling pathways: transduction and amplification.
The IL-17RA and IL-17RC subunits are characterized by two extracellular fibronectin-like domains (FN), and bind to IL-17A, IL-17F and IL-17AF ligands. The intracellular domains encode conserved SEFIR domains that interact with a corresponding SEFIR motif on the adaptor Act1. Both IL-17RA and IL-17RC also have essential “SEFIR-extension” sequences (SEFEX) that are required for functional activity. Act1 additionally contains a TRAF-binding site that enables association with TRAF family proteins. Engagement with TRAF6 drives activation of the classical NF-κB pathway, MAPK:AP1 activation and also activation of Syk kinase:CARMA2 that also activates NF-κB. Collectively these factors trigger transcriptional induction of target genes, TRAF2 and TRAF5 can also be engaged by Act1, and promote a pathway of post-transcriptional mRNA stabilization or through control of multiple RNA binding proteins including HuR and Arid5a. A distal, non-conserved domain in IL-17RA activates the transcription factor C/EBPβ, and has been termed CBAD (C/EBPβ activation domain).
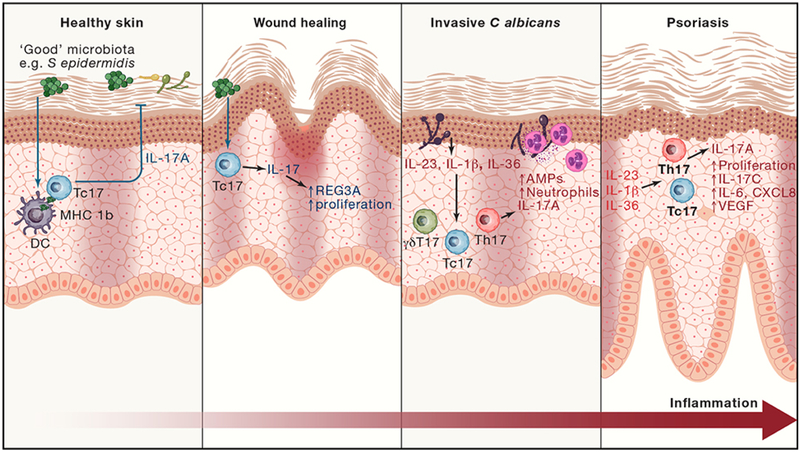
Figure 4: IL-17 functions in skin.
In healthy skin, IL-17 is produced by microbiota-responsive Tc17 cells. Tc17-produced IL-17 regulates microbiota and provides anti-fungal protection. When skin injury breaches the epithelial barrier, IL-17 promotes epithelial cell proliferation. In addition, Tc17 produce tissue repair and immunoregulatory molecules (e.g. amphiregulin, VEGF, IL-10) to promote wound healing. If pathogens such as C albicans become invasive, epithelial injury leads to production of pro-inflammatory cytokines to activate and expand IL-17-producing Th17, Tc17 and γδ T cells. In synergy with TNF, IL-17 now induces heightened production of chemokines to recruit neutrophils, and antimicrobial peptides to combat the infection. In psoriasis, the combined pro-inflammatory and wound-healing effects of IL-17 are chronically activated and amplified, causing pathogenic hyperproliferation of keratinocytes and skin inflammation.
In keratinocytes, several recent studies have suggested involvement of a tyrosine kinase-driven pathway in IL-17-driven signal transduction. The Syk kinase was reported to bind to Act1 and TRAF6 and thus function upstream of NF-κB leading to expression of the chemokine CCL20, a ligand for CCR6 that is expressed on most Type 17 cells, potentially driving a feed-forward circuit of Th17-driven inflammation (Wu et al., 2015c). Consistently, in another study IL-17 was suggested to regulate NF-κB and CCL20 through CARMA2, encoded by CARD14, a psoriasis-linked locus identified in GWAS (Tsoi et al., 2012)) which also interacted with Act1 and TRAF6 (Wang et al., 2018). In the TCR and Dectin signaling pathways, CARD-family proteins are well described to bind to Bcl10 and the paracaspase MALT1 to promote NF-κB activation; consistently, blocking MALT1 showed at least a partial inhibitory effect on IL-17 and TNFα combinatorial signaling in keratinocytes, (Israel et al., 2018), although much more work is warranted in this regard. As noted, IL-17 also induces proliferative signaling in keratinocytes through Act1, a major facet of its impact in psoriasis and perhaps also explaining its involvement in skin tumorigenesis (Chiricozzi and Krueger, 2013; Ha et al., 2014; Langowski et al., 2006; van der Fits et al., 2009). Keratinocyte proliferation is driven by a non-canonical IL-17-mediated TRAF4:ERK5 activation pathway to promote tumorigenesis (Wu et al., 2015b). These studies again point to the importance of considering cell type-specific contextual responses to IL-17, and may explain the finding that IL-17-mediated signals in keratinocytes are overlapping yet distinct from those induced in fibroblasts or other mesenchymal cells (Ha et al., 2014).
Functions of IL-17 family members B through F
IL-17B was originally found to increase during intestinal inflammation and to promote neutrophil migration upon intraperitoneal administration, suggesting a pro-inflammatory role (Bie et al., 2017a; Shi et al., 2000). However, IL-17B has been shown to perform an anti-inflammatory role by blocking IL-25 signaling during mucosal inflammation, by virtue of the shared IL-17RB receptor subunit (Reynolds et al., 2015). Mouse models indicate that IL-17B signaling through IL-17RB promotes survival, proliferation and migration of cancer cells in mouse models (Bie et al., 2017b; Laprevotte et al., 2017; Wu et al., 2015a; Yang et al., 2018). In humans, elevated IL-17B corresponded with poor prognosis in patients with pancreatic, lung and breast cancer (Laprevotte et al., 2017; Wu et al., 2015a; Yang et al., 2018).
Similar to IL-17, IL-17C promotes anti-microbial protective responses and barrier maintenance in skin and intestine (Ramirez-Carrozzi et al., 2011; Song et al., 2011). However, IL-17C is produced by epithelial cells rather than immune cells, hence acts as a rapid local autocrine response to epithelial injury. In addition, IL-17C produced by keratinocytes and cutaneous neurons was recently shown to protect peripheral sensory neurons during Herpes simplex virus reactivation, promoting both survival and growth to replace damaged nerves (Peng et al., 2017). Cutaneous sensory neurons promote IL-17 mediated skin inflammation during infection (Kashem et al., 2015), and IL-17C can drive psoriatic inflammation in mouse models (Johnston et al., 2013; Ramirez-Carrozzi et al., 2011). Hence, future study of the interactions between IL-17 family members and the nervous system during health and disease should yield further interesting insights.
IL-17D is the least well understood IL-17 family member. It is expressed in a wide variety of healthy tissues, and has been found to increase in tumors and during viral infection (Saddawi-Konefka et al., 2016; Seelige et al., 2017; Starnes et al., 2002). IL-17D stimulation of endothelial cells induces classical pro-inflammatory cytokine responses including IL-6, IL-8 and GM-CSF (Starnes et al., 2002), and IL-17D-deficient mice suggest a role for IL-17D in NK cell-mediated tumor and viral surveillance (Saddawi-Konefka et al., 2016).
IL-17E (IL-25) is a little unusual amongst the IL-17 family: while other IL-17 family members promote neutrophilic responses, IL-25 uniquely induces expression of IL-4, IL-5, IL-13, and TSLP, all associated with type 2 immunity. For this reason, IL-25 was given a separate interleukin designation to underscore its distinct function from other IL-17 family members (Fort et al., 2001; Hurst et al., 2002). Early studies showed that IL-25 gene expression was elevated following Aspergillus and Nippostrongylus infection in the lung and gut, and that IL-25 promoted epithelial cell hyperplasia, increased mucus secretion, and airway hyperreactivity (Owyang et al., 2006). IL-25 can also inhibit autoimmunity mediated by Th17 cells. IL-25 treatment elevated production of IL-13 and suppressed expression of IL-1, IL-6, and IL-23 by activated dendritic cells (Kleinschek et al., 2007). In addition to Th2 cells, stromal cell and epithelium, IL-25 was found to activate a population of lineage negative cells subsequently identified as ILC2 (Fort et al., 2001; Hams et al., 2014; Hams et al., 2013; Hurst et al., 2002).
Similarly to IL-17A, C and F, IL-25 plays important roles in both adaptive and innate phases of immunity. IL-25 is produced constitutively by intestinal tuft cells, a population of epithelial chemosensory cells capable of detecting harmful environmental agents (von Moltke et al., 2016). During parasitic helminth infection, intestinal tuft cells produce large amounts of IL-25. This in turn promotes ILC2 cells to secretion IL-13, which programs epithelial crypt progenitors to differentiate toward goblet and tuft cells. This feedforward cellular response between tuft cells and ILC2 greatly enhances mucus production for worm expulsion and type 2 immunity against the parasitic infection. These results indicate that IL-25 is a “barrier surface” cytokine and its production is largely regulated by environmental cues. Recently, IL-25 was also shown to be produced by a subset of thymic epithelial cells that has chemosensory functions, similar to the intestinal tuft cells (Bornstein et al., 2018; Miller et al., 2018). Here, the IL-25-producing thymic epithelial cells provides antigen for TCR selection and development of type 2 innate-like lymphoid cells (iNKT-2) that play important roles in host defense against parasites.
IL-25 can amplify immunological responses to extrinsic environmental factors that when dysregulated may lead to harmful disease outcome. IL-25 biology is closely linked to Th2 cells, eosinophils, mast cells, and airway epithelial cells, all of which can contribute to airway inflammation in asthma. Respiratory viral infection is a major trigger leading to glucocorticoid-resistant asthma exacerbation requiring hospitalization, particularly in young children. Recent reports suggest a link between IL-25 and viral pathogen-associated asthma exacerbation (Beale et al., 2014). IL-25 was found in upper airway bronchial epithelial cells that have features of chemosensory epithelial cells, like the intestinal tuft cells (Kohanski et al., 2018). This raises the possibility that the chemosensory bronchial epithelial cells can promote asthma exacerbation upon sensing pathogen-associated antigens. The requirement of IL-25 in this process was demonstrated by treatment of virus infected mice with anti-IL-17RB neutralizing antibody, which reduced infiltration of eosinophils, neutrophils, and basophils; secretion of mucus; and production of Th2 cytokines in the lung. These results suggest that IL-25 may be a therapeutic target for treatment of severe asthma exacerbation that is driven by excessive responses to harmful environmental agents.
IL-17F shares the most similarities with IL-17 in terms of both cellular sources and function. IL-17F and IL-17A are co-expressed on linked genes and are usually co-produced by Type 17 cells (Akimzhanov et al., 2007). IL-17 and IL-17F exist as homodimers but can also be produced as an IL-17AF heterodimer. All forms of the cytokine induce signals through an obligate dimeric IL-17RA and IL-17RC heterodimer (Figure 1). Since they use the same receptor complex, IL-17A, -AF and -F trigger qualitatively similar signaling pathways, however IL-17A homodimers induce a far more potent signal compared to IL-17F homodimers, with IL-17AF thought to be intermediate signaling strength (Chang and Dong, 2007; Wright et al., 2007). IL-17F contributes to inflammatory responses and protection at barrier surfaces, as evidenced by heightened susceptibility to chronic mucocutaneous candidiasis in humans with an autosomal dominant IL17F deficiency (Puel et al., 2011b). IL-17A is implicated more strongly than IL-17F in driving autoimmunity (Ishigame et al., 2009; Veldoen, 2017), although the role of IL-17AF remains unclear and bispecific Abs targeting both isoforms are under consideration for therapy (Torres et al., 2016).
IL-17A functions: Barrier surface protection and repair
IL-17 signals dominantly in non-hematopoietic cells to induce innate-like acute immune defenses. One hallmark function of IL-17 is induction of chemokines, including CXCL1, CXCL2 and CXCL8 (IL-8), that attract myeloid cells such as neutrophils, to the infected or injured tissue (Onishi and Gaffen, 2010). Additionally, IL-17 induces IL-6 and G-CSF, cytokines that promote myeloid-driven innate inflammation (Gaffen et al., 2014). Together with induction of antimicrobial peptides such as b-defensins, S100A8 and lipocalin 2, these responses protect the host during acute microbial invasion. Accordingly, IL-17 responses defend against extracellular fungal and bacterial pathogenic species including Candida, Cryptococcus, Klebsiella and Staphylococcus, among others. Indeed, genetic defects in the Th17 or IL-17 signaling pathway in humans or in mice lead to severe mucocutaneous Candida infections in humans, which points to the particular importance of IL-17 in immunity to fungi (Conti and Gaffen, 2015; Drummond and Lionakis, 2018; Li et al., 2018).
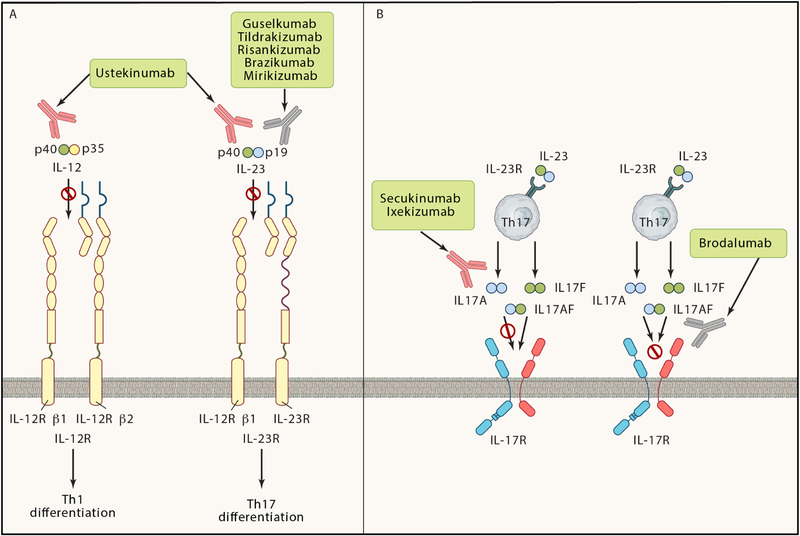
When chronically activated against inappropriate host targets during autoimmune disease, the pro-inflammatory effects of IL-17 contribute to pathogenic inflammation. The most compelling demonstration of IL-17 as a causal driver of chronic inflammation in humans came from the dramatically successful clinical trials in psoriasis (Chiricozzi and Krueger, 2013). There are three antibody agents that block the function of IL-17 in clinical use: secukinumab and ixekizumab inhibit IL-17A (and IL-17A/F) ligands, and brodalumab inhibits IL-17RA. The latter blocks the common IL-17-family receptor subunit and therefore potentially could inhibit multiple members of the IL-17 family. In addition, five biologic agents have been developed to inhibit IL-23, the upstream driver of IL-17 in pathogenic responses (Figure 3). All these IL-17 and IL-23 inhibitors have shown remarkable efficacy in psoriasis, consistently achieving PASI 100 scores (indicative of a complete response) in 40 – 80% of patients. It is not a stretch to say that these IL-23-IL-17 targeting therapies have reset the gold standard for psoriasis treatment.
Figure 3: Current IL-17 or IL-23 targeting biologic therapies.
In psoriatic skin lesions, dysregulated IL-17 production or uncontrolled responses to IL-17 signaling promote pathogenic inflammation. Even so, IL-17 is present in healthy skin (Figure 4). In contrast to psoriasis, colonization with commensal bacteria including Staphylococcus epidermidis induces skin-localized production of IL-17 by non-classical MHC Ib-activated Tc17 cells, without causing overt inflammation (Linehan et al., 2018; Naik et al., 2015). Moreover, commensal-induced IL-17 protects from other infectious agents that require IL-17 for clearance, such as Candida (Naik et al., 2015) indicating that local IL-17 production can lead to a beneficial skin-protective state (Figure 3). Similarly, IL-17 production is increased during inflammatory bowel disease (Kleinschek et al., 2009), yet healthy intestine also contains significant populations of IL-17-producing cells. This microbiota-driven IL-17 acts on local epithelium to promote anti-microbial responses that are necessary and sufficient to maintain a homeostatic balance but without causing overt inflammation in the normal gut (Ivanov et al., 2009; Kumar et al., 2016). Walking this tightrope to maintain an appropriate balance is clearly essential for health.
As well as mediating microbial protection at barrier surfaces, there is increasing evidence that IL-17 contributes to tissue healing following injury, a frequent occurrence at these sites. Skin that has previously been inflamed in a psoriasis-like model shows more rapid wound closure that is partially dependent on RORγt+ T cells (and by inference IL-17) (Naik et al., 2017). Similarly, Tc17 induced by skin commensals accelerated wound healing (Linehan et al., 2018). At least part of these skin healing events are mediated through IL-17 induction of anti-microbial protein REG3A in keratinocytes to promote their proliferation following injury (Lai et al., 2012) (Chen et al., 2019; Ha et al., 2014) . However, this pro-proliferative role likely contributes to the pathogenic effects of IL-17 in psoriasis, as well as potentially in skin cancer (Chen et al., 2019; Lai et al., 2012; Langowski et al., 2006).
Clinical trials of neutralizing antibodies targeting IL-17 and IL-17RA in Crohn’s disease patients showed surprisingly limited efficacy. Of even greater concern were the disease exacerbations observed in some patients treated with secukinumab (anti-IL-17) (Hueber et al., 2012), and increased serum C-reactive protein, an indicator of inflammation, in patients receiving brodalumab (anti-IL-17RA) (Targan et al., 2016). Consistently, mouse studies showed that colitis-associated epithelial injury and intestinal leakage can be exacerbated in absence of IL-17 signaling, and revealed that IL-17 serves a beneficial role in the intestinal epithelium by helping to maintain the epithelial tight-junction barrier during inflammation (Lee et al., 2015; Maxwell et al., 2015; Whibley and Gaffen, 2015). As a side note, the anti-p40 (IL-12 and IL-23) blocker ustekinumab is approved for treatment of IBD, and clinical trials for biologics specifically targeting IL-23 show clinical benefit without encountering adverse events observed when blocking IL-17.. Hence the direct link between IL-23 and IL-17 observed in psoriasis does not appear to extend to inflammatory bowel disease, where IL-23 is likely driving an armory of additional effector molecules such that the specific protective functions of IL-17 in the injured gut apparently counterbalance its pathogenic potential.
A similar dichotomy of protective versus damaging IL-17 functions has been observed in the oral mucosa. Host protection from oral Candida albicans infection is exquisitely reliant on IL-17 signaling, shown in both mouse and human settings (Bär et al., 2012; Conti et al., 2016; Conti et al., 2009; Puel et al., 2011a). Similarly, oral IL-17 production is required to prevent outgrowth of gingivitis-causing bacteria in acute infections (Yu et al., 2007) and thus protect against bacterial inflammation-induced bone loss. In chronic periodontal disease, in contrast, IL-17 drives bone destruction (Eskan et al., 2012). Intriguingly, the mechanical gum injury caused by chewing of hard foods increases gingival IL-17 levels, and increased IL-17 then contributes to periodontitis and bone erosion during aging (Dutzan et al., 2017b). Hence, IL-17 responses that are beneficial following acute mechanical or infectious injury have the capacity to become deleterious when prolonged efforts at wound healing result in tissue remodeling with erosion and/or hyperproliferation, ultimately leading to loss of function.
IL-17 functions in non-barrier stressed tissues
Following success in psoriasis, the next therapeutic applications of IL-17 blockade came in arthritis. IL-17 blockade was effective in psoriatic arthritis (PsA) (McInnes et al., 2014; Mease and McInnes, 2016) as well as ankylosing spondylitis (AS), a disease with previously poor therapeutic options (Baeten et al., 2015; van Mens et al., 2018). In surprising contrast, biologics targeting IL-23 did not show benefit in AS (Baeten et al., 2018), providing another unexpected divergence in the IL-23-IL-17 autoimmunity axis. Both PsA and AS are diseases of connective tissues associated with joints (Lubberts, 2015). Joints and barrier surfaces also have in common a relatively high exposure to mechanical stress during routine use, which may be analogous to oral tissues where mastication drives IL-17-induced damage (see above, (Dutzan et al., 2017a)). Although joints are sterile tissues, dysregulated gut microbiota are found in autoimmune subjects, including those with rheumatoid arthritis (RA) (Scher et al., 2013), and have been demonstrated to contribute to arthritis susceptibility in mice (Maeda et al., 2016; Wu et al., 2010). Although IL-17 is increased in RA (Lubberts, 2015), unlike PsA and AS, IL-17 blockade was not particularly effective in RA clinical trials, at least when compared to standard-of-care biologic therapy (Genovese et al., 2014; Pavelka et al., 2015). There are several possible reasons for this discrepancy: RA is a complex and heterogeneous disease, and IL-17 may be a non-redundant driver in only a subset of patients, or is involved in early stages but less critical during later disease when biologic therapy is typically initiated. These findings collectively indicate that IL-17 is a not simply a ‘catch-all’ master inflammatory factor, and highlights the need to consider concomitant tissue- and immune-specific factors that modulate the role of IL-17 during inflammation.
IL-17 also contributes to several kidney disease settings. Specifically, IL-17 promotes glomerulonephritis following immune complex deposition in the kidney, and is thus thought to contribute to lupus renal disease (Pisitkun et al., 2012; Ramani et al., 2014). Conversely, IL-17 is protective when the kidney is injured by mechanical obstruction or systemic Candida albicans infection (Ramani et al., 2016; Ramani et al., 2018b). Following kidney injury, IL-17 promotes production of bradykinin by activating the kallikrein-kinin system in tubular epithelial cells (Ramani et al., 2018a), resulting in production of matrix metalloproteinases (MMPs) that reduce fibrosis, a scarring event that critically impairs kidney function. Indeed, MMP production is a characteristic response to IL-17, suggesting that regulation of wound healing and fibrotic scarring could be a more general function of IL-17 following injury. IL-17 has been found to be increased in tissues that are undergoing quite varied types of immunopathology and tissue remodeling, ranging from frank neutrophilic inflammation to fibrosis. In this regard, the kidney-specific bradykinin response suggests that the role of IL-17 in tissue remodeling could be tuned by the responding cell type, in addition to amplifying signals mediated by other cytokines present in the local inflammatory milieu, as discussed below.
Many pro-inflammatory cytokines including IL-17 are increased in obese mice and humans. Mice deficient in IL-17 and fed regular chow gain increased fat mass with age, corresponding to a role for IL-17 in regulating glucose homeostasis and inhibiting adipogenesis (Ahmed and Gaffen, 2013; Goswami et al., 2009; Zuniga et al., 2010). IL-17 also indirectly promotes the accumulation of Tregs expressing the IL-33 receptor (ST2) in adipose tissue, through increased numbers of IL-33-producing stromal cells (Kohlgruber et al., 2018). In the healthy state, IL-17 directly influences the metabolic function of adipocytes, increasing expression of thermogenic enzymes and sensitivity to catecholamine (Kohlgruber et al., 2018). Hence, IL-17-deficient mice are susceptible to hypothermia, despite increased total body fat and accumulation of lipid droplets within adipocytes – in other words, while fuel can be stored, it cannot efficiently be burned to generate extra heat when needed in the absence of IL-17. Overall then, increased IL-17 in obesity may reflect an attempt by the immune system to correct the pathologic nature of the increased fat tissue and the associated metabolic stress. Rather than absolute function, it is more likely the shift in balance between the beneficial effects of IL-17 in adipose tissue (thermogenic temperature regulation, adipocyte regulation), and detrimental effects (long-term reduced insulin sensitivity and induction of pro-inflammatory IL-6) that determine the final outcome of obesity-related inflammatory co-morbidities.
The brain is perhaps the most extreme example of a sterile tissue with limited capacity for regeneration and healing. To date, most evidence points to a pathogenic role for IL-17 when produced in the brain. IL-17 promotes neuroinflammation and neurodegeneration in rodent models of multiple sclerosis and stroke (Gelderblom et al., 2012; Shichita et al., 2009). Increased generation of IL-17-producing T cells during intestinal dysbiosis exacerbates central nervous system (CNS)-targeted autoimmune disease (Kumar et al., 2016) and impairs recovery following brain ischemia (stroke) (Benakis et al., 2016). IL-17 activates CNS-resident cells causing hyperexcitability of neurons (Siffrin et al., 2010), production of chemokines and cytokines by astrocytes and oligodendocytes and proliferation and ultimately death of oligodendrocyte precursors (Kang et al., 2013). Oligodendrocyte preservation is an aspirational goal to treat multiple sclerosis (MS), as failed repair of CNS lesions is a major problem for long-term disability. Phase II trials of secukinumab were reported to be successful in MS, but larger trials have yet to be initiated (Patel and Kuchroo, 2015).
IL-17 functions in the brain may extend beyond classical autoimmune inflammation. Cognitive dysfunction induced by high salt diet is linked to increased intestinal IL-17 production leading to brain endothelial damage (Faraco et al., 2018). Increased systemic IL-17 during pregnancy leads to development of autism-like behaviors in pup offspring (Choi et al., 2016), which can also be regulated by maternal microbiota (Kim et al., 2017). Furthermore, Th17 cells exacerbated depression-like symptoms in mice, and infiltrated the pre-frontal cortex and particularly hippocampus in depressed mice compared to controls(Beurel et al., 2013; Beurel and Lowell, 2018). It can be difficult to extrapolate these findings to humans, who have far more complex brain development and higher-order cognitive functioning than mice. However, it is well-known that patients with autoimmune disease have increased risk of cognitive co-morbidities such as depression and fatigue. Also, traumatic or ischemic brain injury patients frequently develop dysbiosis during hospitalization, which could alter immune responses in the inflamed brain (Simon et al., 2017). One further interesting and nascent area of research is the involvement of proteins that control circadian rhythm in regulating IL-17 production by Th17 cells, including melatonin (Farez et al., 2015) and ERB-REV1a (Amir et al., 2018; Yu et al., 2013). The influence of IL-17 on neurocognitive functions and mental health, like many cytokines in the brain, are only just beginning to be explored, but will no doubt reveal interesting and unexpected roles for IL-17 in brain homeostasis.
Balancing IL-17 effects in health and pathology at the level of signaling
Whether the outcomes of IL-17 signaling are beneficial or detrimental depend not only on the amount of IL-17 produced but also on how IL-17 signals are received and transmitted within the responding cell. As noted, the predominance of IL-17 signaling occurs in none-hematopoietic cells expressing the heterodimeric IL-17RA:IL-17RC receptor. IL-17 induces both positive and negative regulators of its own signaling pathways, resulting in complex feedback loops that can amplify or attenuate the inflammatory response (Figure 2). Outcomes of IL-17R activation are also strongly influenced by the biology of the cellular target, as well as concomitant signals from other cytokines, resulting in synergistic activation and nuanced tuning of downstream effector functions.
One curious aspect of IL-17 biology is that this cytokine is consistently a modest activator of signaling in vitro, regardless of the cell system analyzed (Fossiez et al., 1996; Ruddy et al., 2004; Shen et al., 2005; Veldoen, 2017; Yao et al., 1995a). Nonetheless, the activities of IL-17 in vivo are striking, in part because IL-17 signals potently in cooperation with other cytokines or inflammatory effectors (Miossec, 2003). Cooperative signaling among cytokines is biologically relevant in the injured or autoimmune environment, where numerous inflammatory effectors are produced and have potential to interact. One of the best-studied examples of synergy is with TNFα (Chiricozzi et al., 2011; Ruddy et al., 2004); consequently, bi-specific Abs are now in development for therapy (Fleischmann et al., 2017; Klunder et al., 2017; Kontermann and Brinkmann, 2015; Robert et al., 2017; Torres et al., 2016). However, IL-17 synergy is surprisingly promiscuous in that IL-17 is able to synergize with a variety of mediators that activate diverse signaling pathways. In addition to synergizing with strong NF-κB activators such as TNFα, lipopolysaccharide (LPS), IL-1β and lymphotoxin, IL-17 cooperates with IFNγ (activator of STAT1), IL-13 (STAT6), TGFβ (SMADs), fibroblast growth factor (FGF)2 (Ras:Raf) and a Candida albicans-derived pore-forming toxin from Candida albicans (c-Fos) (Faour et al., 2003; Miossec, 2003; Ruddy et al., 2004; Song et al., 2015; Verma et al., 2017). The molecular mechanisms through which many of these synergistic events tune IL-17 signaling are still unresolved, but likely act through modulating expression of positive and negative regulators of either IL-17 signaling or downstream pre- and post-transcriptional events, discussed below. It is intriguing to speculate that barrier surface dysbiosis, which is associated with susceptibility to autoimmune disease, could be due in part to altering the cytokine-tuning milieu of IL-17-responding cells, rather than directly affecting IL-17 production or signaling per se.
IL-17 induces RNA-binding proteins to tune signaling outcomes
More recent developments in studies of IL-17 signaling have revealed a remarkable capacity to direct a variety of post-transcriptional events. Regulation of mRNA allows for flexible and nuanced gene expression in response to rapidly changing environmental or developmental conditions, and hence is vital for immune homeostasis (Fu and Blackshear, 2017; Kafasla et al., 2014; Seko et al., 2006). Mature polyadenylated mRNAs contain recognition motifs for RNA binding proteins (RBPs), typically located in 5’ and 3’ untranslated regions (UTRs), which coordinate RNA nuclear import-export, capping, splicing, polyadenylation, stability-decay and translation. Post-transcriptional control of mRNA is central to IL-17 signaling since numerous canonical IL-17 target genes are encoded by intrinsically unstable transcripts, particularly chemokine and cytokine genes (e.g., Cxcl1, Cxcl5, Il6) (Amatya et al., 2017; Hamilton et al., 2012). The IL-17-driven pathway that controls RNA stability is initiated by phosphorylation of Act1 by Ikki (IKKe), directing its association to TRAF2 and TRAF5. and mobilizing multiple RBPs (Amatya et al., 2018; Bulek et al., 2011; Datta et al., 2010; Qu et al., 2012; Sun et al., 2011) (Figure 4). Tristretrapolin (TTP) is one of the best characterized RNA-stabilizing proteins in the immune system, but surprisingly does not participate in IL-17-mediated signaling (Datta et al., 2010). Rather, IL-17 employs the RBPs HuR and Arid5a to prolong the half-life of certain target transcripts. These RBPs bind 3’ UTR motifs and counteracting the activity of de-stabilizing ribonucleases (see below) (Amatya et al., 2018; Herjan et al., 2013; Puel and Casanova, 2018). Interestingly, Act1 itself also functions as an RBP in cooperation with HuR, binding IL-17-induced mRNA transcripts via its SEFIR domain (Herjan et al., 2018). Act1 also interacts with the DDX3X RNA helicase to increase stability of client mRNA transcripts (Somma et al., 2015). Some RBPs are multifunctional; Arid5a and Act1 both act not only to extend the half-life of certain transcripts, but facilitate translation of others (Amatya et al., 2018; Herjan et al., 2018).
The actions of positively-acting RBPs can be offset by RBPs that promote RNA decay, which is critical in restraining potentially destructive inflammatory signaling (Fu and Blackshear, 2017). HuR, in cooperation with Act1, promotes Cxcl1 stability in part by sterically displacing the destabilizing RBP ASF:SF2 on the 3’ UTR (Herjan et al., 2018; Herjan et al., 2013; Sun et al., 2011). Regnase-1 (MCPIP1) is a potent endoribonuclease (Akira, 2013; Fu and Blackshear, 2017) whose expression is induced by IL-17 and that degrades IL-17-induced mRNA transcripts, thus acting as a feedback inhibitor. Regnase-1 mRNA (Zc3h12a) is itself controlled at the level of mRNA stability by DDX3X (Somma et al., 2015). Regnase-1 binds to IL-17- and LPS-induced gene transcripts via stem-loop structures in the 3’ UTR in a site overlapping with Arid5a, another IL-17-induced RBP (Amatya et al., 2018; Masuda et al., 2013). Both Regnase-1 and Arid5a regulate expression of the transcription factor IkBz, but in different ways; whereas Regnase-1 degrades Nfkbiz mRNA encoding IkBz, Arid5a mainly controls its translation (Amatya et al., 2018; Garg et al., 2015). Thus regulation of RBPs can indirectly impact expression of genes whose transcripts are not intrinsically unstable by virtue of controlling transcription factors such as IκBζ.
Mice with a Regnase-1 deficiency illustrate the perils of having unconstrained IL-17 signaling responses. Whereas beneficial IL-17 signaling is enhanced in Regnase-1-deficient mouse settings, as demonstrated by heightened resistance to fungal infection, this is concomitant with exacerbated autoimmunity in models of EAE and imiquimod-psoriasis (Garg et al., 2015; Monin et al., 2017). In humans, Regnase-1 expression is elevated in psoriatic lesions and other conditions of IL-17-driven inflammation, indicating that its activity can be offset in inflammatory environments (Monin et al., 2017). Regnase-1 binds similar RNA sites to the RBPs Roquin-1 and Roquin-2, which also restrict IL-17 signaling by degrading target genes (Jeltsch et al., 2014). However, Regnase-1 and Roquins are thought to target different transcripts by virtue of distinct subcellular localization: Regnase-1 targets actively-translating transcripts during acute inflammation by associating with polysomes, whereas Roquins tend to target translationally inactive mRNAs (Mino et al., 2015). Thus subcellular compartmentalization provides an additional nuanced layer of regulation during IL-17-mediated effector responses. Interestingly, Regnase-1 and Roquins regulate IL-17 production by regulating Th17 differentiation (Jeltsch et al., 2014), thereby tuning both the production of and response to IL-17.
Roles of IL-17 in pathogenesis of cancer
Following the initial discovery of the IL-23-Th17 immune axis, it was proposed that IL-17 may support cancer development by promoting chronic tissue inflammation. Indeed, elevated IL-17 signature genes can be found in multiple human malignancies including cervical cancer, esophageal cancer, gastric cancer, hepatocellular carcinoma, and colorectal cancer (CRC)(Le Gouvello et al., 2008; Miyahara et al., 2008). For many tumor types, particularly at mucosal barriers, gene signatures of Th1 cells (IFNG, STAT1, TBX21) and CD8+ cytotoxic T cells (PRF1, GZMB) are linked to better overall and relapse-free patient survival, whereas Th17 cell signatures (RORC, IL17, IL23, STAT3) correspond to worse patient outcomes (Tosolini et al., 2011). This dichotomy is even clearer in checkpoint therapy responders (e.g. anti-CTLA4 and anti-PD1 treatments), which have a strong anti-tumor Th1 and CD8+ cytotoxic T cell signature (Gopalakrishnan et al., 2018).
One key factor promoting unfavorable IL-17 immunity in fighting cancer at the barrier surface is microbial dysbiosis. In mice, segmented filamentous bacteria (SFB) form direct physical contact with mouse intestinal epithelial cells and thereby promote intestinal IL-17 signature genes(Ivanov et al., 2009). While SFB is not thought to be causative in pathogenesis of IBD or cancer, other microbes with similar IL-17-inducing activities, including Staphylococcus saprophyticus, Bifidobacterium adolescentis, and enterotoxigenic Bacteroides fragilis (ETBF), are thought to contribute to the disease process(Atarashi et al., 2015; Tan et al., 2016). A recent study demonstrated that IL-17 produced in response to ETBF colonization in the intestine of MinApc+/Δ716 mice promotes colon cancer initiation and progression (Housseau et al., 2016). The concept that microbial colonization can predispose cancer development is further supported by the observation that Helicobacter pylori–associated gastritis often paves the way for gastric cancer in an IL-17 dependent manner (Bagheri et al., 2015). In humans, the link between specific classes of microbes and CRC development is emerging. Mucus-invasive bacterial biofilms of B fragilis, F nucleatum, Peptostreptococcus stomatis, Gemella morbilliform, and Parvimonas micra associate with tumors in patients with familial adenomatous polyposis(Dejea et al., 2018; Dejea et al., 2014). Whether these colorectal tumor polymicrobial biofilms are related to Th17 pathway remains to be determined. Nevertheless, these results suggest that the composition of the gut microbiome likely alters the balance between favorable Th1 and CD8+ cytotoxic T cell response versus unfavorable tolerogenic or Th17 immune responses and could thus mediate patient response to immunotherapy. Indeed, recent studies provided direct evidence that the microbiome community can influence responsiveness to checkpoint blockade therapy (Gopalakrishnan et al., 2018; Routy et al., 2018).
Patients with colorectal carcinoma (CRC) and DNA mismatch repair deficiency (Lynch syndrome) tend to have tumors with high mutational load, which can promote development of tumor antigen-specific CD8+ cytotoxic T cells. However, these tumor-infiltrating T cells have an exhausted phenotype with high surface expression of PD1 and are ineffective cytotoxic killers. Remarkably, many of these patients are highly responsive to checkpoint blockade treatment (Diaz and Le, 2015; Le et al., 2017; Le et al., 2015) where the tumor infiltrating CD8+ cytotoxic T cells can be reactivated by anti-PD1 antagonists to effectively eradicate tumors. For this reason, the Food and Drug Administration approved anti-PD1 for CRC patients with DNA mismatch repair deficiency syndrome. However, only about 8% of the colon cancer patients have DNA mismatch repair deficiency resulting in “MicroSatellite Instability” (MSI), the rest of the CRC patients are “MicroSatellite “Stable” (MSS) and are completely unresponsive to anti-PD1 treatment. Of interest, the MSS patient population shows elevated level of IL-17 and associated expression of stromal factors that improve tumor cell survival, proliferation, and angiogenesis including PGE1, PGE2, VEGF, and MIP-2(Amicarella et al., 2017; Chung et al., 2013). Increased IL17A expression in CRC correlates with increased expression of vascular endothelial growth factor (VEGF) and enhanced tumor blood vessel density (Liu et al., 2011b). As angiogenesis is a rate-limiting step during cancer growth, anti-angiogenic agents that target VEGF receptor signaling pathway is an important therapeutic option for many cancer types. However, similar to other anticancer agents, most patients develop acquired resistance to anti-angiogenic drugs. There is increasing evidence that resistance to anti-VEGF therapies could be in part due to IL-17 promoting multiple pathways supporting wound healing related mechanisms (Chung et al., 2013). IL-17 is known to activate stromal cells to produce angiogenic and immune-suppressive molecules such as prokineticin2, matrix metalloproteinase 9 and proinflammatory S100A8 and S100A9 molecules (calprotectin), thereby mediating resistance to anti-VEGF treatment (Chung et al., 2013). In addition, as discussed above, IL-17 promotes proliferation of skin epithelial cells to promote tumorigenesis (Chen et al., 2019; Langowski et al., 2006). These ideas support the concept that combining anti-VEGF with anti-IL-17 or anti-IL-23 therapy could improve patient outcomes in some cancers.
Concluding remarks
IL-17 has come a long way in 25 years: from an obscure cytokine belonging to a poorly-understood cytokine subfamily to a signature of a key T helper cell population and a key therapeutic target in human autoimmune disease. It will be interesting to follow the progress of targeting this cytokine in other diseases currently still in the testing phase, not only from the standpoint of treating patients but also as these results reveal unexpected biology of different autoimmune conditions in humans. IL-17 functions have proven to be more varied, adaptable and often more subtle than the initial revelation that it can be a critical orchestrator of devastating tissue damage. Our understanding of these nuances is still in its infancy, particularly in the areas of synergistic signaling and tuning of IL-17 responses by diverse cytokines and microbial stimuli. Similarly, IL-17 has been observed in a diverse range of tissues and immunopathologic conditions, yet its context-specific contributions to both health and disease are only just beginning to be appreciated (Figure 5).
Figure 5: IL-17 protective and pathologic functions.
While IL-17 has received the lion’s share of attention, other IL-17 family members appear to have diverse roles in preventing and promoting cancer, protection from viruses and local tissue inflammation. The functions of these members, particularly IL-17B, IL-17C and IL-17D, have barely been studied. This has been in part due to limited tools, but also because the functional importance of these cytokines was unclear and perhaps because they can be produced and act on non-immune cells, thus escaping immunologists’ attention. However, recent evidence associating these IL-17 family members with human disease, for example the association of IL-17C with psoriasis and IL-17B with cancer, should spur further investigation of their biology.
One of the most compelling therapeutic advances in medicine over the past decade has been in the targeting of immune checkpoint inhibitors for cancer immunotherapy, for which Tasuku Honjo and James Allison won the 2018 Nobel Prize in Medicine and Physiology. Perhaps unsurprisingly, unleashing T cell activation against tumors comes with a high risk for collateral tissue damage, with many patients developing some form of autoimmune inflammation that can include encephalitis, myasthenia gravis, adrenal insufficiency, thyroid diseases, type 1 diabetes, reactive arthritis, pneumonitis, myocarditis, colitis, autoimmune hepatitis, vitiligo or psoriasis. Thus we are faced with a dilemma: it is becoming clear that autoimmunity is the “Achilles’ heel of cancer immunotherapy” (June et al., 2017). Some autoimmune-like inflammation is considered a good clinical prognostic indicator, but it remains unclear whether this represents inflammation that is involved in tumor-targeting or merely a by-product of systemic immune activation. The role of IL-17 in driving either response in checkpoint inhibitor-treated cancer patients is similarly unclear but could provide a potential avenue to target side effects if not required for anti-tumor efficacy. A recent case report (Esfahani and Miller, 2017) describes a male patient with metastatic colon cancer who experienced severe flare of previously mild psoriasis following treatment with pembrolizumab (Anti-PD1). Anti-IL-17 was administered and cleared the psoriasis, but unfortunately the cancer progressed, suggesting that IL-17 may have anti-tumor effects. Since immune-related adverse effects occurs in more than half of the patients responding to pembrolizumab treatment, it is impossible to determine cause with this sample size. With the rise of cancer immunotherapy, along with sophisticated tools such as single-cell gene expression analysis, the next 25 years will no doubt reveal more surprising functions of IL-17 in human tissue homeostasis, repair and pathogenesis.
Acknowledgements:
Funding from NIH to MJM: AI110822 and SLG: DE0225500 and AI107825.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Interests:
DJC is an employee of Merck & Co. SLG has consulted for Janssen, Eli Lilly and Merck.
References
- Ahmed M, and Gaffen SL (2013). IL-17 inhibits adipogenesis in part via C/EBPalpha, PPARgamma and Kruppel-like factors. Cytokine 61, 898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimzhanov AM, Yang XO, and Dong C (2007). Chromatin remodeling at IL17-IL-17F cytokine gene locus during inflammatory helper T cell differentiation. The Journal of biological chemistry 282, 5969–5972. [DOI] [PubMed] [Google Scholar]
- Akira S (2013). Regnase-1, a Ribonuclease Involved in the Regulation of Immune Responses. Cold Spring Harb Symp Quant Biol 78, 51–60. [DOI] [PubMed] [Google Scholar]
- Albanesi C, Cavani A, and Girolomoni G (1999). IL-17 is produced by nickel-specific T lymphocytes and regulates ICAM-1 expression and chemokine production in human keratinocytes: Synergistic or antagonistic effects with IFN-g and TNF-a. J. Immunol 162, 494–502. [PubMed] [Google Scholar]
- Amatya N, EE C, Cruz JA, Aggor F, Garg A, Berman A, Gudjonsson JE, Atasoy U, and Gaffen SL (2018). IL-17 integrates multiple self-reinforcing, feed-forward mechanisms through the RNA-binding protein Arid5a. Science Signaling 11, eaat4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatya N, Garg AV, and Gaffen SL (2017). IL-17 Signaling: The Yin and the Yang. Trends Immunol 38, 310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amicarella F, Muraro MG, Hirt C, Cremonesi E, Padovan E, Mele V, Governa V, Han J, Huber X, Droeser RA, et al. (2017). Dual role of tumour-infiltrating T helper 17 cells in human colorectal cancer. Gut 66, 692–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir M, Chaudhari S, Wang R, Campbell S, Mosure SA, Chopp LB, Lu Q, Shang J, Pelletier OB, He Y, et al. (2018). REV-ERBalpha Regulates TH17 Cell Development and Autoimmunity. Cell Rep 25, 3733–3749 e3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonysamy MA, Fanslow WC, Fu F, Li W, Qian S, Troutt AB, and Thomson AW (1999). Evidence for a role of IL-17 in organ allograft rejection: IL-17 promotes the functional differentiation of dendritic cell progenitors. J. Immunol 162, 577–584. [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Ando M, Kamada N, Nagano Y, Narushima S, Suda W, Imaoka A, Setoyama H, Nagamori T, et al. (2015). Th17 Cell Induction by Adhesion of Microbes to Intestinal Epithelial Cells. Cell 163, 367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeten D, Ostergaard M, Wei JC, Sieper J, Jarvinen P, Tam LS, Salvarani C, Kim TH, Solinger A, Datsenko Y, et al. (2018). Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann Rheum Dis 77, 1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeten D, Sieper J, Braun J, Baraliakos X, Dougados M, Emery P, Deodhar A, Porter B, Martin R, Andersson M, et al. (2015). Secukinumab, an Interleukin-17A Inhibitor, in Ankylosing Spondylitis. N Engl J Med 373, 2534–2548. [DOI] [PubMed] [Google Scholar]
- Bagheri N, Azadegan-Dehkordi F, Shirzad H, Rafieian-Kopaei M, Rahimian G, and Razavi A (2015). The biological functions of IL-17 in different clinical expressions of Helicobacter pylori-infection. Microb Pathog 81, 33–38. [DOI] [PubMed] [Google Scholar]
- Bär E, Gladiator A, Bastidas S, Roschitzki B, Acha-Orbea H, Oxenius A, and LeibundGut-Landmann S (2012). A novel Th cell epitope of Candida albicans mediates protection from fungal infection. J Immunol 188, 5636–5643. [DOI] [PubMed] [Google Scholar]
- Beale J, Jayaraman A, Jackson DJ, Macintyre JDR, Edwards MR, Walton RP, Zhu J, Man Ching Y, Shamji B, Edwards M, et al. (2014). Rhinovirus-induced IL-25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation. Sci Transl Med 6, 256ra134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, Sita G, Racchumi G, Ling L, Pamer EG, et al. (2016). Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat Med 22, 516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Harrington LE, and Jope RS (2013). Inflammatory T helper 17 cells promote depression-like behavior in mice. Biol Psychiatry 73, 622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, and Lowell JA (2018). Th17 cells in depression. Brain Behav Immun 69, 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bie Q, Jin C, Zhang B, and Dong H (2017a). IL-17B: A new area of study in the IL-17 family. Mol Immunol 90, 50–56. [DOI] [PubMed] [Google Scholar]
- Bie Q, Zhang B, Sun C, Ji X, Barnie PA, Qi C, Peng J, Zhang D, Zheng D, Su Z, et al. (2017b). IL-17B activated mesenchymal stem cells enhance proliferation and migration of gastric cancer cells. Oncotarget 8, 18914–18923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein C, Nevo S, Giladi A, Kadouri N, Pouzolles M, Gerbe F, David E, Machado A, Chuprin A, Toth B, et al. (2018). Single-cell mapping of the thymic stroma identifies IL-25-producing tuft epithelial cells. Nature 559, 622–626. [DOI] [PubMed] [Google Scholar]
- Buckley KM, Ho ECH, Hibino T, Schrankel CS, Schuh NW, Wang G, and Rast JP (2017). IL17 factors are early regulators in the gut epithelium during inflammatory response to Vibrio in the sea urchin larva. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulek K, Liu C, Swaidani S, Wang L, Page RC, Gulen MF, Herjan T, Abbadi A, Qian W, Sun D, et al. (2011). The inducible kinase IKKi is required for IL-17-dependent signaling associated with neutrophilia and pulmonary inflammation. Nature immunology 12, 844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SH, and Dong C (2007). A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res 17, 435–440. [DOI] [PubMed] [Google Scholar]
- Chang SH, Park H, and Dong C (2006). Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. The Journal of biological chemistry 281, 35603–35607. [DOI] [PubMed] [Google Scholar]
- Chen F, Cao A, Yao S, Evans-Marin HL, Liu H, Wu W, Carlsen ED, Dann SM, Soong L, Sun J, et al. (2016). mTOR Mediates IL-23 Induction of Neutrophil IL-17 and IL-22 Production. J Immunol 196, 4390–4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Cai G, Liu C, Zhao J, Gu C, Wu L, Hamilton TA, Zhang CJ, Ko J, Zhu L, et al. (2019). IL-17R-EGFR axis links wound healing to tumorigenesis in Lrig1(+) stem cells. The Journal of experimental medicine 216, 195–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiricozzi A, Guttman-Yassky E, Suarez-Farinas M, Nograles KE, Tian S, Cardinale I, Chimenti S, and Krueger JG (2011). Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. The Journal of investigative dermatology 131, 677–687. [DOI] [PubMed] [Google Scholar]
- Chiricozzi A, and Krueger JG (2013). IL-17 targeted therapies for psoriasis. Expert Opin Investig Drugs 22, 993–1005. [DOI] [PubMed] [Google Scholar]
- Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, Hoeffer CA, Littman DR, and Huh JR (2016). The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351, 933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung AS, Wu X, Zhuang G, Ngu H, Kasman I, Zhang J, Vernes JM, Jiang Z, Meng YG, Peale FV, et al. (2013). An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat Med 19, 1114–1123. [DOI] [PubMed] [Google Scholar]
- Conti H, Bruno V, Childs E, Daugherty S, Hunter J, Mengesha B, Saevig D, Hendricks M, Coleman BM, Brane L, et al. (2016). IL-17RA signaling in oral epithelium is critical for protection against oropharyngeal candidiasis. Cell Host Microbe 20, 606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti H, Shen F, Nayyar N, Stocum E, JN S, Lindemann M, Ho A, Hai J, Yu J, Jung J, et al. (2009). Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. The Journal of experimental medicine 206, 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti HR, and Gaffen SL (2015). IL-17-Mediated Immunity to the Opportunistic Fungal Pathogen Candida albicans. J Immunol 195, 780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz J, Childs E, Amatya N, Garg A, Beyaert R, Kane LP, Aneskievich BJ, Ma A, and Gaffen SL (2017). Interleukin-17 signaling triggers degradation of the constitutive NF-κB inhibitor ABIN-1. ImmnoHorizons 1, 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cua DJ, and Tato CM (2010). Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol 10, 479–489. [DOI] [PubMed] [Google Scholar]
- Datta S, Novotny M, Pavicic PG Jr., Zhao C, Herjan T, Hartupee J, and Hamilton T (2010). IL-17 regulates CXCL1 mRNA stability via an AUUUA/tristetraprolin-independent sequence. J Immunol 184, 1484–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejea CM, Fathi P, Craig JM, Boleij A, Taddese R, Geis AL, Wu X, DeStefano Shields CE, Hechenbleikner EM, Huso DL, et al. (2018). Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science 359, 592–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejea CM, Wick EC, Hechenbleikner EM, White JR, Mark Welch JL, Rossetti BJ, Peterson SN, Snesrud EC, Borisy GG, Lazarev M, et al. (2014). Microbiota organization is a distinct feature of proximal colorectal cancers. Proc Natl Acad Sci U S A 111, 18321–18326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz LA Jr., and Le DT (2015). PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 373, 1979. [DOI] [PubMed] [Google Scholar]
- Drummond RA, and Lionakis MS (2018). Organ-specific mechanisms linking innate and adaptive antifungal immunity. Semin Cell Dev Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, et al. (2006). A Genome-Wide Association Study Identifies IL23R as an Inflammatory Bowel Disease Gene. Science 314, 1461–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutzan N, Abusleme L, Bridgeman H, Greenwell-Wild T, Zangerle-Murray T, Fife ME, Bouladoux N, Linley H, Brenchley L, Wemyss K, et al. (2017a). On-going Mechanical Damage from Mastication Drives Homeostatic Th17 Cell Responses at the Oral Barrier. Immunity 46, 133–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutzan N, Abusleme L, Bridgeman H, Greenwell-Wild T, Zangerle-Murray T, Fife ME, Bouladoux N, Linley H, Brenchley L, Wemyss K, et al. (2017b). On-going Mechanical Damage from Mastication Drives Homeostatic Th17 Cell Responses at the Oral Barrier. Immunity 46, 133–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esfahani K, and Miller WH Jr. (2017). Reversal of Autoimmune Toxicity and Loss of Tumor Response by Interleukin-17 Blockade. N Engl J Med 376, 1989–1991. [DOI] [PubMed] [Google Scholar]
- Eskan MA, Jotwani R, Abe T, Chmelar J, Lim JH, Liang S, Ciero PA, Krauss JL, Li F, Rauner M, et al. (2012). The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nature immunology 13, 465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faour WH, Mancini A, He QW, and Di Battista JA (2003). T-cell-derived interleukin-17 regulates the level and stability of cyclooxygenase-2 (COX-2) mRNA through restricted activation of the p38 mitogen-activated protein kinase cascade: role of distal sequences in the 3’-untranslated region of COX-2 mRNA. The Journal of biological chemistry 278, 26897–26907. [DOI] [PubMed] [Google Scholar]
- Faraco G, Brea D, Garcia-Bonilla L, Wang G, Racchumi G, Chang H, Buendia I, Santisteban MM, Segarra SG, Koizumi K, et al. (2018). Dietary salt promotes neurovascular and cognitive dysfunction through a gut-initiated TH17 response. Nat Neurosci 21, 240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farez MF, Mascanfroni ID, Mendez-Huergo SP, Yeste A, Murugaiyan G, Garo LP, Balbuena Aguirre ME, Patel B, Ysrraelit MC, Zhu C, et al. (2015). Melatonin Contributes to the Seasonality of Multiple Sclerosis Relapses. Cell 162, 1338–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann RM, Wagner F, Kivitz AJ, Mansikka HT, Khan N, Othman AA, Khatri A, Hong F, Jiang P, Ruzek M, and Padley RJ (2017). Safety, Tolerability, and Pharmacodynamics of ABT-122, a Tumor Necrosis Factor- and Interleukin-17-Targeted Dual Variable Domain Immunoglobulin, in Patients With Rheumatoid Arthritis. Arthritis Rheumatol 69, 2283–2291. [DOI] [PubMed] [Google Scholar]
- Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, et al. (2001). IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 15, 985–995. [DOI] [PubMed] [Google Scholar]
- Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin J-J, Garrone P, Garcia E, Saeland S, et al. (1996). T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med 183, 2593–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, and Blackshear PJ (2017). RNA-binding proteins in immune regulation: a focus on CCCH zinc finger proteins. Nat Rev Immunol 17, 130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffen SL (2011). Life before seventeen: Cloning of the IL-17 receptor. J Immunol 187, 4389–4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffen SL, Jain R, Garg AV, and Cua DJ (2014). The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol 14, 585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg A, Ahmed M, Vallejo A, Ma A, and Gaffen S (2013). The deubiquitinase A20 mediates feedback inhibition of Interleukin-17 receptor signaling. Science Signaling 6, ra44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg AV, Amatya N, Chen K, Cruz JA, Grover P, Whibley N, Conti HR, Hernandez Mir G, Sirakova T, Childs EC, et al. (2015). MCPIP1 Endoribonuclease Activity Negatively Regulates Interleukin-17-Mediated Signaling and Inflammation. Immunity 43, 475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom M, Weymar A, Bernreuther C, Velden J, Arunachalam P, Steinbach K, Orthey E, Arumugam TV, Leypoldt F, Simova O, et al. (2012). Neutralization of the IL-17 axis diminishes neutrophil invasion and protects from ischemic stroke. Blood 120, 3793–3802. [DOI] [PubMed] [Google Scholar]
- Genovese MC, Greenwald M, Cho CS, Berman A, Jin L, Cameron GS, Benichou O, Xie L, Braun D, Berclaz PY, and Banerjee S (2014). A phase II randomized study of subcutaneous ixekizumab, an anti-interleukin-17 monoclonal antibody, in rheumatoid arthritis patients who were naive to biologic agents or had an inadequate response to tumor necrosis factor inhibitors. Arthritis Rheumatol 66, 1693–1704. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, et al. (2018). Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami J, Hernandez-Santos N, Zuniga L, and Gaffen S (2009). A bone-protective role for IL-17 receptor signaling in ovariectomy-induced bone loss. Eur J Immunol 39, 2831–2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha HL, Wang H, Pisitkun P, Kim JC, Tassi I, Tang W, Morasso MI, Udey MC, and Siebenlist U (2014). IL-17 drives psoriatic inflammation via distinct, target cell-specific mechanisms. Proc Natl Acad Sci U S A 111, E3422–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T, Li X, Novotny M, Pavicic PG Jr., Datta S, Zhao C, Hartupee J, and Sun D (2012). Cell type- and stimulus-specific mechanisms for post-transcriptional control of neutrophil chemokine gene expression. J Leukoc Biol 91, 377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hams E, Armstrong ME, Barlow JL, Saunders SP, Schwartz C, Cooke G, Fahy RJ, Crotty TB, Hirani N, Flynn RJ, et al. (2014). IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc Natl Acad Sci U S A 111, 367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hams E, Locksley RM, McKenzie AN, and Fallon PG (2013). Cutting edge: IL-25 elicits innate lymphoid type 2 and type II NKT cells that regulate obesity in mice. J Immunol 191, 5349–5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Q, Das S, Hirano M, Holland SJ, McCurley N, Guo P, Rosenberg CS, Boehm T, and Cooper MD (2015). Characterization of Lamprey IL-17 Family Members and Their Receptors. J Immunol 195, 5440–5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, and Weaver CT (2005). Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 6, 1123–1132. [DOI] [PubMed] [Google Scholar]
- Herjan T, Hong L, Bubenik J, Bulek K, Qian W, Liu C, Li X, Chen X, Yang H, Ouyang S, et al. (2018). IL-17-receptor-associated adaptor Act1 directly stabilizes mRNAs to mediate IL-17 inflammatory signaling. Nature immunology 19, 354–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herjan T, Yao P, Qian W, Li X, Liu C, Bulek K, Sun D, Yang WP, Zhu J, He A, et al. (2013). HuR is required for IL-17-induced Act1-mediated CXCL1 and CXCL5 mRNA stabilization. J Immunol 191, 640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housseau F, Wu S, Wick EC, Fan H, Wu X, Llosa NJ, Smith KN, Tam A, Ganguly S, Wanyiri JW, et al. (2016). Redundant Innate and Adaptive Sources of IL17 Production Drive Colon Tumorigenesis. Cancer Res 76, 2115–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, Wehkamp J, Feagan BG, Yao MD, Karczewski M, et al. (2012). Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 61, 1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, et al. (2002). New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol 169, 443–453. [DOI] [PubMed] [Google Scholar]
- Hymowitz SG, Filvaroff EH, Yin JP, Lee J, Cai L, Risser P, Maruoka M, Mao W, Foster J, Kelley RF, et al. (2001). IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. Embo J 20, 5332–5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante-Duarte C, Horton HF, Byrne MC, and Kamradt T (2000). Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol 165, 6107–6115. [DOI] [PubMed] [Google Scholar]
- Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, et al. (2009). Differential roles of interleukin-17A and −17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 30, 108–119. [DOI] [PubMed] [Google Scholar]
- Israel L, Bardet M, Huppertz A, Mercado N, Ginster S, Unterreiner A, Schlierf A, Goetschy JF, Zerwes HG, Roth L, et al. (2018). A CARD10-Dependent Tonic Signalosome Activates MALT1 Paracaspase and Regulates IL-17/TNF-alpha-Driven Keratinocyte Inflammation. The Journal of investigative dermatology 138, 2075–2079. [DOI] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. (2009). Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeltsch KM, Hu D, Brenner S, Zoller J, Heinz GA, Nagel D, Vogel KU, Rehage N, Warth SC, Edelmann SL, et al. (2014). Cleavage of roquin and regnase-1 by the paracaspase MALT1 releases their cooperatively repressed targets to promote T(H)17 differentiation. Nat Immunol 15, 1079–1089. [DOI] [PubMed] [Google Scholar]
- Johansen C, Mose M, Ommen P, Bertelsen T, Vinter H, Hailfinger S, Lorscheid S, Schulze-Osthoff K, and Iversen L (2015). IkappaBzeta is a key driver in the development of psoriasis. Proc Natl Acad Sci U S A 112, E5825–5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston A, Fritz Y, Dawes SM, Diaconu D, Al-Attar PM, Guzman AM, Chen CS, Fu W, Gudjonsson JE, McCormick TS, and Ward NL (2013). Keratinocyte overexpression of IL-17C promotes psoriasiform skin inflammation. J Immunol 190, 2252–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June CH, Warshauer JT, and Bluestone JA (2017). Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat Med 23, 540–547. [DOI] [PubMed] [Google Scholar]
- Kafasla P, Skliris A, and Kontoyiannis DL (2014). Post-transcriptional coordination of immunological responses by RNA-binding proteins. Nature immunology 15, 492–502. [DOI] [PubMed] [Google Scholar]
- Kang Z, Wang C, Zepp J, Wu L, Sun K, Zhao J, Chandrasekharan U, DiCorleto PE, Trapp BD, Ransohoff RM, and Li X (2013). Act1 mediates IL-17-induced EAE pathogenesis selectively in NG2+ glial cells. Nature neuroscience 16, 1401–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsen JR, Borregaard N, and Cowland JB (2010). Induction of neutrophil gelatinase-associated lipocalin expression by co-stimulation with interleukin-17 and tumor necrosis factor-alpha is controlled by IkappaB-zeta but neither by C/EBP-beta nor C/EBP-delta. The Journal of biological chemistry 285, 14088–14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashem SW, Riedl MS, Yao C, Honda CN, Vulchanova L, and Kaplan DH (2015). Nociceptive Sensory Fibers Drive Interleukin-23 Production from CD301b+ Dermal Dendritic Cells and Drive Protective Cutaneous Immunity. Immunity 43, 515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kim H, Yim YS, Ha S, Atarashi K, Tan TG, Longman RS, Honda K, Littman DR, Choi GB, and Huh JR (2017). Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 549, 528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, Turner SP, Raskin L, Desai B, Faubion WA, de Waal Malefyt R, et al. (2009). Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med 206, 525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschek MA, Owyang AM, Joyce-Shaikh B, Langrish CL, Chen Y, Gorman DM, Blumenschein WM, McClanahan T, Brombacher F, Hurst SD, et al. (2007). IL-25 regulates Th17 function in autoimmune inflammation. J Exp Med 204, 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunder B, Mohamed MF, and Othman AA (2017). Population Pharmacokinetics of Upadacitinib in Healthy Subjects and Subjects with Rheumatoid Arthritis: Analyses of Phase I and II Clinical Trials. Clin Pharmacokinet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohanski MA, Workman AD, Patel NN, Hung LY, Shtraks JP, Chen B, Blasetti M, Doghramji L, Kennedy DW, Adappa ND, et al. (2018). Solitary chemosensory cells are a primary epithelial source of IL-25 in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol 142, 460–469 e467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlgruber AC, Gal-Oz ST, LaMarche NM, Shimazaki M, Duquette D, Nguyen HN, Mina AI, Paras T, Tavakkoli A, von Andrian U, et al. (2018). gammadelta T cells producing interleukin-17A regulate adipose regulatory T cell homeostasis and thermogenesis. Nat Immunol 19, 464–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontermann RE, and Brinkmann U (2015). Bispecific antibodies. Drug Discov Today 20, 838–847. [DOI] [PubMed] [Google Scholar]
- Kostulas N, Pelidou SH, Kivisakk P, Kostulas V, and Link H (1999). Increased IL-1beta, IL-8, and IL-17 mRNA expression in blood mononuclear cells observed in a prospective ischemic stroke study. Stroke 30, 2174–2179. [DOI] [PubMed] [Google Scholar]
- Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, et al. (1999). IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Invest 103, 1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Monin L, Castillo P, Elsegeiny W, Horne W, Eddens T, Vikram A, Good M, Schoenborn AA, Bibby K, et al. (2016). Intestinal Interleukin-17 Receptor Signaling Mediates Reciprocal Control of the Gut Microbiota and Autoimmune Inflammation. Immunity 44, 659–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Li D, Li C, Muehleisen B, Radek KA, Park HJ, Jiang Z, Li Z, Lei H, Quan Y, et al. (2012). The antimicrobial protein REG3A regulates keratinocyte proliferation and differentiation after skin injury. Immunity 37, 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, Basham B, McClanahan T, Kastelein RA, and Oft M (2006). IL-23 promotes tumour incidence and growth. Nature 442, 461–465. [DOI] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, and Cua DJ (2005). IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 201, 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprevotte E, Cochaud S, du Manoir S, Lapierre M, Dejou C, Philippe M, Giustiniani J, Frewer KA, Sanders AJ, Jiang WG, et al. (2017). The IL-17B-IL-17 receptor B pathway promotes resistance to paclitaxel in breast tumors through activation of the ERK1/2 pathway. Oncotarget 8, 113360–113372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et al. (2017). Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357, 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et al. (2015). PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 372, 2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gouvello S, Bastuji-Garin S, Aloulou N, Mansour H, Chaumette MT, Berrehar F, Seikour A, Charachon A, Karoui M, Leroy K, et al. (2008). High prevalence of Foxp3 and IL17 in MMR-proficient colorectal carcinomas. Gut 57, 772–779. [DOI] [PubMed] [Google Scholar]
- Lee JS, Tato CM, Joyce-Shaikh B, Gulen MF, Cayatte C, Chen Y, Blumenschein WM, Judo M, Ayanoglu G, McClanahan TK, et al. (2015). Interleukin-23-Independent IL-17 Production Regulates Intestinal Epithelial Permeability. Immunity 43, 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Casanova JL, and Puel A (2018). Mucocutaneous IL-17 immunity in mice and humans: host defense vs. excessive inflammation. Mucosal Immunol 11, 581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Vinh DC, Casanova JL, and Puel A (2017). Inborn errors of immunity underlying fungal diseases in otherwise healthy individuals. Curr Opin Microbiol 40, 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan JL, Harrison OJ, Han SJ, Byrd AL, Vujkovic-Cvijin I, Villarino AV, Sen SK, Shaik J, Smelkinson M, Tamoutounour S, et al. (2018). Non-classical Immunity Controls Microbiota Impact on Skin Immunity and Tissue Repair. Cell 172, 784–796 e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Qian W, Qian Y, Giltiay NV, Lu Y, Swaidani S, Misra S, Deng L, Chen ZJ, and Li X (2009). Act1, a U-box E3 ubiquitin ligase for IL-17 signaling. Sci Signal 2, ra63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Swaidani S, Qian W, Kang Z, Sun P, Han Y, Wang C, Gulen MF, Yin W, Zhang C, et al. (2011a). A CC’ Loop Decoy Peptide Blocks the Interaction Between Act1 and IL-17RA to Attenuate IL-17- and IL-25-Induced Inflammation. Sci Signal 4, ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Duan Y, Cheng X, Chen X, Xie W, Long H, Lin Z, and Zhu B (2011b). IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem Biophys Res Commun 407, 348–354. [DOI] [PubMed] [Google Scholar]
- Lubberts E (2015). The IL-23-IL-17 axis in inflammatory arthritis. Nat Rev Rheumatol 11, 415–429. [DOI] [PubMed] [Google Scholar]
- Luzza F, Parrello T, Monteleone G, Sebkova L, Romano M, Zarrilli R, Imeneo M, and Pallone F (2000). Up-regulation of IL-17 is associated with bioactive IL-8 expression in Helicobacter pylori-infected human gastric mucosa. J Immunol 165, 5332–5337. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Kurakawa T, Umemoto E, Motooka D, Ito Y, Gotoh K, Hirota K, Matsushita M, Furuta Y, Narazaki M, et al. (2016). Dysbiosis Contributes to Arthritis Development via Activation of Autoreactive T Cells in the Intestine. Arthritis Rheumatol 68, 2646–2661. [DOI] [PubMed] [Google Scholar]
- Maitra A, Shen F, Hanel W, Mossman K, Tocker J, Swart D, and Gaffen SL (2007). Distinct functional motifs within the IL-17 receptor regulate signal transduction and target gene expression. Proc. Natl. Acad. Sci, USA 104, 7506–7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda K, Ripley B, Nishimura R, Mino T, Takeuchi O, Shioi G, Kiyonari H, and Kishimoto T (2013). Arid5a controls IL-6 mRNA stability, which contributes to elevation of IL-6 level in vivo. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell JR, Zhang Y, Brown WA, Smith CL, Byrne FR, Fiorino M, Stevens E, Bigler J, Davis JA, Rottman JB, et al. (2015). Differential Roles for Interleukin-23 and Interleukin-17 in Intestinal Immunoregulation. Immunity 43, 739–750. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Mease PJ, Ritchlin CT, Rahman P, Gottlieb AB, Kirkham B, Kajekar R, Delicha EM, Pricop L, and Mpofu S (2017). Secukinumab sustains improvement in signs and symptoms of psoriatic arthritis: 2 year results from the phase 3 FUTURE 2 study. Rheumatology (Oxford) 56, 1993–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes IB, Sieper J, Braun J, Emery P, van der Heijde D, Isaacs JD, Dahmen G, Wollenhaupt J, Schulze-Koops H, Kogan J, et al. (2014). Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis 73, 349–356. [DOI] [PubMed] [Google Scholar]
- Mease P, and McInnes IB (2016). Secukinumab: A New Treatment Option for Psoriatic Arthritis. Rheumatol Ther 3, 5–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CN, Proekt I, von Moltke J, Wells KL, Rajpurkar AR, Wang H, Rattay K, Khan IS, Metzger TC, Pollack JL, et al. (2018). Thymic tuft cells promote an IL-4-enriched medulla and shape thymocyte development. Nature 559, 627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mino T, Murakawa Y, Fukao A, Vandenbon A, Wessels HH, Ori D, Uehata T, Tartey S, Akira S, Suzuki Y, et al. (2015). Regnase-1 and Roquin Regulate a Common Element in Inflammatory mRNAs by Spatiotemporally Distinct Mechanisms. Cell 161, 1058–1073. [DOI] [PubMed] [Google Scholar]
- Miossec P (2003). Interleukin-17 in rheumatoid arthritis: if T cells were to contribute to inflammation and destruction through synergy. Arthritis and rheumatism 48, 594–601. [DOI] [PubMed] [Google Scholar]
- Miyahara Y, Odunsi K, Chen W, Peng G, Matsuzaki J, and Wang RF (2008). Generation and regulation of human CD4+ IL-17-producing T cells in ovarian cancer. Proc Natl Acad Sci U S A 105, 15505–15510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monin L, Gudjonsson JE, Childs EE, Amatya N, Xing X, Verma AH, Coleman BM, Garg AV, Killeen M, Mathers A, et al. (2017). MCPIP1/Regnase-1 Restricts IL-17A- and IL-17C-Dependent Skin Inflammation. J Immunol 198, 767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S, Bouladoux N, Linehan JL, Han SJ, Harrison OJ, Wilhelm C, Conlan S, Himmelfarb S, Byrd AL, Deming C, et al. (2015). Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature 520, 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S, Larsen SB, Gomez NC, Alaverdyan K, Sendoel A, Yuan S, Polak L, Kulukian A, Chai S, and Fuchs E (2017). Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature 550, 475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novatchkova M, Leibbrandt A, Werzowa J, Neubuser A, and Eisenhaber F (2003). The STIR-domain superfamily in signal transduction, development and immunity. Trends Biochem Sci 28, 226–229. [DOI] [PubMed] [Google Scholar]
- Onishi RM, and Gaffen SL (2010). Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology 129, 311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owyang AM, Zaph C, Wilson EH, Guild KJ, McClanahan T, Miller HR, Cua DJ, Goldschmidt M, Hunter CA, Kastelein RA, and Artis D (2006). Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med 203, 843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel DD, and Kuchroo VK (2015). Th17 Cell Pathway in Human Immunity: Lessons from Genetics and Therapeutic Interventions. Immunity 43, 1040–1051. [DOI] [PubMed] [Google Scholar]
- Pavelka K, Chon Y, Newmark R, Lin SL, Baumgartner S, and Erondu N (2015). A study to evaluate the safety, tolerability, and efficacy of brodalumab in subjects with rheumatoid arthritis and an inadequate response to methotrexate. J Rheumatol 42, 912–919. [DOI] [PubMed] [Google Scholar]
- Peng T, Chanthaphavong RS, Sun S, Trigilio JA, Phasouk K, Jin L, Layton ED, Li AZ, Correnti CE, De van der Schueren W, et al. (2017). Keratinocytes produce IL-17c to protect peripheral nervous systems during human HSV-2 reactivation. J Exp Med 214, 2315–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisitkun P, Ha HL, Wang H, Claudio E, Tivy CC, Zhou H, Mayadas TN, Illei GG, and Siebenlist U (2012). Interleukin-17 cytokines are critical in development of fatal lupus glomerulonephritis. Immunity 37, 1104–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A, and Casanova J-L (2018). Arid5a makes the IL-17A/F-responsive pathway less arid. Science Signaling 11, eaau8876. [DOI] [PubMed] [Google Scholar]
- Puel A, Cypowji S, Bustamante J, Wright J, Liu L, Lim H, Migaud M, Israel L, Chrabieh M, Audry M, et al. (2011a). Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 332, 65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, Migaud M, Israel L, Chrabieh M, Audry M, et al. (2011b). Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 332, 65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, et al. (2007). The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nature immunology 8, 247–256. [DOI] [PubMed] [Google Scholar]
- Qian Y, Qin J, Cui G, Naramura M, Snow EC, Ware CF, Fairchild RL, Omori SA, Rickert RC, Scott M, et al. (2004). Act1, a negative regulator in CD40- and BAFF-mediated B cell survival. Immunity 21, 575–587. [DOI] [PubMed] [Google Scholar]
- Qu F, Gao H, Zhu S, Shi P, Zhang Y, Liu Y, Jallal B, Yao Y, Shi Y, and Qian Y (2012). TRAF6-Dependent Act1 Phosphorylation by the IkappaB Kinase-Related Kinases Suppresses Interleukin-17-Induced NF-kappaB Activation. Molecular and cellular biology 32, 3925–3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani K, Garg AV, Jawale CV, Conti HR, Whibley N, Jackson EK, Shiva SS, Horne W, Kolls JK, Gaffen SL, and Biswas PS (2016). The Kallikrein-Kinin System: A Novel Mediator of IL-17- Driven Anti-Candida Immunity in the Kidney. PLoS Pathog 12, e1005952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani K, Jawale CV, Verma AH, Coleman BM, Kolls JK, and Biswas PS (2018a). Unexpected kidney-restricted role for IL-17 receptor signaling in defense against systemic Candida albicans infection. JCI Insight 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani K, Pawaria S, Maers K, Huppler AR, Gaffen SL, and Biswas PS (2014). An essential role of interleukin-17 receptor signaling in the development of autoimmune glomerulonephritis. J Leukoc Biol 96, 463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani K, Tan RJ, Zhou D, Coleman BM, Jawale CV, Liu Y, and Biswas PS (2018b). IL-17 Receptor Signaling Negatively Regulates the Development of Tubulointerstitial Fibrosis in the Kidney. Mediators Inflamm 2018, 5103672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Carrozzi V, Sambandam A, Luis E, Lin Z, Jeet S, Lesch J, Hackney J, Kim J, Zhou M, Lai J, et al. (2011). IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat Immunol 12, 1159–1166. [DOI] [PubMed] [Google Scholar]
- Reynolds JM, Lee YH, Shi Y, Wang X, Angkasekwinai P, Nallaparaju KC, Flaherty S, Chang SH, Watarai H, and Dong C (2015). Interleukin-17B Antagonizes Interleukin-25-Mediated Mucosal Inflammation. Immunity 42, 692–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert R, Juglair L, Lim EX, Ang C, Wang CJH, Ebert G, Dolezal O, and Mackay CR (2017). A fully humanized IgG-like bispecific antibody for effective dual targeting of CXCR3 and CCR6. PLoS One 12, e0184278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, et al. (2018). Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97. [DOI] [PubMed] [Google Scholar]
- Rouvier E, Luciani M-F, Mattei M-G, Denizot F, and Golstein P (1993). CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a Herpesvirus Saimiri gene. J. Immunol 150, 5445–5456. [PubMed] [Google Scholar]
- Ruddy MJ, Wong GC, Liu XK, Yamamoto H, Kasayama S, Kirkwood KL, and Gaffen SL (2004). Functional cooperation between interleukin-17 and tumor necrosis factor-a is mediated by CCAAT/enhancer binding protein family members. The Journal of biological chemistry 279, 2559–2567. [DOI] [PubMed] [Google Scholar]
- Saddawi-Konefka R, Seelige R, Gross ET, Levy E, Searles SC, Washington A Jr., Santosa EK, Liu B, O’Sullivan TE, Harismendy O, and Bui JD (2016). Nrf2 Induces IL-17D to Mediate Tumor and Virus Surveillance. Cell Rep 16, 2348–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C, Rostron T, Cerundolo V, Pamer EG, Abramson SB, et al. (2013). Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife 2, e01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwandner R, Yamaguchi K, and Cao Z (2000). Requirement of tumor necrosis factor-associated factor (TRAF)6 in interleukin 17 signal transduction. J. Exp. Med 191, 1233–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelige R, Washington A Jr., and Bui JD (2017). The ancient cytokine IL-17D is regulated by Nrf2 and mediates tumor and virus surveillance. Cytokine 91, 10–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seko Y, Cole S, Kasprzak W, Shapiro BA, and Ragheb JA (2006). The role of cytokine mRNA stability in the pathogenesis of autoimmune disease. Autoimmun Rev 5, 299–305. [DOI] [PubMed] [Google Scholar]
- Shen F, Ruddy MJ, Plamondon P, and Gaffen SL (2005). Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-alpha-induced genes in bone cells. J Leukoc Biol 77, 388–399. [DOI] [PubMed] [Google Scholar]
- Shi Y, Ullrich SJ, Zhang J, Connolly K, Grzegorzewski KJ, Barber MC, Wang W, Wathen K, Hodge V, Fisher CL, et al. (2000). A novel cytokine receptor-ligand pair. Identification, molecular characterization, and in vivo immunomodulatory activity. J Biol Chem 275, 19167–19176. [DOI] [PubMed] [Google Scholar]
- Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, Iwaki T, Okada Y, Iida M, Cua DJ, et al. (2009). Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nature medicine 15, 946–950. [DOI] [PubMed] [Google Scholar]
- Shin HC, Benbernou N, Esnault S, and Guenounou M (1999). Expression of IL-17 in human memory CD45RO+ T lymphocytes and its regulation by protein kinase A pathway. Cytokine 11, 257–266. [DOI] [PubMed] [Google Scholar]
- Siffrin V, Radbruch H, Glumm R, Niesner R, Paterka M, Herz J, Leuenberger T, Lehmann SM, Luenstedt S, Rinnenthal JL, et al. (2010). In vivo imaging of partially reversible th17 cell-induced neuronal dysfunction in the course of encephalomyelitis. Immunity 33, 424–436. [DOI] [PubMed] [Google Scholar]
- Simon DW, McGeachy MJ, Bayir H, Clark RS, Loane DJ, and Kochanek PM (2017). The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol 13, 171–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somma D, Mastrovito P, Grieco M, Lavorgna A, Pignalosa A, Formisano L, Salzano AM, Scaloni A, Pacifico F, Siebenlist U, and Leonardi A (2015). CIKS/DDX3X Interaction Controls the Stability of the Zc3h12a mRNA Induced by IL-17. J Immunol 194, 3286–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonder SU, Paun A, Ha HL, Johnson PF, and Siebenlist U (2012). CIKS/Act1-mediated signaling by IL-17 cytokines in context: implications for how a CIKS gene variant may predispose to psoriasis. J Immunol 188, 5906–5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonder SU, Saret S, Tang W, Sturdevant DE, Porcella SF, and Siebenlist U (2011). IL-17-induced NF-kappaB activation via CIKS/Act1: physiologic significance and signaling mechanisms. J Biol Chem 286, 12881–12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Dai D, He X, Zhu S, Yao Y, Gao H, Wang J, Qu F, Qiu J, Wang H, et al. (2015). Growth Factor FGF2 Cooperates with Interleukin-17 to Repair Intestinal Epithelial Damage. Immunity 43, 488–501. [DOI] [PubMed] [Google Scholar]
- Song X, Zhu S, Shi P, Liu Y, Shi Y, Levin SD, and Qian Y (2011). IL-17RE is the functional receptor for IL-17C and mediates mucosal immunity to infection with intestinal pathogens. Nat Immunol 12, 1151–1158. [DOI] [PubMed] [Google Scholar]
- Starnes T, Broxmeyer HE, Robertson MJ, and Hromas R (2002). Cutting edge: IL-17D, a novel member of the IL-17 family, stimulates cytokine production and inhibits hemopoiesis. J Immunol 169, 642–646. [DOI] [PubMed] [Google Scholar]
- Sun D, Novotny M, Bulek K, Liu C, Li X, and Hamilton T (2011). Treatment with IL-17 prolongs the half-life of chemokine CXCL1 mRNA via the adaptor TRAF5 and the splicing regulatory factor SF2 (ASF). Nature immunology 12, 853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamassia N, Arruda-Silva F, Calzetti F, Lonardi S, Gasperini S, Gardiman E, Bianchetto-Aguilera F, Gatta LB, Girolomoni G, Mantovani A, et al. (2018). A Reappraisal on the Potential Ability of Human Neutrophils to Express and Produce IL-17 Family Members In Vitro: Failure to Reproducibly Detect It. Frontiers in immunology 9, 795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan TG, Sefik E, Geva-Zatorsky N, Kua L, Naskar D, Teng F, Pasman L, Ortiz-Lopez A, Jupp R, Wu HJ, et al. (2016). Identifying species of symbiont bacteria from the human gut that, alone, can induce intestinal Th17 cells in mice. Proc Natl Acad Sci U S A 113, E8141–E8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targan SR, Feagan B, Vermeire S, Panaccione R, Melmed GY, Landers C, Li D, Russell C, Newmark R, Zhang N, et al. (2016). A Randomized, Double-Blind, Placebo-Controlled Phase 2 Study of Brodalumab in Patients With Moderate-to-Severe Crohn’s Disease. Am J Gastroenterol 111, 1599–1607. [DOI] [PubMed] [Google Scholar]
- Taylor PR, Leal SM Jr., Sun Y, and Pearlman E (2014). Aspergillus and Fusarium corneal infections are regulated by Th17 cells and IL-17-producing neutrophils. J Immunol 192, 3319–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunissen MB, Koomen CW, de Waal Malefyt R, Wierenga EA, and Bos JD (1998). Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol 111, 645–649. [DOI] [PubMed] [Google Scholar]
- Tohyama M, Shirakata Y, Hanakawa Y, Dai X, Shiraishi K, Murakami M, Miyawaki S, Mori H, Utsunomiya R, Masuda K, et al. (2018). Bcl-3 induced by IL-22 via STAT3 activation acts as a potentiator of psoriasis-related gene expression in epidermal keratinocytes. Eur J Immunol 48, 168–179. [DOI] [PubMed] [Google Scholar]
- Torres T, Romanelli M, and Chiricozzi A (2016). A revolutionary therapeutic approach for psoriasis: bispecific biological agents. Expert opinion on investigational drugs 25, 751–754. [DOI] [PubMed] [Google Scholar]
- Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pages F, and Galon J (2011). Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res 71, 1263–1271. [DOI] [PubMed] [Google Scholar]
- Tsang M, Friesel R, Kudoh T, and Dawid I (2002). Identification of Sef, a novel modulator of FGF signalling. Nature Cell Biol. 4, 165–169. [DOI] [PubMed] [Google Scholar]
- Tsoi LC, Spain SL, Ellinghaus E, Stuart PE, Capon F, Knight J, Tejasvi T, Kang HM, Allen MH, Lambert S, et al. (2015). Enhanced meta-analysis and replication studies identify five new psoriasis susceptibility loci. Nat Commun 6, 7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoi LC, Spain SL, Knight J, Ellinghaus E, Stuart PE, Capon F, Ding J, Li Y, Tejasvi T, Gudjonsson JE, et al. (2012). Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat Genet 44, 1341–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP, and Lubberts E (2009). Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol 182, 5836–5845. [DOI] [PubMed] [Google Scholar]
- van Mens LJJ, van de Sande MGH, Menegatti S, Chen S, Blijdorp ICJ, de Jong HM, Fluri IA, Latuhihin TE, van Kuijk AWR, Rogge L, et al. (2018). Brief Report: Interleukin-17 Blockade With Secukinumab in Peripheral Spondyloarthritis Impacts Synovial Immunopathology Without Compromising Systemic Immune Responses. Arthritis Rheumatol 70, 1994–2002. [DOI] [PubMed] [Google Scholar]
- Veldoen M (2017). Interleukin 17 is a chief orchestrator of immunity. Nature immunology 18, 612–621. [DOI] [PubMed] [Google Scholar]
- Verma A, Richardson J, Zhou C, Coleman BM, Moyes D, Ho J, Huppler AR, Ramani K, McGeachy MJ, Mufazalov IA, et al. (2017). Oral epithelial cells orchestrate innate Type 17 responses to Candida albicans through the virulence factor Candidalysin. Sci Immunol 2, eeam8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Moltke J, Ji M, Liang HE, and Locksley RM (2016). Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529, 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Zhang S, Zheng G, Huang J, Songyang Z, Zhao X, and Lin X (2018). Gain-of-Function Mutation of Card14 Leads to Spontaneous Psoriasis-like Skin Inflammation through Enhanced Keratinocyte Response to IL-17A. Immunity 49, 66–79 e65. [DOI] [PubMed] [Google Scholar]
- Werner JL, Gessner MA, Lilly LM, Nelson MP, Metz AE, Horn D, Dunaway CW, Deshane J, Chaplin DD, Weaver CT, et al. (2011). Neutrophils produce interleukin 17A (IL-17A) in a dectin-1-and IL-23-dependent manner during invasive fungal infection. Infect Immun 79, 3966–3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whibley N, and Gaffen SL (2015). Gut-Busters: IL-17 Ain’t Afraid of No IL-23. Immunity 43, 620–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JF, Guo Y, Quazi A, Luxenberg DP, Bennett F, Ross JF, Qiu Y, Whitters MJ, Tomkinson KN, Dunussi-Joannopoulos K, et al. (2007). Identification of an Interleukin 17F/17A Heterodimer in Activated Human CD4+ T Cells. The Journal of biological chemistry 282, 13447–13455. [DOI] [PubMed] [Google Scholar]
- Wu HH, Hwang-Verslues WW, Lee WH, Huang CK, Wei PC, Chen CL, Shew JY, Lee EY, Jeng YM, Tien YW, et al. (2015a). Targeting IL-17B-IL-17RB signaling with an anti-IL-17RB antibody blocks pancreatic cancer metastasis by silencing multiple chemokines. J Exp Med 212, 333–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, and Mathis D (2010). Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 32, 815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Chen X, Zhao J, Martin B, Zepp JA, Ko JS, Gu C, Cai G, Ouyang W, Sen G, et al. (2015b). A novel IL-17 signaling pathway controlling keratinocyte proliferation and tumorigenesis via the TRAF4-ERK5 axis. J Exp Med 212, 1571–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu NL, Huang DY, Tsou HN, Lin YC, and Lin WW (2015c). Syk mediates IL-17-induced CCL20 expression by targeting Act1-dependent K63-linked ubiquitination of TRAF6. The Journal of investigative dermatology 135, 490–498. [DOI] [PubMed] [Google Scholar]
- Yang YF, Lee YC, Lo S, Chung YN, Hsieh YC, Chiu WC, and Yuan SF (2018). A positive feedback loop of IL-17B-IL-17RB activates ERK/beta-catenin to promote lung cancer metastasis. Cancer Lett 422, 44–55. [DOI] [PubMed] [Google Scholar]
- Yao Z, Fanslow WC, Seldin MF, Rousseau A-M, Painter SL, Comeau MR, Cohen JI, and Spriggs MK (1995a). Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity 3, 811–821. [DOI] [PubMed] [Google Scholar]
- Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, and Armitage RJ (1995b). Cutting Edge: Human IL-17: A novel cytokine derived from T cells. J. Immunol 155, 5483–5486. [PubMed] [Google Scholar]
- Yu JJ, Ruddy MJ, Wong GC, Sfintescu C, Baker PJ, Smith JB, Evans RT, and Gaffen SL (2007). An essential role for IL-17 in preventing pathogen-initiated bone destruction: recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals. Blood 109, 3794–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Rollins D, Ruhn KA, Stubblefield JJ, Green CB, Kashiwada M, Rothman PB, Takahashi JS, and Hooper LV (2013). TH17 cell differentiation is regulated by the circadian clock. Science 342, 727–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong B, Liu X, Wang X, Chang S, Liu X, Wang A, Reynolds J, and Dong C (2012). Negative regulation of IL-17-mediated signalling and inflammation by the ubiquitin-specific protease USP25. Nature immunology 13, 1110–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga LA, Shen WJ, Joyce-Shaikh B, Pyatnova EA, Richards AG, Thom C, Andrade SM, Cua DJ, Kraemer FB, and Butcher EC (2010). IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J Immunol 185, 6947–6959. [DOI] [PMC free article] [PubMed] [Google Scholar]